Institute of Oceanology, Chinese Academy of Sciences
Article Information
- ZHU Lin(朱琳), TANG Xuexi(唐学玺), WANG Ying(王影), SUI Yadong(隋亚栋), XIAO Hui(肖慧)
- Use of antioxidant enzymes of clam Ruditapes philippinarum as biomarker to polycyclic aromatic hydrocarbon pollution
- Journal of Oceanology and Limnology, 34(2): 416-421
- http://dx.doi.org/10.1007/s00343-015-4327-z
Article History
- Received Nov. 20, 2014
- accepted in principle Apr. 5, 2015
2 Yellow Sea Fisheries Research Institute, Chinese Academy of Fishery Sciences, Qingdao 266071, China
Polycyclic aromatic hydrocarbons (PAHs), which constitute a series of widely studied pollutants, are persistent, bioaccumulated and toxic (PBT) for freshwater and marine ecosystems (Jin, 1992; Iglesias-Groth et al., 2010). In marine ecosystems, PAHs can be absorbed by organisms through their respiratory passages, surface infiltration, and foodchain transfer. They can remain within the body because they cannot be metabolized (Jin, 1992) and are poisonous to the organisms (Piccardo et al., 2001; Aksmann and Tukaj, 2004; Zbigniew and Wojciech, 2006).
The clam R . philippinarum is the native species in the Indian-Pacific region which has been widely cultivated as an important commercial shellfish in Chinese coastal waters. We selected this species as the target organism because the quality and yield of R . philippinarum is highly susceptible to pollutants (Yang, 2007).
The antioxidant enzyme system has a crucial role in inhibiting the oxidation reactions in vivo once the organism is threatened by pollutants (Chong and Wang, 2000; Zhang et al., 2007a, b, 2008). Superoxide dismutase (SOD), catalase (CAT) and glutathione peroxidase (GSH-Px) are vital antioxidant enzymes which have important roles in detoxification mechanisms in the body.
In the present study, changes in the activities of the antioxidant enzymes SOD, CAT and GSH-Px were monitored and the results used to evaluate the tolerance of R . philippinarum exposed to anthracene. The enzymes examined may be an indicator of oxidative stress induced by PAHs.
2 MATERIAL AND METHODThe samples of R . philippinarum used in the present study were fresh (caught <12 h before purchase). They were purchased from the Tuandao seafood market of Qingdao (Shandong Province, China). Animals were maintained in filtered and aerated seawater at 19℃, 30 of salinity and pH 8.0. The animals were allowed to acclimatize for 7 days and the most robust clams (shell length, 3–3.5 cm; wet weight, 6–9 g) were collected before the experiments described below. During the acclimation period, they were constantly fed with the microalgae Isochrysis galbana Parke 8701 and Platymonas helgolandica Kylin var. tsingtaoensis at a daily ration. After the acclimation period, the clams were placed in glass tanks containing 3 L of filtered seawater. There were 30 individuals for each tank.
The organic compound, anthracene was used in this study because of its contamination potential to coastal waters and subsequent threat to the economy of coastal areas (Yang, 2007). The concentrations of anthracene assayed were 0.007, 0.07 and 0.7 mg/L and the actual measured concentrations were 0.007 08, 0.070 4 and 0.689 mg/L respectively (Cai et al., 2006). Anthracene is poorly soluble in water, so a small amount of dimethyl sulfoxide (DMSO) was added to help dissolution. And a seawater control group and a DMSO negative control group were also employed in the experiment. At 0, 3, 6, 9, 12 and 15 days, samples of three specimens were taken from each tank and used to analyze the enzymatic activities in the mantle, visceral mass, and muscle tissue.
Enzyme extracts were prepared by homogenizing previously stored deep-frozen tissue samples in a buffer of ice-cold 0.86% physiological saline (w/v =1:9) with a homogenizer. The homogenate was centrifuged at 1 097 × g for 15 min. The supernatant was collected and used as the enzyme source. The activities of different enzymes were determined with tissues extracts equivalent to 100 mg of protein. Protein content per mg of tissue was determined using a Coomassie Brilliant Blue Protein Assay kit purchased from Nanjing Jiancheng Bioengineering Institute (Nanjing, China). The red-brown Coomassie brilliant blue combined with the amino group of proteins and transformed into a blue combination which had absorbance at 595 nm. The activities of antioxidant enzymes were determined using commercial kits purchased from Nanjing Jiancheng Bioengineering Institute (Nanjing, China). The assay for total SOD is based on its ability to inhibit the oxidation of oxymine by the xanthine-xanthine oxidase system. The red product (nitrite) produced by the oxidation of oxymine has absorbance at 550 nm. A unit of SOD was defined as the enzyme amount that reduced the absorbance at 550 nm by 50% for 1 mg of tissue proteins in a reaction mixture of 1 mL. The enzymatic decomposition of H2O2 by catalase can be stopped by adding ammonium molybdate. The remaining H2O2 by catalase can be stopped by adding ammonium molybdate. combines with ammonium molybdate, and the products have absorbance at 405 nm. One unit of CAT activity was defined as 1 mg of tissue proteins consuming 1 μmol H2O2 by catalase can be stopped by adding ammonium molybdate. at 405 nm for 1 s. The activities of CAT were demonstrated with unit per mg proteins. The activity of GSH-Px was determined by quantifying the rate of H2O2 by catalase can be stopped by adding ammonium molybdate. -induced oxidation of reduced glutathione (GSH) to oxidized glutathione (GSSH). A yellow product which had absorbance at 412 nm could be formed as GSH reacted with dithiobisnitrobenzoic acid. One unit of GSH-Px was defined as the amount that reduced the level of GSH by 1 μmol/L in 1 min per mL.
All data were tested using a two way ANOVA with anthracene concentrations (factor "concentration") and time (factor "time") were treated as fixed factors (SPSS 13.0). All values cited in this paper were obtained from fully independent samples. Data were initially examined with Levene’s test for homogeneity and the Shapiro-Wilk test for normality. The Duncan post-hoc test were used if ANOVA indicated a significant effect. Differences among anthracene treatment means were considered significant at the 5% level.
3 RESULT AND DISCUSSIONIn all treatments, the DMSO control was not different from the control (P >0.05)(Figs. 1–3). Therefore, a small quantity of DMSO (less than 0.1%) used as a carrier solvent did not affect enzymatic activities. Antioxidant enzymes showed significant variance in the combined effect of anthracene concentrations and time (Table 1, P<0.05).
|
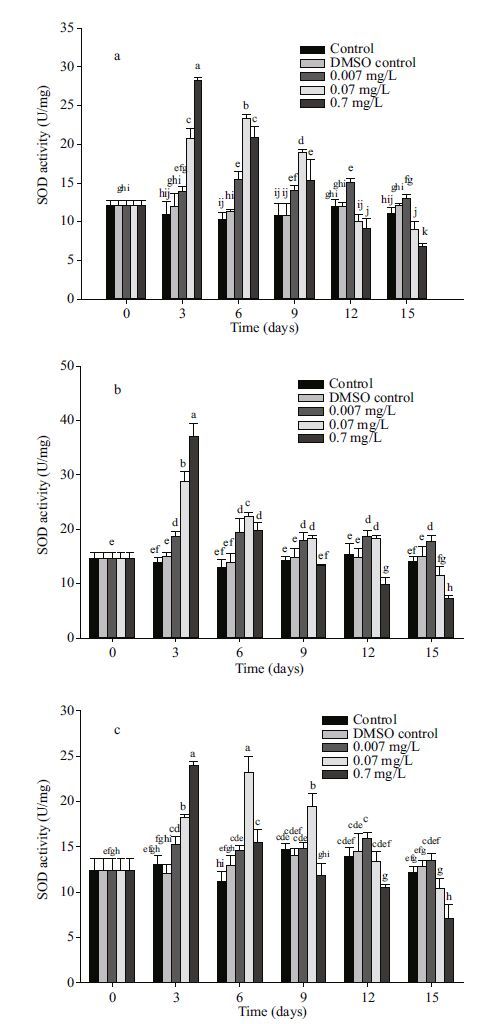
| Figure 1 Changes in SOD activity in different tissues after exposure to anthracene a. mantle; b. visceral mass; c. muscle. Means±SD, n=4. Different letters indicate significant differences according to two-way ANOVA (P<0.05). |
|
Significant changes in SOD activity were observed in all three organs with the increase in concentration and time of anthracene treatment when compared to controls (Fig. 1). At the concentration of 0.007 mg/L anthracene, SOD activities increased above the control level for the mantle and the visceral mass for 15 days while for the muscle tissue merely during the first 6 days. At 0.07 mg/L anthracene, SOD activities first increased and then decreased down to the control level at the 12 d in the mantle and the muscle tissue or at the 15 d in the visceral mass. The highest SOD activities were observed at 0.7 mg/L anthracene concentraton in all three organs at day 3. Then a marked decline in SOD activity was observed in all the organs. Activity of SOD increased 26.1%(at 0.007 mg/L), 89.0%(at 0.07 mg/L), 156.7%(at 0.7 mg/L), 34.5%(at 0.007 mg/L), 106.9%(at 0.07 mg/L), 166.1%(at 0.7 mg/L), 17%(at 0.007 mg/L), 39.5%(at 0.07 mg/L) and 83.1%(at 0.7 mg/L) for mantle, visceral mass and muscle tissue, respectively, compared to the control at day 3. So the sensitivity of the three organs to anthracene stress were in the order visceral mass > mantle > muscle tissue.
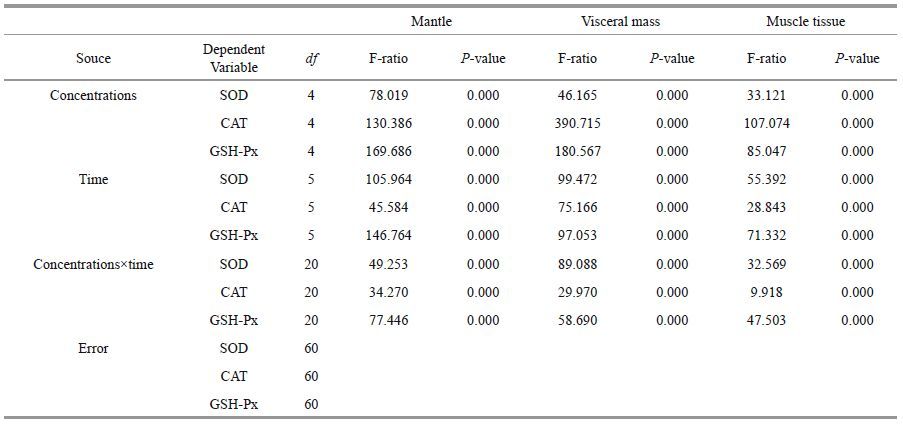
Anthracene concentrations and exposure time affected CAT activity in combination (Fig. 2). At 0.007 mg/L anthracene, CAT activity increased at day 3, 3 or 9 in different organs respectively in mantle, visceral mass and muscle tissue. At 0.7 mg/L anthracene, CAT activities first increased above control activity and then decreased below the control in the mantle while they decreased straightly below the control at day 9 and day 3 in the visceral mass and the muscle tissue for each. It concluded that the CAT activites of mantle and the visceral mass were more susceptible to anthracene stress.
|
| Figure 2 Changes in CAT activity in different tissues after exposure to anthracene a. mantle; b. visceral mass; c. muscle. Means±SD, n=4. Different letters indicate significant differences according to two-way ANOVA (P<0.05). |
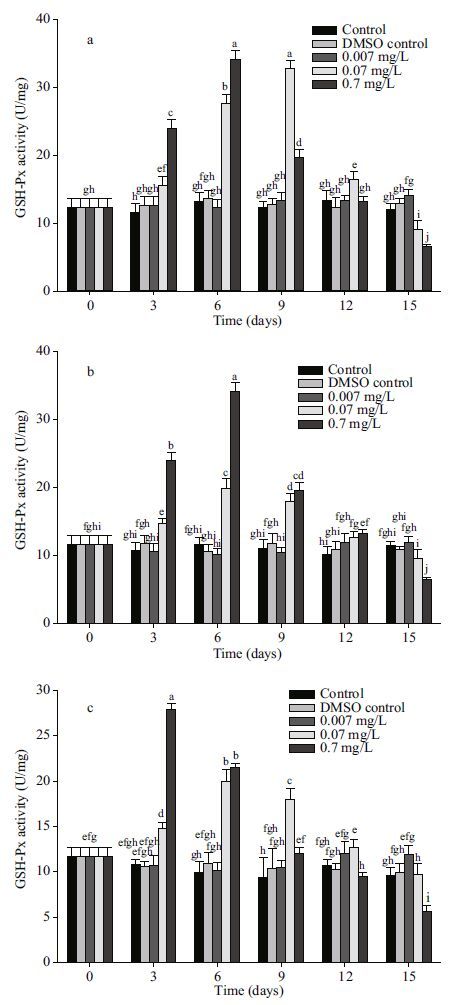
There were no significant changes in the activities of GSH-Px at a 0.007 mg/L anthracene concentration in all organs when compared to control. The effect of anthracene on GSH-Px activitity at moderate and high anthracene concentrations was similar to that on SOD activity. At 0.07 mg/L anthracene, GSH-Px activities increased above the control activity for the first 12 days in all the organs. At high anthracene concentration (0.7 mg/L), GSH-Px activities first increased and then decreased below the control. Activity of GSH-Px increased 34.1%(at 0.07 mg/L), 107.5%(at 0.7 mg/L), 35.1%(at 0.07 mg/L), 122.7%(at 0.7 mg/L), 36.8%(at 0.07 mg/L) and 158.3%(at 0.7 mg/L) for mantle, visceral mass and muscle tissue, respectively, compared to the control at the 3 d. So the sensitivities of the three oragns to anthracene stress were in the order muscle tissue > visceral mass > mantle.
PAHs can induce the formation of harmful free radicals such as OH•, O 2 • – and RO• radicals, which induces lipid peroxidation (Halliwell and Gutteridge, 1984). Consequently, the activity of one or more antioxidant enzymes would generally increases in order to meet the stress conditions. The effects of antioxidant enzymes in PAHs stress have been demonstrated in several studies (Doyottea et al., 1997; Yu et al., 2007; Zhang et al., 2007a) . A study showed that after two weeks of exposure to PAH contaminated sediment, the total oxyradical scavenging capacityassay (TOSC) of the arctic bivalve Mya truncata showed no change but it always kept high value to protect biomolecules (Camus et al., 2003). But Wang et al.(2011)showed that the activities of SOD, AHH and GST of the gill and the digestive glands in clams Ruditapes philippinarum in benzo[a]pyrene-exposure treatment were higher than in control group during the experimental period. Our results demonstrated that the dose-effect relationship and time-effect relationship exist between anthracene and the three antioxidant enzymes of R . philippinarum . The effects of anthracene on the activities of the three antioxidant enzymes could be divided into two phases: induction and suppression. SOD converts O 2 • – radicals into H2O2 by catalase can be stopped by adding ammonium molybdate. and O 2 . In a certain range of concentration and time, SOD will be induced, increasing its activity, producing hormesis, and actively scavenging oxygen free radicals: this was observed in the low concentration group. However, with an increase in concentration and time, the ability of SOD to scavenge oxygen free radicals will be limited gradually. Like in the middle- and high- concentration group, in general, the SOD activities began with a brief induction and then rapidly decreased. The same thing happened to CAT activities and GSH-Px activities whereas the effect on CAT was completely inhibited in the high toxic stress to visceral mass and muscle tissue. In the low-concentration group, CAT activity showed obvious increase while GSH-Px activity did not show significant fluctuation. This finding indicated that CAT was more reactive to reactive oxygen species (ROS) than GSH-Px and that it may play an important part in removal of the ROS. This conclusion was similar to that of Porte et al.(1991)and Niyogi et al.(2001). Furthermore, increasing anthracene concentration induced a sharp rise in enzyme activities. GSH-Px and CAT catalyze the decomposition of H2O2 by catalase can be stopped by adding ammonium molybdate. . But GSH-Px reacted not as quickly as CAT at low concentration toxic stress. In other words, the toxicant concentration for induction or inhibition effects for GSH-Px was higher than that for CAT. This mechanism may be a recessive form of protection to peroxide damage. GSH-Px and CAT cooperate to catalyze and remove free radicals so that the organism has a greater tolerance to the poison and better adapts to the environment (Porte et al., 1991; Niyogi et al., 2001).
|
| Figure 3 Changes in GSH-Px activity in different tissues after exposure to anthracene a. mantle; b. visceral mass; c. muscle. Means±SD, n=4. Different letters indicate significant differences according to two-way ANOVA (P<0.05). |
In general, the SOD activities of the visceral mass, the CAT activities of the mantle and the visceral mass, and the GSH-Px activity of the muscle tissue were more sensitive and might be chosen as sensitive indicators of anthracene stress in R . philippinarum .
| Aksmann A, Tukaj Z, 2004. The effect of anthracene and phenanthrene on the growth, photosynthesis, and SOD activity of the green alga Scenedesmus armatus depends on the PAR irradiance and CO2 level. Arch. Environ. Con.Tox., 47 (2) : 147 –184. |
| Cai Z Q, Lu Z B, Zhu Y X, Hong L Y, Zhang Y, 2006. Simultaneous determination of dissolved anthracene and pyrene in an aqueous solution by synchronous fluorimetry. Chin. J. Anal. Lab., 25 (4) : 61 –64. |
| Camus L, Birkely S R, Jones M B, Børseth J F, Grøsvik B E, Gulliksen B, Lønne O J, Regoli F, Depledge M H, 2003. Biomarker responses and PAH uptake in Mya truncata following exposure to oil-contaminated sediment in an Arctic fjord(Svalbard). Sci. Tot. Environ., 308 (1-3) : 221 –234. Doi: 10.1016/S0048-9697(02)00616-2 |
| Chong K, Wang W X, 2000. Bioavailability of sediment-bound Cd, Cr and Zn to the green mussel Perna viridis and the Manila clam Ruditapes philippinarum. J. Exp. Mar. Biol.Ecol., 255 (1) : 75 –92. Doi: 10.1016/S0022-0981(00)00296-3 |
| Doyottea A, Cossua C, Jacquina M-C, Babutb M, Vasseur P, 1997. Antioxidant enzymes, glutathione and lipid peroxidation as relevant biomarkers of experimental or field exposure in the gills and the digestive gland of the freshwater bivalve Unio tumidus. Aquat. Toxicol., 39 (2) : 93 –110. Doi: 10.1016/S0166-445X(97)00024-6 |
| Halliwell B, Gutteridge J M C, 1984. Oxygen toxicity, oxygen radicals, transition metals and disease. Biochem. J., 219 (1) : 1 –14. Doi: 10.1042/bj2190001 |
| Iglesias-Groth S, Manchado A, Rebolo R, González Hernández J I, García-Hernández D A, Lambert D L, 2010. A search for interstellar anthracene towards the Perseus anomalous microwave emission region. Mon. Not. R. Astron. Soc., 407 (4) : 2157 –2165. Doi: 10.1111/mnr.2010.407.issue-4 |
| Jin X C, 1992. Sediment Pollution Chemistry. China Environmental Science Press, Beijing, China. |
| Niyogi S, Biswas S, Sarker S, Datta A G, 2001. Seasonal variation of antioxidant and biotransformation enzymes in barnacle, Balanus balanoides, and their relation with polyaromatic hydrocarbons. Mar. Environ. Res., 52 (1) : 13 –26. Doi: 10.1016/S0141-1136(00)00257-9 |
| Piccardo M T, Coradeghini R, Valerio F, 2001. Polycyclic aromatic hydrocarbon pollution in native and caged mussels. M ar. P ollut. B ull., 42 (10) : 951 –956. |
| Porte C, Sole M, Albaigés J, Livingstone D R, 1991. Responses of mixed-function oxygenase and antioxidase enzyme system of Mytilus sp. to organic pollution. Comp.Biochem. Phys. C:Comp. Pharmacol., 100 (1-2) : 183 –186. Doi: 10.1016/0742-8413(91)90150-R |
| Wang L, Pan L Q, Liu N, Liu D, Xu C Q, Miao J J, 2011. Biomarkers and bioaccumulation of clam Ruditapes philippinarum in response to combined cadmium and benzo [α] pyrene exposure. Food Chem. Toxicol., 49 (12) : 3407 –3417. Doi: 10.1016/j.fct.2011.06.015 |
| Yang H S, 2007. Save the beautiful shellfish home. Life World (8) : 28 –30. |
| Yu J, Tang X X, Yang Z, 2007. Effect of anthracene on the antioxidant system of marine microalgae. Chin. J. Appl.Environ. Biol., 7 (4) : 340 –343. |
| Zbigniew T, Wojciech P, 2006. Individual and combined effect of anthracene, cadmium, and chloridazone on growth and activity of SOD izoformes in three Scenedesmus species. Ecotox. Environ. Safe., 65 (3) : 323 –331. Doi: 10.1016/j.ecoenv.2005.12.001 |
| Zhang P Y, Tang X X, Dong S L, 2007a. Effects of anthracene on differences of antioxidant enzymes activities and peroxide of membrane lipid of different tissues of Crassostrea gigas. Mar. Environ. Sci., 26 (5) : 434 –437. |
| Zhang X D, Wu T X, Cai L S, Zhu Y F, 2007b. Effects of α-tocopheryl acetate supplementation in preslaughter diet on antioxidant enzyme activities and fillet quality of commercial-size Sparus macrocephalus. J. Zhejiang Univ. Sci. B, 8 (9) : 680 –685. Doi: 10.1631/jzus.2007.B0680 |
| Zhang Y, Tang H R, Luo Y, 2008. Variation in antioxidant enzyme activities of two strawberry cultivars with shortterm low temperature stress. World J. Agr. Sci., 4 (4) : 458 –462. |
 2016, Vol. 34
2016, Vol. 34


