Institute of Oceanology, Chinese Academy of Sciences
Article Information
- LI Cuiping(李翠萍), YU Huahua(于华华), LI Rongfeng(李荣锋), XING Ronge(邢荣娥), LIU Song(刘松), LI Pengcheng(李鹏程)
- Investigation into the hemolytic activity of tentacle venom from jellyfish Cyanea nozakii Kishinouye
- Journal of Oceanology and Limnology, 34(2): 382-385
- http://dx.doi.org/10.1007/s00343-015-4322-4
Article History
- Received Nov. 18, 2014
- accepted in principle Jul. 14, 2015
2 Qingdao University of Technology, Qingdao 266300, China
Jellyfish of the Phylum Cnidaria possess nematocysts that produce venoms used for capturing prey and in self-defense against predators. The toxin from jellyfish had many bioactivities, such as enzymatic activity, cardiac toxicity, neurotoxicity, hemolysis, lethality, insecticidal activity and antioxidant activity (Radwan et al., 2000; Ramasamy et al., 2003; Li et al. 2005; Radwan et al., 2005; Yu et al., 2005, 2006; Xiao et al., 2009). Swimmers and fishermen are commonly stung by these jellyfish, resulting in local edema, tingling, shortness of breath, depressed blood pressure and even death. Such effects arise from the complex mixture of biologically active molecules that make up jellyfish venoms (Chung et al., 2001). Cyan ea nozakii Kishinouye (C . nozakii) is a giant cnidarians of class Scyphomedusae, order Semaeostomeae and family Cyaneidae. In recent years, C . nozakii has become more widely distributed in the East China Sea, the Yellow Sea and the Bohai Sea, and is abundant in late summer to early autumn. Because the mesogloea of C . nozakii is very thin, its nutritional and commercial value is low. Moreover, the toxin from mass aggregations of C . nozakii pollutes seawater resulting in death of the halobios and seriously damages the environment and commercial fisheries (Zhong et al., 2004; Dong et al., 2005). The bioactivity of venom from jellyfish species, such as Rhopilema esculentum Kishinouye, Aurelia aurita, Chironex fleckeri and Cassiopea xamachana have been reported previously (Radwan et al., 2001; Torres et al., 2001; Winter et al., 2007; Li et al., 2013; Yu et al., 2007, 2014). We have previously studied the effects of temperature, pH, proteases, divalent cations and EDTA on the hemolytic activity of nematocyst venom from C . nozakii(Feng et al., 2010a, b). Carbohydrate and protein inhibitors can affect venom activity; however, to date, there are no reports on the effect of carboxylmethyl chitosan, benzamidine or glycerol on the hemolytic activity of C . nozakii venom. In this paper, we investigated the hemolytic activity of tentacle venom from the jellyfish C . nozakii and the effects of additives, including carboxylmethyl chitosan, benzamidine and glycerol, on its activity.

|
| Figure 1 Dose-response curves for the hemolytic activity of CNV against dove and chicken erythrocytes |
The jellyfish C . nozakii was collected at the First Bathing Beach in Qingdao, Shandong Province, China, in August 2007. The oral arms with tentacles were manually excised in-vivo, packed in polythene bags, and frozen immediately at -20℃ until use. The frozen oral arms were autolyzed in cold (4℃) sodium phosphate buffer (PBS; pH 7.4, 10 mmol/L) for 2 days. After filtering any residual tentacles, the resultant fluid was clarified by centrifugation at 15 000 ×g for 20 min at 4℃ and used as crude protein (CNV). Sample protein concentrations were determined by the Bradford (1976) method using bovine serum albumin (BSA) as standard.
2.2 Hemolytic activity assay and cooperativity analysisThe hemolytic activity assay was performed according to the method previously described for venom of the jellyfish Casrybdes marsuoialis(Rottini et al., 1995). Briefly, 0.5 mL of a 0.05% suspension of erythrocytes in Krebs-Ringer phosphate buffer (KRP), pH 7.4, was incubated at 37℃ for 30 min with different amounts of CNV (total volume of reactive system was 5.0 mL). After centrifugation, the hemolytic activity
was evaluated spectrophotometrically at 414 nm by assaying the hemoglobin released in the supernatant. Reference samples were employed using hypotonic lysis with water as a 100% lysis reference and the supernatant of 0.05% erythrocyte suspension (0.5 mL) incubated with 4.5 mL KRP at 37℃ for 30 min as the 0% reference. Dose-response curves were analyzed using the following equation: H = H max /[1+(K /[CNV])n ], where H is the value of hemolysis fraction at each CNV concentration, H max is the maximal hemolysis fraction, K is the CNV concentration that produces 50% hemolysis and n is Hill’s cooperativity coefficient.
2.3 Assay of the effect of carboxylmethyl chitosan, benzamidine and glycerol on hemolytic activityDifferent amounts of carboxylmethyl chitosan, benzamidine and glycerol were added to the reactive hemolytic activity system and hemolytic activity determined according to the method described above. Chicken erythrocytes were used in this experiment.
The effect was characterizes as: Hemolytic ratio= A1 / A0, where A1 is the absorbance at A414 nm with the effect of the additives and A0 is the A414 nm without the effect of additives.
3 RESULT AND DISCUSSION 3.1 Hemolytic activity assay and cooperativity analysisFigure 1 shows two dose-response curves of the hemolytic activity of CNV. The curves were sigmoid which were suggestive of the cooperative phenomenon (Rottini et al., 1995). Cooperativity can be quantitatively analyzed via the Hill equation. When such an elaboration was performed on the data reported in Fig. 1, the K values (HU 50) for dove and chicken erythrocytes were 34 and 59 μg/mL, respectively, with Hill coefficient (n) values of 6.2 and 5.02, respectively. A Hill index greater than 1 is regarded as an indication of cooperativity between at least two molecules. The venom showed higher hemolytic activity against dove erythrocytes compared with chicken erythrocytes. In an earlier study, Gusmani et al.(1997)found discrepancies in the hemolytic activity of venom from the jellyfish Rhopolema nomadica against sheep, human and rabbit erythrocytes. This difference in susceptibility to lysis may be correlated with sphingomyelin levels in the lipid bilayers of each erythrocyte, as sphingomyelin is regarded as the favored receptor for hemolysis (Uechi et al., 2005).
3.2 Effect of carboxylmethyl chitosan on the hemolytic activity of CNVFigure 2 shows the complex effect of carboxylmethyl chitosan on the hemolytic activity of CNV. At a concentration of 0.06%, the hemolytic ratio was 1.0. At concentrations <0.06%, hemolytic activity was inhibited by carboxylmethyl chitosan but was enhanced at concentrations >0.06%. In general, hemolytic activity increased with increasing concentrations of carboxylmethyl chitosan. In previous studies, Chung et al.(2001)reported that 10 mmol/L d-fucose had no significant inhibitory effect on the hemolytic activity of crude venom from Carybedea alata . Rottini et al.(1995)reported that an inhibitory ratio of 5 mmol/L l-fucose on the hemolytic activity of venom from Carybdea marsuoialis of was 2%. It has been suggested that jellyfish venoms cause pore-like structures in target membranes resulting in rapid cell lysis. It was reported that Carybdea marsuoialis toxin can induce pores of a diameter ranging from 0.84 to 1.08 nm (Rottini et al., 1995). Although the molecular diameter of carboxylmethyl chitosan is greater than that of sucrose, fucose and glucose, it enhanced hemolytic activity at concentrations >0.06%, which may be attributed to the charge of the amido groups in carboxylmethyl chitosan.
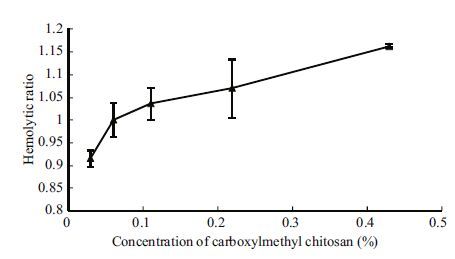
|
| Figure 2 Effect of carboxylmethyl chitosan on the hemolytic activity of CNV |
Previous studies have recorded that carbohydrates could inhibit the hemolytic activity of some jellyfish venom (Rottini et al., 1995; Chung et al., 2001); however, to our knowledge, there are no reports on the hemolysis being increased by carbohydrates. This paper is the first to report that polysaccharides can enhance the hemolysis of jellyfish venom.
3.3 Effect of benzamidine on the hemolytic activity of CNVChung et al.(2001)reported that protease could degrade the protein of hemolysis from jellyfish and, thus, decrease hemolytic activity. As it was confirmed that C . nozakii venom contained protease (the determination of the effects of glycerol on protease activity of CNV was consistent with the method of Li et al.(2005). At a concentration of 0.15 mol/L glycerol, protease activity improved 1.25-fold), so the effect of a protease inhibitor on hemolytic activity was also assayed. Figure 3 shows the effect of benzamidine on the hemolytic activity of CNV. Hemolytic activity is slightly inhibited by benzamidine and, as benzamidine is a serine protease inhibitor, this result may be explained by the fact that no serine protease is in CNV .
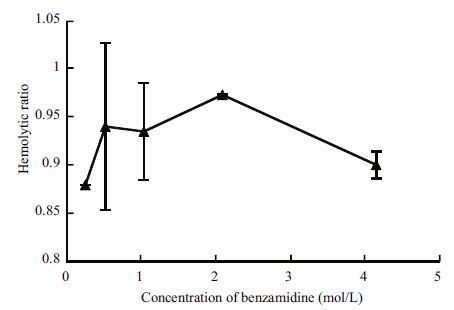
|
| Figure 3 Effect of benzamidine on the hemolytic activity of CNV |
Figure 4 clearly shows that hemolytic activity was affected by glycerol. At glycerol concentrations <0.18 mol/L, hemolytic activity was reduced slightly but, at concentrations >0.18 mol/L, hemolytic activity was enhanced with increasing concentrations; at a concentration of 0.36 mol/L, the hemolytic ratio was 1.4. Glycerol improves protease activity of CNV; thus, at glycerol concentrations <0.2 mol/L, protease activity increased and accelerated the hydrolyzing active proteins, resulting in reduced hemolytic activity. However, glycerol has strong co-solvency and can change the thermodynamic characteristics of a solution. A balance can be established when hydration of the protein surface is complete and totally combined with the co-solvent. In this case, the balance would stabilize the protein. Thus, at glycerol concentrations >0.2 mol/L, the effect of stabilization on the active protein may be higher than the effect of increased protease activity; therefore, hemolytic activity is gradually increased.

|
| Figure 4 Effect of glycerol on the hemolytic activity of CNV |
In conclusion, C . nozakii venom has hemolytic activity which, in addition, is affected by a number of chemical factors. The venom had stronger hemolytic activity against chicken erythrocytes compared with dove erythrocytes. Carboxylmethyl chitosan and glycerol increased hemolytic activity at concentrations greater than 0.06% and 0.2 mol/L, respectively. Further studies on the hemolytic mechanism and its biochemistry are currently in progress.
| Bradford M M, 1976. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem., 72 (1-2) : 248 –254. Doi: 10.1016/0003-2697(76)90527-3 |
| Chung J J, Ratnapala L A, Cooke I M, Yanagihara A A, 2001. Partial purification and characterization of a hemolysin(CAH1) from Hawaiian box jellyfish(Carybdea alata)venom. Toxicon, 39 (7) : 981 –990. Doi: 10.1016/S0041-0101(00)00237-3 |
| Dong J, Liu C Y, Li W Q, Yu H B, Wang B, Wang Y Q, 2005. The morphology and structure of jellyfish(Cyanea nozakii Kishinouye). Fish. Sci., 24 (2) : 22 –23. |
| Feng J H, Yu H H, Li C P, Xing R E, Liu S, Wang L, Cai S B, Li P C, 2010a. Isolation and characterization of lethal proteins in nematocyst venom of the jellyfish Cyanea nozakii Kishinouye. Toxicon, 55 (1) : 118 –125. Doi: 10.1016/j.toxicon.2009.07.008 |
| Feng J H, Yu H H, Xing R E, Liu S, Wang L, Cai S B, Li P C, 2010b. Partial characterization of the hemolytic activity of the nematocyst venom from the jellyfish Cyanea nozakii Kishinouye. Toxico l. in Vitro, 24 (6) : 1750 –1756. Doi: 10.1016/j.tiv.2010.02.010 |
| Gusmani L, Avian M, Galil B, Patriarca P, Rottini G, 1997. Biologically active polypeptides in the venom of the jellyfish Rhopilema nomadica. Toxicon, 35 (5) : 637 –648. Doi: 10.1016/S0041-0101(96)00182-1 |
| Li C P, Yu H H, Liu S, Xing R E, Guo Z Y, Li P C, 2005. Factors affecting the protease activity of venom from jellyfish Rhopilema esculentum Kishinouye. Bioorg. Med.Chem. Lett., 15 (24) : 5370 –5374. Doi: 10.1016/j.bmcl.2005.09.010 |
| Li R F, Yu H H, Xing R E, Liu S, Qing Y K, Li K C, Li B, Meng X T, Cui J H, Li P C, 2013. Isolation and in vitro partial characterization of hemolytic proteins from the nematocyst venom of the jellyfish Stomolophus meleagris. Toxicol. in Vitro., 27 (6) : 1620 –1625. Doi: 10.1016/j.tiv.2013.04.004 |
| Radwan F F Y, Burnett J W, Bloom D A, Coliano T, Eldefrawi M E, Erderly H, Aurelian L, Torres M, Heimer-de la Cotera E P, 2001. A comparison of the toxinological characteristics of two Cassiopea and Aurelia species. Toxicon, 39 (2-3) : 245 –257. Doi: 10.1016/S0041-0101(00)00121-5 |
| Radwan F F Y, Gershwin L A, Burnett J W, 2000. Toxinological studies on the nematocyst venom of Chrysaora achlyos. Toxicon, 38 (11) : 1581 –1591. Doi: 10.1016/S0041-0101(00)00092-1 |
| Radwan F F Y, Román L G, Baksi K, Burnett J W, 2005. Toxicity and mAChRs binding activity of Cassiopea xamachana venom from Puerto Rican coasts. Toxicon, 45 (1) : 107 –112. Doi: 10.1016/j.toxicon.2004.10.002 |
| Ramasamy S, Isbister G K, Seymour J E, Hodgson W C, 2003. The in vitro effects of two chirodropid(Chironex fleckeri and Chiropsalmus sp. ) venoms:efficacy of box jellyfish antivenom. Toxicon, 41 (6) : 703 –711. |
| Rottini G, Gusmani L, Parovel E, Avian M, Patriarca P, 1995. Purification and properties of a cytolytic toxin in venom of the jellyfish Carybdea marsupi a lis. Toxicon, 33 (3) : 315 –326. Doi: 10.1016/0041-0101(94)00174-7 |
| Torres M, Aguilar M B, Falcón A, Sánchez L, Radwan F F Y, Burnett J W, Heimer-de la Cotera E P, Arellano R O, 2001. Electrophysiological and hemolytic activity elicited by the venom of the jellyfish Cassiopea xamachana. Toxicon, 39 (9) : 1297 –1307. Doi: 10.1016/S0041-0101(01)00081-2 |
| Uechi G, Toma H, Arakawa T, Sato Y, 2005. Biochemical and physiological analyses of a hemolytic toxin isolated from a sea anemone Actineria villosa. Toxicon, 45 (6) : 761 –766. Doi: 10.1016/j.toxicon.2005.01.015 |
| Winter K L, Isbister G K, Seymour J E, Hodgson WC, 2007. An in vivo examination of the stability of venom from the Australian box jellyfish Chironex fleckeri. Toxicon, 49 (6) : 804 –809. Doi: 10.1016/j.toxicon.2006.11.031 |
| Xiao L, He Q, Guo Y F, Zhang J, Nie F, Li Y, Ye X F, Zhang L M, 2009. Cyanea capillata tentacle-only extract as a potential alternative of nematocyst venom:its cardiovascular toxicity and tolerance to isolation and purification procedures. Toxicon, 53 (1) : 146 –152. Doi: 10.1016/j.toxicon.2008.10.023 |
| Yu H H, Li C P, Li R G, Xing R E, Liu S, Li P C, 2007. Factors influencing hemolytic activity of venom from the jellyfish Rhopilema Esculentum Kishinouye. Food Chem. Toxico l., 45 (7) : 1173 –1178. Doi: 10.1016/j.fct.2006.12.025 |
| Yu H H, Li R F, Dong X L, Xing R E, Liu S, Li P C, 2014. Efficacy of venom from tentacle of jellyfish Stomolophus meleagris(Nemopilema nomurai) against the cotton bollworm Helicoverpa armigera. Biomed Res. Int., 2014 : 315853 . |
| Yu H H, Liu X G, Dong X L, Li C P, Xing R E, Liu S, Li P C, 2005. Insecticidal activity of proteinous venom from tentacle of jellyfish Rhopilema esculentum Kishinouye. Bioorg. Med. Chem. Lett., 15 (22) : 4949 –4952. Doi: 10.1016/j.bmcl.2005.08.015 |
| Yu H H, Liu X G, Xing R E, Guo Zh Y, Wang P B, Li C P, Li P C, 2006. In vitro determination of antioxidant activity of proteins from jellyfish Rhopilema esculentum. Food Chem., 95 (1) : 123 –130. Doi: 10.1016/j.foodchem.2004.12.025 |
| Zhong X M, Tang J H, Liu P T, 2004. A study on the relationship between Cyanea nozakii Kishinouye breaking out and ocean ecosystem. Mod. Fish. Inf., 19 (3) : 15 –17. |
 2016, Vol. 34
2016, Vol. 34


