Institute of Oceanology, Chinese Academy of Sciences
Article Information
- QIU Nianwei(邱念伟), ZHOU Feng(周峰), LIU Qian(刘倩), ZHAO Wenqian(赵文倩)
- Determination of photosynthetic parameters in two seawatertolerant vegetables
- Journal of Oceanology and Limnology, 34(2): 412-415
- http://dx.doi.org/10.1007/s00343-015-4337-x
Article History
- Received Dec. 11, 2014
- accepted in principle Jun. 24, 2015
2 School of Biochemical and Environmental Engineering, Nanjing Xiaozhuang University, Nanjing 211171, China
With the development of photosynthetic measurement technologies, photosynthetic instruments, such as CO2 and H2O infrared analyzers, have been more widely applied for plant research in laboratory. These instruments can be used to determine dozens of photosynthetic parameters (Pons and Welschen, 2002). Net photosynthetic rate (P N), transpiration rate (E), stomatal conductance (g s), and intercellular CO2 concentration (C i) are the four most common photosynthetic parameters. P N, E, and g s are usually expressed as CO 2 or H 2 O mass per unit leaf area per unit time (PP Systems Inc, 2010). Photosynthetic instruments can automatically calculate their values according to the fixed area of standard leaf chamber. However, if the leaf is too small, narrow, or anomalistic, the leaf area does not fit the fixed area of the standard leaf chamber. Photosynthetic parameters should be recalculated according to the actual leaf area (Donahue et al., 1997) resulting from image analyses, from leaf area meters, or by copy-weighing (Pandey and Singh, 2011; Ali et al., 2012). However, for some non-flat leaves (e.g. needle, linear, or cylindrical leaves), the gauged leaf area differs greatly from the actual leaf area. For example, the leaves of Suaeda salsa plants are bar-type, half-cylindrical, and succulent. Similarly, the photosynthetic organ of Salicornia bigelovii is a succulent bar-type stem with multiple knots, i.e., its leaves are reduced to scales. Both plants are seawatertolerant plants and have been developed for the highly successful vegetables. However, their photosynthetic area is difficult to be measured. Therefore, this new method is developed to measure the photosynthetic parameters using fresh mass rather than leaf area for these non-flat and irregular photosynthetic organs. The new method is a complement to the traditional method, which expends the use of photosynthetic instruments.
2 MATERIAL AND METHOD 2.1 Experimental materials and detailsSuaeda salsa and Salicornia bigelovii were used to test photosynthetic parameters per unit fresh mass compared to conventional method. Both species seedlings were grown in pots of sand moistened with half-strength seawater.
When the seedlings had four to five branches, gas exchange analysis was carried out using a Ciras-2 portable photosynthetic system (Hansatech, Hitchin, United Kingdom). Leaf P N, g s, and E were determined at a CO 2 concentration of 380 μmol/mol, 60% relative humidity, and a saturation light intensity of 1 000 μmol/(m2 ∙s). The leaf chamber were standard chamber (2 cm×6 cm). Leaf area analysis was performed using a LI-3000C Portable Area Meter (Li- Cor Biosciences, Lincoln, NE, USA). The fresh mass of photosynthetic materials was determined using a balance with a sensitivity of 0.1 mg.
2.2 Method for measuring photosynthetic parametersPhotosynthetic parameters are usually measured with photosynthetic tissues attached to plant (in-situ) using special leaf chamber (such as 6400-05 conifer chamber). But the succulent leaf or young stem of S . salsa and S . bigelovii is very friable, and would easily be crushed by the leaf chamber. We proposed a new method with photosynthetic materials isolated from plant body. The differences of P N acquired by usual method and the new method were compared in this paper. This new method is very simple and convenient and don’t need special leaf chamber. Concrete operations described below.
Cut offa section of young stem with leaves, and immediately placed the tissue into the leaf chamber for photosynthetic determination. Before the determination, S . salsa and S . bigelovii seedlings needed to be illuminated sufficiently to activate photosynthesis. When photosynthetic parameter reached a relatively stable value in the instrument, the reading was taken immediately, because the values for the tissue separated from plant would be lasted for only 1–3 min due to water loss. Sequentially the fresh tissue should be weighted as soon as possible. The fresh mass and the photosynthetic parameter reading were used to recalculate actual photosynthetic parameters based on the fresh mass.
The light/CO2 /temperature response curves of the plants also can be measured by this method. It is important to note that photosynthetic tissues must be attached to plant (in-situ) to determine these response curves, because the determination needs more time. These response curves can be measured with special 6400-05 conifer chamber (2 cm×6 cm)(Edward, 1989).
2.3 Illations and calculations of photosynthetic parametersAccording to the photosynthetic yield formula (Zhu et al., 2010), the calculation and illation of net photosynthetic rate (P N) is illustrated as follows. The calculation for E and g s is similar to P N . The Abbreviations of photosynthetic parameter below were provided in footnote.
Yield= P N ×A×t, or P N (R)× A f ×t, or P N (A)× A g ×t, or P N (FM)× FM ×t,
P N (R) is from the reading and Af is the fixed area of the standard leaf chamber (6 cm2). The values of Ag and FM can be determined by an area meter and an electronic balance respectively. We can obtain the following equations:
PN (A)= PN (R)× Af / Ag [unit: μmol (CO2)/(m2 ∙s)], PN (FM)= PN (R)× Af / FM [unit: μmol (CO2)/(g (FW)∙s) or μmol (CO2)/(kg (FW)∙s) or μmol (CO2)/(g (FW)∙h)].
For example, when the fresh mass of foliar tissue in the chamber is 0.175 g, PN (R)is 6.1 μmol (CO2)/(m2 ∙s), Af is 6 cm2, and the relevant calculations are as follows:
PN (FM)=6.1×6×10-4 /0.175=0.0209 μmol (CO2)/(g (FW)∙s), =6.1×6×10-4 /(0.175×10-3)=20.9 μmol (CO2)/(kg (FW)∙s), or=6.1×6×10-4 ×3600/0.175=75.3 μmol (CO2)/(g (FW)∙h).
It is important to note the units of the parameters in the calculation. The unit of Af and Ag is square centimeter (cm2), and that of FM is gram (g). Because the unit of PN (R) is μmol (CO2)/(m2 ∙s), the unit of Af should be changed from cm2 to 10-4 m2 . The value of PN (FM) in μmol (CO2)/(g (FW)∙s) is too small. Therefore, units of μmol (CO2)/(kg (FW)∙s) or μmol (CO2)/(g (FW)∙h) are more suitable in this instance. The calculation of PN (FM) does not require a value of leaf area. The P N value with the unit μmol (CO2)/(kg (FW)∙s) is the closest to that of μmol (CO2)/(m2 ∙s).
PN (FM) = PN (R) × Af ×10-4 /(FM ×10-3)= PN (R) × Af ×0.1/ FM [unit: μmol (CO2)/(kg (FW)∙s)].
The same procedure may be easily adapted to obtain E(FM) and g s(FM), as follows:
E(FM)= E(R)× Af ×0.1/ FM [unit: mmol (H2O)/(kg (FW)∙s)], Es(FM)= Es(R)× Af ×0.1/ FM [unit: mmol (H2O)/(kg (FW)∙s)].
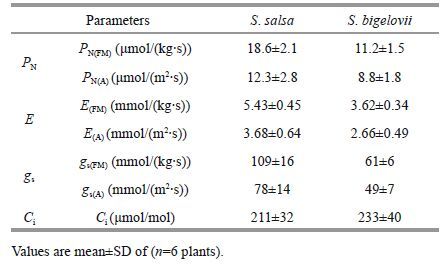
3 RESULT AND DISCUSSION 3.1 Reliability analysis of measurement resultsTable 1 shows the results of photosynthetic parameters in both species seedlings in the new method (PN (FM) , E(FM), Es(FM)) as well as conventional method (PN (A) , E(A), Es(A)). The values of /(kg (FW)∙s) in the new method were greater than that of /(m2 ∙s) in conventional one, but their coefficients of variability (CV) were reversed. These CV information implied that the values of /(kg (FW)∙s) were more accurate and reliable than that of /(m2 ∙s), e.g. P N for the two halophytes.
For S . salsa, CV of PN (FM) =2.1/18.6=0.113, CV of PN (A) =2.8/12.3=0.228,
For S . bigelovii, CV of PN (FM) =1.5/11.2=0.134, CV of PN (A) =1.8/8.8=0.205.
Figure 1 shows the results of net photosynthetic rate (PN (FM) ) of the two halophytes in the new method with photosynthetic materials isolated from or attached to plant body (in-situ). The PN (FM) value of these two materials had no significant difference. So the measurement results of isolated photosynthetic materials is reliable by fast determination in few minutes.

|
| Figure 1 Net photosynthetic rate of the two halophytes in the new method with photosynthetic materials isolated from or attached to plant body Values are mean±SD of (n =6 plants). |
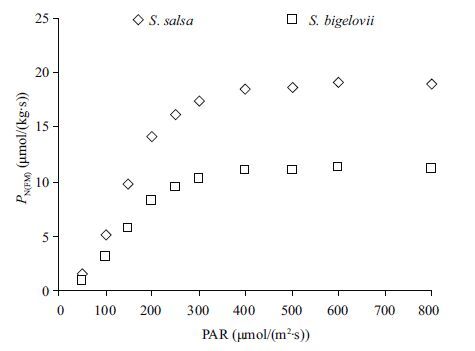
The light response curve also can be measured by this method. Because the ratio of fresh mass to area of a certain photosynthetic material is a constant, so the shape of light response curve measured by this method is similar to the common method (Fig. 2).
|
| Figure 2 The light response curve of the two halophytes |
There are some limitations in this method. The photosynthetic parameters based on unit fresh mass are not comparable among different plants due to morphological differences. If there are morphological differences in the branch (stem with leaves) taken from different parts of the same plant or the plants in different age, the comparison is not recommended too. The other limitation of this method is that the measured photosynthetic rates of different materials is not correlated with their illumination area, because the ratios of fresh weight to area of these heteromorphic materials are different. Therefore, this method is optimized for the comparison of same photosynthetic materials of same plant, e.g. the leaves of Suaeda salsa tree under different saline conditions.
To obtain accurate and reliable photosynthetic parameters by this method, the notes on measurements of photosynthetic parameters are listed as follows.
(1) Do not place too much photosynthetic tissue in the leaf chamber so that the tissue is fully illuminated.
(2) The seedlings should be exposed to saturated light for more than 30 min to activate photosynthetic enzymes prior to the determination.
(3) The reading from the photosynthetic instrument must be recorded quickly when that is relatively stable.
(4) The fresh mass must be measured as soon as possible after the reading is taken.
(5) A better tissue for the photosynthetic measurement is still attached to the plant body, except for very friable photosynthetic one.
(6) The light/CO2 /temperature response curve must be determined with photosynthetic tissues attached to plant (in situ).
4 CONCLUSIONFor non-flat photosynthetic tissues, the measurements of photosynthetic parameters are both simple and rapid using photosynthetic instruments in this new method, and the values are more accurate and reliable compared to conventional one, especially for friable succulent photosynthetic tissues of seawater-tolerant vegetables.
| Ali M M, Al-Ani A, Eamus D, Tan D K Y, 2012. A new imageprocessing-based technique for measuring leaf dimensions. American-Eurasian J. Agric. Environ. Sci., 12 (12) : 1588 –1594. |
| Donahue R A, Poulson M E, Edwards G E, 1997. A method for measuring whole plant photosynthesis in Arabidopsis thaliana. P hotosyn. Res., 52 (3) : 263 –269. |
| Edwards N T, 1989. Pine needle holders for use in gas exchange measurements. Tree Physiol., 5 (4) : 507 –509. Doi: 10.1093/treephys/5.4.507 |
| Pandey S K, Singh H, 2011. A simple, cost-effective method for leaf area estimation. J. Botany, 2011 : 658240 . |
| Pons T L, Welschen R A M, 2002. Overestimation of respiration rates in commercially available clamp-on leaf chambers. Complications with measurement of net photosynthesis.Plant Cell Environ., 25 (10) : 1367 –1372. |
| PP Systems Inc. 2010. CIRAS gas exchange equations(For CIRAS-1 and CIRAS-2). www.ppsystems.com, Version 3.0:1-3. |
| Zhu X G, Long S P, Ort D R, 2010. Improving photosynthetic efficiency for greater yield. Annu. Rev. Plant Biol., 61 : 235 –261. Doi: 10.1146/annurev-arplant-042809-112206 |
 2016, Vol. 34
2016, Vol. 34