Institute of Oceanology, Chinese Academy of Sciences
Article Information
- ZHANG Hongyu(张宏宇), XIE Zeping(谢则平), LOU Tingting(娄婷婷), JIANG Peng(姜鹏)
- Isolation, identification, and cytotoxicity of a new isobenzofuran derivative from marine Streptomyces sp. W007
- Journal of Oceanology and Limnology, 34(2): 386-390
- http://dx.doi.org/10.1007/s00343-015-4326-0
Article History
- Received Nov. 19, 2014
- accepted in principle Mar. 10, 2016
2 Key Laboratory of Experimental Marine Biology, Institute of Oceanology, Chinese Academy of Sciences, Qingdao 266071, China;
3 School of Pharmacy, Binzhou Medical University, Yantai 264003, China;
4 Tianjin Entry & Exit Inspection and Quarantine Bureau, Tianjin 300461, China
Microbial natural products are an important source of both existing and novel drugs(Solanki et al., 2008). Among the producers of commercially important metabolites, actinomycetes have proven to be a prolific source, with a surprisingly small group of taxa accounting for the vast majority of compounds. For instance, chemical studies of the single genus Streptomyces has led to the discovery of actinomycin D, bleomycin, doxorubicin, chromomycin, carzinophilin, mithramycin, mitomycin C, sarkomycin, streptozocin, and neocarzinostatin during the past several decades(Bérdy, 2005).
In our previous search for new, biologically active metabolites from marine streptomycetes, two new angucyclinone antibiotics, including 3-hydroxy-1- keto-3-methyl-8-methoxy-1, 2, 3, 4-tetrahydro-benz[α] anthracene with significant inhibitory activity against human lung adenocarcinoma cell line A549 and kiamycin with high cytotoxicity against human leukemia cell line HL-60, and two new isobenzofuran derivatives were obtained from marine Streptomyces sp. W007(Zhang et al., 2011; Xie et al., 2012a, 2012b; Zhang et al., 2014). To obtain more structurally-new analogues, further screening of Streptomyces sp. W007 was carried out, and another novel isobenzofuran derivative(1) was isolated and identified. In this letter, we describe the isolation, structure elucidation, and bioactivities of the compound.
2 MATERIAL AND METHOD 2.1 General experimental proceduresNMR spectra were recorded on an AVANCE ⅢTM 500 spectrometers(Bruker Corp., Billerica, MA, USA) and tetramethylsilane(TMS) used as internal standard. Electrospray ionization(ESI) was recorded on a LCQ Fleet mass spectrometer(Thermo Fisher Scientific Inc., Waltham, MA, USA). Column chromatography was carried out on silica gel(200– 300 mesh), Sephadex LH-20(Amersham Biosciences AB, Uppsala, Sweden), and a PU-2087 HPLC instrument(Jasco Inc., Easton, MD, USA) equipped with a Zorbax Eclipse XDB-C18 column(Agilent Technologies, Santa Clara, CA, USA). Precoated silica gel plates(F-254, 0.2 mm, Merck Millipore, Billerica, MA, USA) were used for analytical TLC. All reagents were analytical grade.
2.2 Strain production and fermentationStreptomyces sp. W007, isolated from marine sediments of Jiaozhou Bay, Qingdao, was characterized according to the 16S rDNA(accession No. JN180126) in GenBank. Well-grown agar cultures of W007 were used to inoculate 1 L flasks each containing 300 mL M2+ medium and incubated at 180 r/min agitation and 28℃ for 2 d. The resulting culture was then used to inoculate 100 flasks each containing 300 mL M2+ medium and inoculum size was at 20%(v/v), and the flasks were held at 28℃ for 4d(pH 7.8 and 220 r/min agitation).
2.3 Extraction and isolation of compound 1The W007 crude extract was obtained according to a previously described method(Zhang et al., 2011). In the W007 crude extract isolation process, five fractions, 1–5, were obtained. Fraction 4 was further purified by Sephadex LH-20 to afford D1–D3, among which D2 was purified by reverse column chromatography with a stepwise gradient of methanol/ water(2/8–8/2) and detection by TLC to produce four fractions(E1–E4). E2–E4 were then subjected to semi-preparative HPLC eluted with CH 3 OH/H 2 O(80/20, v/v) and CH 3 OH/H 2 O(75/25, v/v) to afford compound 1(retention time 19.21 min, 3.63 mg).
2.4 Cancer cell lines and cytotoxicity testsPreliminary screening of cytotoxicities was carried out using human cell lines of lung cancer A549, gastric cancer BGC-823, and breast cancer MCF7 according to the methyl-thiazol-tetrazolium(MTT) assay previously described by Wang et al.(2008, 2011). Human cancer cell lines A549, BGC-823, and MCF7 were cultured in RPMI-1640 media supplemented with 10% fetal calf serum as previously described(Wang et al., 2008). Cell viability after treatment with various chemicals was evaluated by MTT assay. Briefly, cells were seeded into 96-well plates and then treated with test chemicals at the desired concentrations for 72 h. MTT solution was added to the wells, incubated for 2 h, the medium was removed, and then DMSO was added to each well. The plates were gently agitated until the color reaction was uniform and the OD 570 was determined using a microplate reader(Wellscan MK3, Labsystems Dragon, Helsinki, Finland). Media only-treated cells with DMSO served as indicators of 100% cell viability.
3 RESULT AND DISCUSSION 3.1 Compound 1 structural elucidationCompound 1(Figs. 1 and 2) was obtained as a colorless powder and showed a quasi-molecular ion [M + Na] + at m/z 373.2, [M - H] - at m/z 349.1 by ESIMS, which was consistent with the molecular formula C 21 H 18 O 5 (calc'd for 350.4). Its 1 H-NMR spectrum exhibited a methyl singlet at δ H 2.15(3H, d, J =1.5 Hz), two methoxyls at δ H 3.72(3H, s) and 3.58(3H, s), 7 aromatic methines signals at δ H 8.19(1H, d, J =1.8 Hz), 8.01(1H, d, J =8 Hz), 7.91(1H, dd, J =1.8, 8.05 Hz), 7.39(1H, t, 7.75 Hz), 7.06(1H, d, J =7.55 Hz), 6.96(1H, d, J =8.05 Hz), and 6.88(1H, q, J =1.5Hz). Two oxymethine protons at δ H 6.31(1H, s) and 6.20(1H, s) belonged to a heterocyclic ring, typical of an epoxide structure(Sasaki et al., 1988; Tsukuda et al., 1996; Puder et al., 2000). Additionally, 1, 2, 3-trisubstituted benzene protons at δ H 6.96, 7.39, and 7.06 and 1, 2, 4-trisubstituted benzene protons at δ H 8.01, 7.91, and 8.19 were observed. The 13 C-NMR of compound 1 displayed 21 signals(Table 1), which were classified as one methyl(δ C 15.36), two methoxyls(δ C 54.93 and 55.13), two methines of an epoxide structure(δ C 83.67 and 108.20), seven aromatic methines(δ C 111.28, 115.23, 125.05, 125.96, 130.58, 132.68, and 135.35), seven aromatic quaternary carbons(δ C 148.12, 131.47, 147.66, 139.58, 154.33, 132.19, and 129.53), and two ketone carbonyl carbons(δ C 184.88 and 184.46). As the molecular formula required 13 double bond equivalents, together with the partial structures mentioned above, compound 1 must have been tetracyclic. The methoxy signal(δ H 3.72) and H-6 triplet showed HMBC couplings with the same carbon at δ C 154.33 and the methoxy signal(δ H 3.58) showed HMBC couplings with the carbon at δ C 108.20, which led to assignment of the methoxy group(δ H 3.72) to C-4 and the other methoxy group(δ H 3.58) to C-1. Further HMBC signals with key correlations from H-1 to C-7(and vice versa) and from H-3 to C-4, 3a, 7a, 1, and 1, 2, 3-trisubstituted benzene protons at δ H 6.96, 7.39, and 7.06(H-H COSY correlations of H-5 to H-6 and H-6 to H-7) resulted in a dibasic isobenzofuran substructure(rings A and B in 1). The presence of the latter moiety was confirmed by HMBC correlations in the 1 H-NMR spectrum, with 1, 2, 4-trisubstituted benzene protons observed at δ8.01, 7.91, and 8.19, which was also supported by 1H- 1 HCOSY correlations of H-5' to H-6'. Further HMBC signals with key correlations from H-5' to C-4', 7'a, from H-6' to C-4'a, 7', from H-7' to C-1', 4'a, 6', from H-2' to C-4', 7'a, 3'-CH 3, and from 3'-CH 3 to C-2', 3', 4' resulted in a 3-methyl-1, 4-naphthoquinone substructure(rings C and D in 1). C-3 of isobenzofuran connected with C-6'a of 3-methyl-1, 4-naphthoquinone moiety on the basis of HMBC correlations from H-3 to C-5', 6', 7'(and vice versa) and from H-3 to C-6'a, 7'a. Based on this structural evidence, an isobenzofuran core was judged to be present in 1, containing a 3-methyl-1, 4-naphthoquinone moiety.
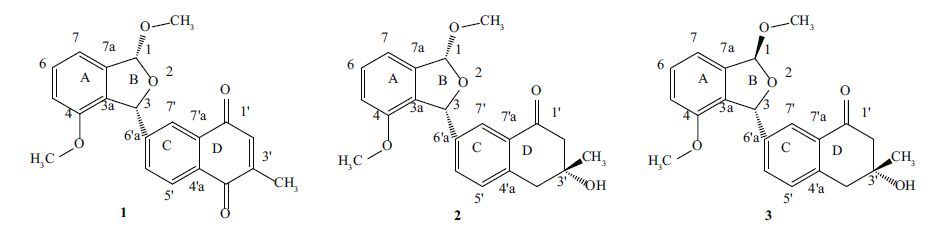
|
| Figure 1 Structures of compounds 1-3 |
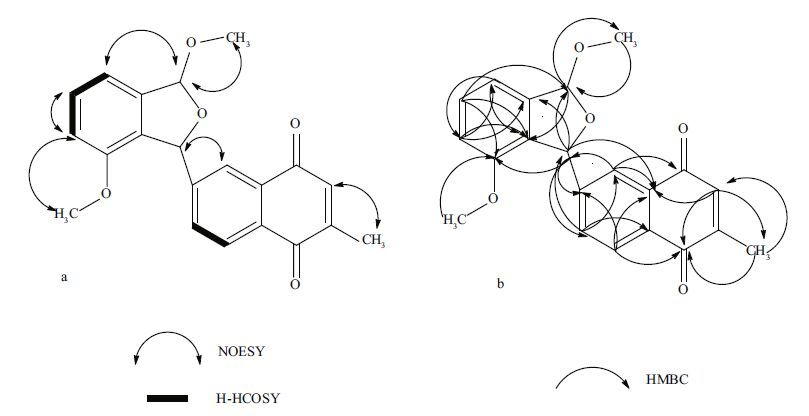
|
| Figure 2 a. key 1 H- 1 H COSY and selected NOE correlations of compound 1; b. HMBC correlations of compound 1 |
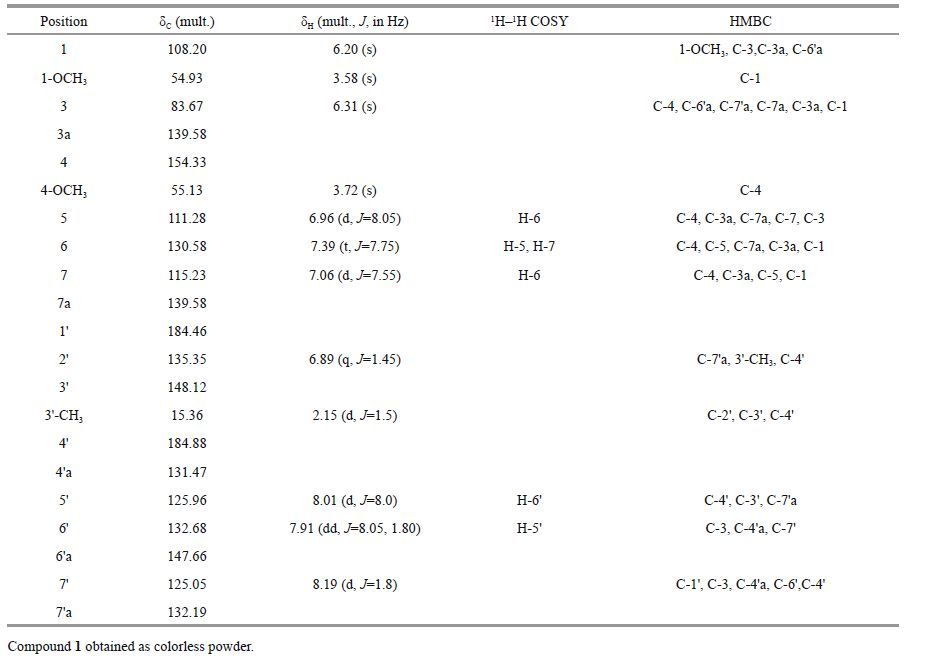
|
After identifying the skeletal structure of 1, the major feature was its stereochemistry for protons H-1 and H-3 on the two chiral centers. It has been reported that, in 2, 5-dihydrofurans and phthalans, 3 J H, H coupling constants are consistently larger for trans isomers than for cis(Barfield et al., 1975), and in compound 1, both H-1 and H-3 appeared as singlets and no COSY correlation existed(3 J H, H =0.00 Hz). These findings suggested compound 1 could be assigned a relative cis configuration. Furthermore, compound 1 also exhibited no NOE’s between H-3 and a methoxy group or between H-1 and H-6'. Another novel isobenzofuran derivative(compound 2 in Fig. 1) and its isomer(compound 3 in Fig. 1) previously reported had the same chiral centers of H-1 and H-3 as observed in compound 1 and their absolute configurations have been determined(Zhang et al., 2014). Therefore, according to previously reported data(NMR J -couplings and NOE’s, biogenetic facts, specific CD spectrum bands, and overall CD profiles), the same(1R* and 3R*) configuration was assigned to compound 1 as to compound 2 . By comparison of NMR data with the known analogues pestacin(Harper et al., 2003), isopestacin(Strobel et al., 2002), and 4-methoxy-3- H-isobenzofuran-1-one(Fotso et al., 2008), compound 1 was elucidated as 1, 4-dimethoxy-3-(3-methyl-1, 4-naphthoquinone)-isobenzofuran.
3.2 compound 1 cytotoxicitiesIn cytotoxicity tests, compound 1 exhibited 27.0% inhibition against human gastric cancer cell line BGC-823 at 10 -5 mol/L; compound 1 showed no effective cytotoxicity against human cells of gastric cancer BGC-823, lung cancer A549 and breast cancer MCF7, as shown in Table 2, compared with previously reported adriamycin and 1, 4-dimethoxy-3-(3- hydroxy-3-methyl-1-tetralone)-1(3H)-isobenzofuran(Xie et al., 2012).
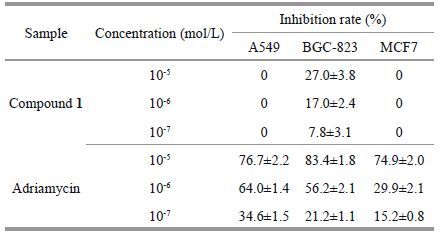
|
In this chemical and bioactive research, a novel isobenzofuran derivative (1) was isolated from the secondary metabolites of marine Streptomyces sp. W007 and its cytotoxicities were evaluated. Results showed that compound 1 did not show inhibitory activities against human gastric cancer cells BGC-823, breast cancer cells MCF7, and lung cancer cells A549.
| Barfield M, Spear R J, Sternhell S, 1975. Interproton spin-spin coupling across a dual path in 2, 5-dihydrofurans and phthalans. J. Am. Chem. Soc., 97 (18) : 5160 –5167. Doi: 10.1021/ja00851a023 |
| Bérdy J, 2005. Bioactive microbial metabolites. J. Antibiot., 58 (1) : 1 –26. Doi: 10.1038/ja.2005.1 |
| Fotso S, Mahmud T, Zabriskie T M, et al, 2008. Rearranged and unrearranged angucyclinones from Indonesian Streptomyces spp. J. Antibiot., 61 (7) : 449 –456. Doi: 10.1038/ja.2008.61 |
| Harper J K, Arif A M, Ford E J, Strobel G A, Porco J A Jr, Tomer D P, Oneill K L, Heider E M, Grant D M, 2003. Pestacin:a 1, 3-dihydro isobenzofuran from Pestalotiopsis microspora possessing antioxidant and antimycotic activities. Tetrahedron, 59 (14) : 2471 –2476. Doi: 10.1016/S0040-4020(03)00255-2 |
| Puder C, Zeeck A, Beil W, 2000. New biologically active rubiginones from Streptomyces sp. J. Antibiot., 53 (4) : 329 –336. Doi: 10.7164/antibiotics.53.329 |
| Sasaki T, Yoshida J, Itoh M, Gomi S, Shomura T, Sezaki M, 1988. New antibiotics SF2315A and B produced by an Excellospora sp. I. Taxonomy of the strain, isolation and characterization of antibiotics. J. Antibiot., 41 (7) : 835 –842. |
| Solanki R, Khanna M, Lal R, 2008. Bioactive compounds from marine actinomycetes. Indian J. Microbiol., 48 (4) : 410 –431. Doi: 10.1007/s12088-008-0052-z |
| Strobel G, Ford E, Worapong J, Harper J K, Arif A M, Grant D M, Fung P C W, Chau R M W, 2002. Isopestacin, an isobenzofuranone from Pestalotiopsis microspora, possessing antifungal and antioxidant activities. Phytochemistry, 60 (2) : 179 –183. Doi: 10.1016/S0031-9422(02)00062-6 |
| Tsukuda E, Tanaka T, Ochiai K, Kondo H, Yoshida M, Agatsuma T, Saitoh Y, Teshiba S, Matsuda Y, 1996. EI-1507-1 and-2, novel interleukin-1 ß converting enzyme inhibitors produced by Streptomyces sp. E-1507. J.Antibiot., 49 (4) : 333 –339. |
| Wang H B, Li H Y, Zuo M X, Zhang Y, Liu H, Fang W S, Chen X G, 2008. Lx2-32c, a novel taxane and its antitumor activities in vitro and in vivo. Cancer Lett., 268 (1) : 89 –97. Doi: 10.1016/j.canlet.2008.03.051 |
| Wang H B, Ma X J, Ren S M, Buolamwini J K, Yan C H, 2011. A small-molecule inhibitor of MDMX activates p53 and induces apoptosis. Mol. Cancer Ther., 10 (1) : 69 –79. Doi: 10.1158/1535-7163.MCT-10-0581 |
| Xie Z P, Liu B, Wang H P, Yang S X, Zhang H Y, Wang Y P, Ji N Y, Qin S, Laatsch H, 2012a. Kiamycin, a unique cytotoxic angucyclinone derivative from a marine Streptomyces sp. Mar. Drugs, 10 (3) : 551 –558. |
| Xie Z P, Zhang H Y, Li F C, Liu B, Yang S X, Wang H P, Pu Y, Chen Y, Qin S, 2012b. A new isobenzofuranone derivative from a marine Streptomyces sp. Chinese Chem. Lett., 23 (8) : 941 –944. Doi: 10.1016/j.cclet.2012.06.001 |
| Zhang H Y, Wang H P, Cui H L, Li Z G, Xie Z P, Pu Y, Li F C, Qin S, 2011. A new anthracene derivative from marine Streptomyces sp. W007 exhibiting highly and selectively cytotoxic activities. Mar. Drugs, 9 (9) : 1502 –1509. |
| Zhang S M, Zhang H Y, Yang S X, Qu C L, Xie Z P, Pescitelli G, 2014. Isolation, stereochemical study, and cytotoxic activity of isobenzofuran derivatives from a marine Streptomyces sp. Chirality, 27 (1) : 82 –87. |
 2016, Vol. 34
2016, Vol. 34


