Institute of Oceanology, Chinese Academy of Sciences
Article Information
- Ji WANG(王稷), Ye LI(李晔), Juan DAI(戴娟), Xiurong SU(苏秀榕), Chenghua LI(李成华), Lingling SHEN(沈灵灵)
- Long-term effects of di-octyl phthalate on the expression of immune-related genes in Tegillarca granosa
- Journal of Oceanology and Limnology, 34(3): 423-429
- http://dx.doi.org/10.1007/s00343-015-4297-1
Article History
- Received: Nov. 24, 2014
- Accepted: Jan. 30, 2015
Phthalate esters (PAEs) are an important additive that increase flexibility in polyvinyl chloride (PVC) resins. PAEs are increasingly used as plasticizers,wire insulators,and pesticide carriers. As a result of their widespread use,PAEs are commonly found in the environment (Jobling et al.,1995) . For example,the concentration of PAEs ranges from 0.1–300 μg/L at the seawater surface (Mayer et al.,1972; Giam et al.,1978; Fatoki and Vernon,1990; Gledhill et al.,1980) and 0.1 ng/g–100 μg/g in river sediments (Thurén,1986; Tan,1995) . Di-octyl phthalate (DOP) is a major component of PAEs and is widely used in several industries (e.g.,food packaging materials,medical devices,etc.) because of its utility and cost effectiveness. Because DOP is not chemically bound to PVC resin,it can leak from disposable plastic products into the environment. Thus,DOP is frequently adsorbed into soil,sediment,and suspended solids because of its low water solubility (approximately 0.003 mg/L) and high octanol/water partition coeffcient (logKow=7.94) (Staples et al.,1997) . The estimated concentration of DOP in Laizhou Bay seawater and sediments typically ranged from 2.002 to 43.891×10-9 g/L and 4.454 to 4 389.243×10-9 g/kg,respectively. In Quanzhou Bay,the concentration of DOP in seawater and sediments ranged from 18.77 to 191.51 ng/L and 171.50 to 1 435.61 μg/kg,respectively (Sung et al.,2003; Zhuang et al.,2011) .
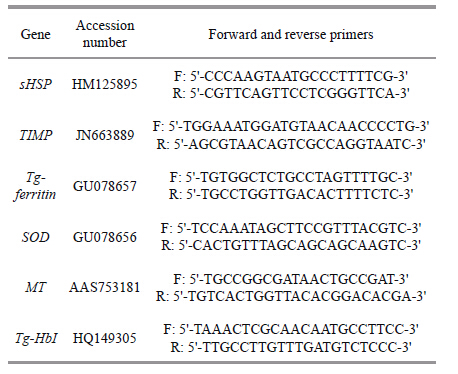
In recent years,a number of researchers have evaluated the effects of DOP pollution on marine animals. Hobson et al. (1984) reported that DOP could be absorbed,metabolized,and accumulated at high levels in the tissue of penaeid shrimp. Sung et al. (2003) demonstrated that DOP damages the hemocytes of prawns and impairs their immune defense system. Additionally,Park and Kwak (2009,2012) and Caldwell (2012) found that DOP can alter the activity of some proteins involved in apoptosis,signaling,and metabolism. Furthermore,DOP induces the expression of many genes,including cytoskeletal proteins,alcohol dehydrogenases,and heat shock proteins in lower aquatic animals such as Table 1 Sequences of oligonucleotide primers used for qRT- PCR Accession Chironomus riparius and Tigriopus japonicas ( deLafontaine et al.,2000; Oh and Lim,2009; Li et al.,2010) .
T. granosa is an economically important bivalve shellfish that is found on beaches along the Chinese coast. Outbreaks of pathogens and environmental degradation have resulted in dramatically decreased production of T. granosa. Immune-related genes are thought to be promising biomarkers for early monitoring of environmental conditions because of their rapid response to environmental perturbations and contamination (Brown et al.,1995; deLafontaine et al.,2000; Lyons et al.,2003) .
Although the acute toxicity of PAHs has been evaluated in marine animals (Sung et al.,2003; Chen and Sung,2005; Lu et al.,2013) ,there is little information about the long-term immune responses of organisms to DOP exposure. To address this,we evaluated the in vivo effects of long-term DOP exposure on the expression of immune-related genes in T. granosa. We identified several genes that were promising candidates for biomarkers of DOP.
2 MATERIAL AND METHOD 2.1 ClamsClams (T. granosa,average weight being 8.12±0.36 g) were purchased from a clam farm in Ningbo (Zhejiang Province,China) . The clams were acclimated for a week before beginning the experiment. The temperature was maintained at 20– 22°C throughout the experiment,and the salinity of the seawater was maintained at 30.
2.2 Treatment protocolsDOP (analytical purity >99.0%) was first dissolved in absolute ethanol and subsequently diluted with an equal volume of seawater to obtain a stock solution of 4 g/L. The clams were assigned randomly into four groups (30 individuals/group) . The DOP stress experiments were performed by continuously feeding the clams one of three doses of DOP (final concentrations of 2.6,7.8,or 31.2 mg/L) . The control group was exposed to the same volume of ethanol and seawater. Water was charged twice within 1 week and the corresponding volume of DOP was supplied. We collected individuals from the experimental and control groups on days 7 and 14 and removed the visceral mass. We performed five replicates of each experimental and control group.
2.3 Analysis of expression profiles of candidate genes using quantitative real-time PCRTotal RNA was isolated from the visceral mass of T. granosa using TRIzol reagent (Invitrogen) . First- strand cDNA synthesis was performed using an MMLV First-Strand cDNA Synthesis Kit according to the manufacturer’s instructions (Bio Basic Inc.) . The expression levels of candidate genes of clams exposed to DOP were measured by quantitative real- time PCR (qRT PCR) . We used β-actin as an internal control to verify successful reverse transcription and to calibrate the cDNA template. Two specific primers for each gene were designed to amplify the desired product (Table 1) . Real-time PCR amplification was performed using a Rotor-Gene 6000 real-time PCR detection system. We used the reaction components and thermal profiles suggested by the manufacturer. Dissociation curve analysis of amplification products was performed at the end of each PCR reaction to confirm that only one PCR product was amplified and detected. Finally,the Ct values for the target amplified genes and for the internal control (β-actin) were determined for each sample,then converted into relative expression levels using the 2-∆∆Ct method. All data are expressed as mean±SD. Differences between groups were analyzed by one-way analysis of variance (ANOVA) . Differences were considered statistically significant at P<0.05.
3 RESULT AND DISCUSSIONRecently,significant attention has been paid to the presence of di-octyl phthalate (DOP) in marine ecological systems and its effects on aquatic organisms. Some studies have shown that marine species are exposed to DOP in their natural habitat (Thurén,1986; Tan,1995) . In higher animals,DOP disrupts the oxidative balance by increasing the production of nitric oxide (NO) and reactive oxygen species (ROS) ,leading to lipid oxidation,DNA damage,and activation of the ERK/NFkB signaling pathway (Ghosh et al.,2010) . In crustaceans such as the giant freshwater prawn (Macrobrachium rosenbergii) ,DOP mediates the immune responses of hemocytes by inhibiting O2- generation (Sung et al.,2003) . To better understand the toxic effects of DOP on T. granosa,we evaluated the expression of six genes involved in the immune response using qRT- PCR.
3.1 Small heat shock proteinsHeat shock proteins (HSPs) are a class of functionally related proteins found in virtually all living organisms. They are divided into several families,including HSP90,HSP70,HSP60,HSP40,and small HSP (HSPs) according to their molecular size (Mayer,1997; Santoro,2000) . Stressors,including DOP,that cause oxidative stress also affect the expression of HSPs (Wood et al.,1997; Lee et al.,2006) . The HSPss are small stress-induced proteins that function to prevent stress-induced cell damage by binding and maintaining the denatured proteins in a folding-competent state. A growing body of evidence indicates that increased expression of HSPss in different cell types can increase tolerance to a variety of stresses,including heat,salt,drugs,viral infection,and oxidants (Jaya et al.,2009) .
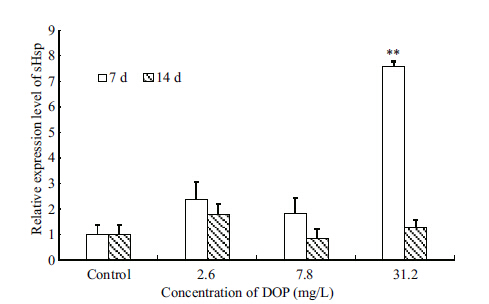
In our study,clam HSPs was up-regulated at all DOP dosage levels (Fig. 1) after 7 d exposure to DOP. Indeed,we observed a 7.60-fold increase in expression when clams were exposed to 31.2 mg/L DOP. This is similar to the effect observed following exposure to other chemicals and heavy metals for 10 d (Comporti,2002; Lesser,2006) . We speculate that the increase isa result of disturbances in the normal redox state and oxidative stress. Interestingly,the effects of DOP on the expression of HSPs were negligible by day 14.
|
| Figure 1 Mean±SD (n=5) expression of sHsp in the viscera of T. granosa after exposure to DOP for 7 and 14 days |
Tissue inhibitors of metalloproteinases (TIMPs) were originally characterized as inhibitors of matrix metalloproteinases (MMPs) ,but are also multifunctional proteins that are vital to the regulation of ECM (extracellular matrix) metabolism. The ECM plays a role in the defense against pathogens and viral infection. Because of their role in regulating ECM metabolism,TIMPs are also involved in the defense against infection by pathogens (Lepore et al.,1996; Okamoto et al.,1997; Miyoshi and Shinoda,2000) . The expression of TIMP is induced by shell damage or the pollutants in the aquatic environment (Montagnani et al.,2007) .
To determine the effect of DOP on TIMP expression,we conducted a time-course analysis on expression levels of the gene in clams following exposure to different concentrations of DOP. The mRNA expression of TIMP in T. granosa was up-regulated significantly after the clams were stimulated by DOP,reaching a peak (24.7-fold compared with the control group) at day 7 at the highest concentration of DOP (31.2 mg/L) (Fig. 2) . The relative expression of TIMP exhibited the same pattern as HSPs—expression increased significantly after 7 d exposure and was highest in the group exposed to 31.2 mg/L DOP. Similarly,TIMP expression in the three experimental groups was not different from the control group after 14 d. Because the effects of DOP exposure on TIMP expression are poorly understood,we compared our findings with the response of TIMP to bacterial challenge (Okamoto et al.,1997; Montagnani et al.,2007) . It was suggested that TIMP appears to play an important role in the immune regulation of clams exposed to short-term stressors,including DOP. Organisms can increase TIMP mRNA expression to inhibit proteases that participate in ECM destruction and inactivate protease inhibitors and activate the latent form of MMP.

|
| Figure 2 Mean±SD (n=5) expression of TIMP in the viscera of T. granosa after exposure to DOP for 7 and 14 days |
Ferritin is a ubiquitous and highly conserved iron- binding protein and the principal protein for iron storage and metabolism. Additionally,ferritin has a protective role against oxidative damage (Zheng et al.,2010) . Oxidative stress is an important component of the stress response in marine organisms (Vaughan,1997; Livingstone,2003) . Bivalve mollusks produce ROS following exposure to heavy metals,high temperatures,and other environmental disturbances (Abele et al.,2002) . Generally,ferritin is thought of as a “protective” protein that functions to protect cells from oxidative stress (Larade and Storey,2004) . Additionally,antioxidant enzymes also play important roles in eliminating ROS. In this process,superoxide dismutase (SOD) is a key enzyme that catalyzes the dismutative reaction of O2- and transforms it into hydrogen peroxide and oxygen (Lee et al.,2007; Li et al.,2008) .
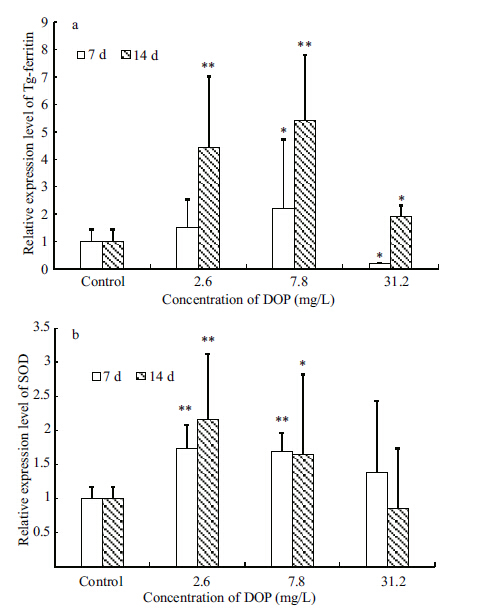
The changes in expression of Tg-ferritin and SOD mRNA were similar,with up-regulation occurring in clams exposed to 2.6 and 7.8 mg/L DOP (Fig. 3) . At day 14,ferritin and SOD expression had increased 5.43-fold and 2.16-fold,respectively,relative to the control. At the highest concentration of DOP (31.2 mg/L) ,Tg-ferritin and SOD were down- regulated. There is evidence that hosts can differentiate the hazard and adopt different strategies to control ROS at a sustainable level by regulating the expression or activities of antioxidant enzymes. We speculate that Tg-ferritin and SOD are acute response proteins involved in the response to DOP exposure in T. granosa and that both proteins regulate the immune response of the host via the same immunologic mechanism.
|
| Figure 3 Mean±SD (n=5) expression of ferritin (a) and SOD (b) in the viscera of T. granosa after exposure to DOP for 7 and 14 days |
Metallothionein (MT) exists in most organisms and represents a group of cysteine-rich,low- molecular-weight intracellular proteins that act to bind and detoxify heavy metal ions (Kägi,1991) . MTs play important roles in the detoxification of essential metals or non-essential metals and they have been used as biomarkers for monitoring heavy metal pollution in many marine organisms,including fish,crabs,and shellfish (Coyle et al.,2002; Berthet et al.,2005) . MT expression has been implicated as a transient response to virtually any form of injury or stress,providing a cytoprotective mechanism against the potential damaging effects of oxygen-derived free radicals through scavenging of reactive metal ions (Ghosh et al.,2010) .
The relative expression of MT was up-regulated then down-regulated as the concentration of DOP increased. In this study,1.60-fold increase was detected at 7.8 mg/L on days 7,while 1.86-fold increase at 2.6 mg/L on days 14 (Fig. 4) . This pattern of MT expression is consistent with prior reports (Maity et al.,2011) . We speculate that DOP exposure induces T. granosa to produce a poison stimulatory effect to induce MT gene expression. When the concentration of MT is sufficiently high to allow for DOP detoxification,MT gene expression was inhibited and exhibited a clear downward trend. The decrease in MT expression may be caused by the induction of other immune protective genes and/or increased tolerance through a different detoxification and adaptive mechanism (Posthuma and van Straalen,1993) .

|
| Figure 4 Mean±SD (n=5) expression of MT in the viscera of T. granosa after exposure to DOP for 7 and 14 days |
Hemoglobin (Hb) is the iron-containing oxygen transport metalloprotein in the red blood cells of vertebrates and some invertebrates (Hardison,1998) ,including some mollusks. In addition to the function of carrying oxygen,Hbs have been implicated in a number of processes,depending on the Hb types and the species (Coates,1975; Terwilliger,1998; Wajcman and Kiger,2002) . In the bivalve mollusk genus Scapharca,two distinct types of Hb,dimeric (HbI) and tetrameric (HbII) ,were found (Chiancone et al.,1981; Gambacurta et al.,2000) . Bao et al. (2011) designated the HbI gene from the bloody clam T. granosa as Tg-HbI.
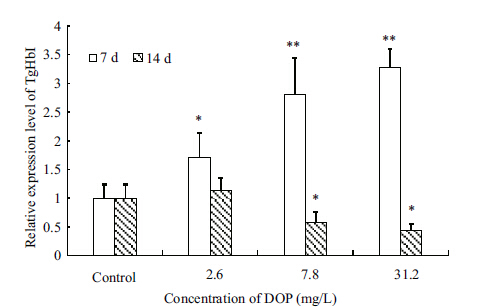
In our experiments,Tg-HbI exhibited two distinct expression patterns at two time points (Fig. 5) . At day 7,Tg-HbI expression was significantly up-regulated and positively correlated with the concentration of DOP,with the peak expression level detected at 31.2 mg/L DOP with a 3.27-fold increase compared with the untreated samples. At day 14,Tg-HbI expression was significantly down-regulated and reached a level that was approximately 0.44-fold that of the untreated samples at the highest concentration of DOP (31.2 mg/L) . This expression pattern suggests that Hb is an inducible protein that is sensitive to the timing of DOP exposure. Previous studies have shown that Hb can be directly activated by microbial proteases and enhanced by pathogen-associated molecules (Bao et al.,2011) . We speculate that the different patterns of Tg-HbI expression upon DOP exposure are related to ROS production and its detoxification. However,to better understand the role of Tg-HbI during DOP exposure,the molecular mechanism and the changes in Tg-HbI activity at the protein level behind this interaction need to be investigated further.
|
| Figure 5 Mean±SD (n=5) expression of Tg-HbI in the viscera of T. granosa after exposure to DOP for 7 and 14 days |
Our results suggest that the expressions of the immune genes Tg-ferritin,SOD,and MT are affected by exposure to DOP for up to 14 d in T. granosa. Conversely,the effects of DOP on other genes,particularly HSPs and TIMP,were limited to the first 7 d of exposure.
| Abele D, Heise K, Pörtner H O, Puntarulo S, 2002. Temperature-dependence of mitochondrial function and production of reactive oxygen species in the intertidal mud clam Mya arenaria. J. Exp. Biol., 205 (13) : 1831 –1841. |
| Bao Y B, Wang Q, Lin Z H, 2011. Hemoglobin of the bloody clam Tegillarca granosa (Tg-HbI) is involved in the immune response against bacterial infection. Fish Shellfish Immun., 31 (4) : 517 –523. Doi: 10.1016/j.fsi.2011.05.029 |
| Berthet B, Mouneyrac C, Pérez T, Amiard-Triquet C, 2005. Metallothionein concentration in sponges (Spongia of ficinalis) as a biomarker of metal contamination. Comp. Biochem. Phys iol. C Toxicol. Pharmacol., 141 (3) : 306 –313. Doi: 10.1016/j.cca.2005.07.008 |
| Brown D C, Bradley B P, Tedengren M, 1995. Genetic and environmental regulation of HSP70 expression. Mar. Environ. Res., 39 (1-4) : 181 –184. Doi: 10.1016/0141-1136(94)00014-G |
| Caldwell J C, 2012. DEHP:genotoxicity and potential carcinogenic mechanisms-a review. Mutat. Res./Rev. Mutat. Res., 751 (2) : 82 –157. Doi: 10.1016/j.mrrev.2012.03.001 |
| Chen W L, Sung H H, 2005. The toxic effect of phthalate esters on immune responses of giant freshwater prawn (Macrobrachium rosenbergii) via oral treatment. Aquat. Toxicol., 74 (2) : 160 –171. Doi: 10.1016/j.aquatox.2005.05.008 |
| Chiancone E, Vecchini P, Verzili D, Ascoli F, Antonini E, 1981. Dimeric and tetrameric hemoglobins from the mollusc Scapharca inaequivalvis:structural and functional properties. J. Mol. Biol., 152 (3) : 577 –592. Doi: 10.1016/0022-2836(81)90270-9 |
| Coates M L, 1975. Hemoglobin function in the vertebrates:an evolutionary model. J. Mol. Evol., 6 (4) : 285 –307. Doi: 10.1007/BF01794636 |
| Comporti M, 2002. Introduction-Serial review:iron and cellular redox status. Free Radic. Biol. Med., 32 (7) : 565 –567. Doi: 10.1016/S0891-5849(02)00758-X |
| Coyle P, Philcox J C, Carey L C, Rofe A M, 2002. Metallothionein:the multipurpose protein. Cell. Mol. Life Sci., 59 (4) : 627 –647. Doi: 10.1007/s00018-002-8454-2 |
| de Lafontaine Y, Gagné F, Blaise C, Costan G, Gagnon P, Chan H M, 2000. Biomarkers in zebra mussels (Dreissena polymorpha) for the assessment and monitoring of water quality of the St Lawrence River (Canada) . Aquat. Toxicol., 50 (1-2) : 51 –71. Doi: 10.1016/S0166-445X(99)00094-6 |
| Fatoki O S, Vernon F, 1990. Phthalate esters in rivers of the Greater Manchester area, U. K. Sci. Total Environ., 95 : 227 –232. Doi: 10.1016/0048-9697(90)90067-5 |
| Gambacurta A, Basili P, Ascoli F, 2000. Scapharca inaequivalvis A and B miniglobin genes:promoter activity of the 5' fl anking regions and in vivo transcription. Gene, 255 (1) : 75 –81. Doi: 10.1016/S0378-1119(00)00321-8 |
| Ghosh J, Das J, Manna P, Sil P C, 2010. Hepatotoxicity of di-(2-ethylhexyl) phthalate is attributed to calcium aggravation, ROS-mediated mitochondrial depolarization, and ERK/NF-k B pathway activation. Free Radic. Bio. Med., 49 (11) : 1779 –1791. Doi: 10.1016/j.freeradbiomed.2010.09.011 |
| Giam C S, Chan H S, Neff G S, Atlas E L, 1978. Phthalate ester plasticizers:a new class of marine pollutant. Science, 199 (4327) : 419 –421. Doi: 10.1126/science.413194 |
| Gledhill W E, Kaley R G, Adams W J, Hicks O, Michael P R, Saeger V W, LeBlanc G A, 1980. An environmental safety assessment of butyl benzyl phthalate. Environ. Sci. Technol., 14 (3) : 301 –305. Doi: 10.1021/es60163a001 |
| Hardison R, 1998. Hemoglobins from bacteria to man:evolution of different patterns of gene expression. J. Exp. Biol., 201 (8) : 1099 –1117. |
| Hobson J F, Carter D E, Lightner D V, 1984. Toxicity of a phthalate ester in the diet of a penaied shrimp. J. Toxicol. Environ. Health, 13 (4-6) : 959 –968. Doi: 10.1080/15287398409530553 |
| Jaya N, Garcia V, Vierling E, 2009. Substrate binding site fl exibility of the small heat shock protein molecular chaperones. Proc. Natl. Acad. Sci. U S A, 106 (37) : 15604 –15609. Doi: 10.1073/pnas.0902177106 |
| Jobling S, Reynolds T, White R, Parker M G, Sumpter J P, 1995. A variety of environmentally persistent chemicals, including some phthalate plasticizers, are weakly estrogenic. Environ. Health Persp ect., 103 (6) : 582 –587. Doi: 10.1289/ehp.95103582 |
| Kägi J H R, 1991. Overview of metallothionein:metallobiochemistry part B metallothionein and related molecules. Methods Enzymol., 205 : 613 –626. Doi: 10.1016/0076-6879(91)05145-L |
| Larade K, Storey K B, 2004. Accumulation and translation of ferritin heavy chain transcripts following anoxia exposure in a marine invertebrate. J. Exp. Biol., 207 (8) : 1353 –1360. Doi: 10.1242/jeb.00872 |
| Lee E, Ahn M Y, Kim H J, Kim I Y, Han S Y, Kang T S, Hong J H, Park K L, Lee B M, Kim H S, 2007. Eff ect of Di (n-butyl) phthalate on testicular oxidative damage and antioxidant enzymes in hyperthyroid rats. Environ. Toxicol., 22 (3) : 245 –255. Doi: 10.1002/(ISSN)1522-7278 |
| Lee S M, Lee S B, Park C H, Choi J, 2006. Expression of heat shock protein and hemoglobin genes in Chironomus tentans (Diptera, chironomidae) larvae exposed to various environmental pollutants:a potential biomarker of freshwater monitoring. Chemosphere, 65 (6) : 1074 –1081. Doi: 10.1016/j.chemosphere.2006.02.042 |
| Lepore L S, Roelvink P R, Granados R R, 1996. Enhancin, the granulosis virus protein that facilitates nucleopolyhedrovirus (NPV) infections, is a metalloprotease. J. Invertebr. Pathol., 68 (2) : 131 –140. Doi: 10.1006/jipa.1996.0070 |
| Lesser M P, 2006. Oxidative stress in marine environments:biochemistry and physiological ecology. Annu. Rev. Physiol., 68 (1) : 253 –278. Doi: 10.1146/annurev.physiol.68.040104.110001 |
| Li C H, Ni D J, Song L S, Zhao J M, Zhang H, Li L, 2008. Molecular cloning and characterization of a catalase gene from Zhikong scallop Chlamys farreri. Fish Shellfish Immunol., 24 (1) : 26 –34. Doi: 10.1016/j.fsi.2007.06.010 |
| Li C H, Wang L L, Ning X X, Chen A Q, Zhang L B, Qin S, Wu H F, Zhao J M, 2010. Identification of two small heat shock proteins with different response profile to cadmium and pathogen stresses in Venerupis philippinarum. Cell Stress Chaperon., 15 (6) : 897 –904. Doi: 10.1007/s12192-010-0198-6 |
| Livingstone D R, 2003. Oxidative stress in aquatic organisms in relation to pollution and aquaculture. Rev. Méd. Vét., 154 (6) : 427 –430. |
| Lu Y L, Zhang P, Li C H, Su X R, Jin C H, Li Y, Xu Y J, Li T W, 2013. Characterisation of immune-related gene expression in clam (Venerupis philippinarum) under exposure to di (2-ethylhexyl) phthalate. Fish Shellfish Immunol., 34 (1) : 142 –146. Doi: 10.1016/j.fsi.2012.10.015 |
| Lyons C, Dowling V, Tedengren M, Gardeström J, Hartl M G, O'Brien N, van Pelt F N M, O'Halloran J, Sheehan D, 2003. Variability of heat shock proteins and glutathione S-transferase in gill and digestive gland of blue mussel, Mytilus edulis. Mar. Environ. Res., 56 (5) : 585 –597. Doi: 10.1016/S0141-1136(03)00044-8 |
| Maity S, Roy S, Bhattacharya S, Chaudhury S, 2011. Metallothionein responses in the earthworm Lampito mauritii (Kinberg) following lead and zinc exposure:a promising tool for monitoring metal contamination. Eur. J. Soil Biol., 47 (1) : 69 –71. Doi: 10.1016/j.ejsobi.2010.10.001 |
| Mayer F L, Stalling D L, Johnson J L, 1972. Phthalate esters as environmental contaminants. Nature, 238 (5364) : 411 –413. Doi: 10.1038/238411a0 |
| Mayer R J, 1997. Stress-inducible cellular responses. Mol. Path ol., 50 (2) : 112 . |
| Miyoshi S I, Shinoda S, 2000. Microbial metalloproteases and pathogenesis. Microbes Infect., 2 (1) : 91 –98. Doi: 10.1016/S1286-4579(00)00280-X |
| Montagnani C, Avarre J C, de Lorgeril J, Quiquand M, Boulo V, Escoubas J M, 2007. First evidence of the activation of Cg-timp, an immune response component of pacific oysters, through a damage-associated molecular pattern pathway. Dev. Comp. Immunol., 31 (1) : 1 –11. Doi: 10.1016/j.dci.2006.04.002 |
| Oh P S, Lim K T, 2009. Phytoglycoprotein (75 kDa) isolated from Cudrania tricuspidata Bureau inhibits expression of interleukin-4 in the presence of di (2-ethylhexyl) phthalate via modulation of p38 mitogen-activated protein kinase in primary-cultured mouse thymocytes. J. Appl. Toxicol., 29 (6) : 496 –504. Doi: 10.1002/jat.v29:6 |
| Okamoto T, Akaike T, Suga M, Tanase S, Horie H, Miyajima S, Ando M, Ichinose Y, Maeda H, 1997. Activation of human matrix metalloproteinases by various bacterial proteinases. J. Biol. Chem., 272 (9) : 6059 –6066. Doi: 10.1074/jbc.272.9.6059 |
| Park K, Kwak I S, 2009. Calponin gene expression in Chironomus riparius exposed to di (2-ethylhexyl) phthalate. Environ. Toxicol., 24 (6) : 555 –562. Doi: 10.1002/tox.v24:6 |
| Park K, Kwak I S, 2012. Gene expression of ribosomal protein mRNA in Chironomus riparius:effects of endocrine disruptor chemicals and antibiotics. Comp. Biochem. Physiol. C Toxicol. Pharmacol., 156 (2) : 113 –120. Doi: 10.1016/j.cbpc.2012.05.002 |
| Posthuma L, van Straalen N M, 1993. Heavy-metal adaptation in terrestrial invertebrates:a review of occurrence, genetics, physiology and ecological consequences. Comp. Biochem. Physiol. C Toxicol. Pharmacol., 106 (1) : 11 –38. |
| Santoro M G, 2000. Heat shock factors and the control of the stress response. Biochem. Pharmacol., 59 (1) : 55 –63. Doi: 10.1016/S0006-2952(99)00299-3 |
| Staples C A, Peterson D R, Parkerton T F, Adams W J, 1997. The environmental fate of phthalate esters:a literature review. Chemosphere, 35 (4) : 667 –749. Doi: 10.1016/S0045-6535(97)00195-1 |
| Sung H H, Kao W Y, Su Y J, 2003. Effects and toxicity of phthalate esters to hemocytes of giant freshwater prawn, Macrobrachium rosenbergii. Aquat. Toxicol., 64 (1) : 25 –37. Doi: 10.1016/S0166-445X(03)00011-0 |
| Tan G H, 1995. Residue levels of phthalate esters in water and sediment samples from the Klang River basin. B ull. Environ. Contam. Tox icol., 54 (2) : 171 –176. |
| Terwilliger N B, 1998. Functional adaptations of oxygentransport proteins. J. Exp. Biol., 201 (8) : 1085 –1098. |
| Thurén A, 1986. Determination of phthalates in aquatic environments. B ull. Environ. Contam. Tox icol., 36 (1) : 33 –40. Doi: 10.1007/BF01623471 |
| Vaughan M, 1997. Oxidative modification of macromolecules minireview series. J. Biol. Chem., 272 (30) : 18513 . Doi: 10.1074/jbc.272.30.18513 |
| Wajcman H, Kiger L, 2002. Hemoglobin, from microorganisms to man:a single structural motif, multiple functions. C. R. Biol., 325 (12) : 1159 –1174. Doi: 10.1016/S1631-0691(02)01537-8 |
| Wood L W, O'Keefe P, Bush B, 1997. Similarity analysis of PAH and PCB bioaccumulation patterns in sedimentexposed Chironomus tentans larvae. Environ. Toxicol. Chem., 16 (2) : 283 –292. Doi: 10.1002/etc.v16:2 |
| Zheng W J, Hu Y H, Sun L, 2010. Identification and analysis of a Scophthalmus maximus ferritin that is regulated at transcription level by oxidative stress and bacterial infection. Comp. Biochem. Physiol. B, 156 (3) : 222 –228. Doi: 10.1016/j.cbpb.2010.03.012 |
| Zhuang W E, Yao W S, Wang X X, Huang D R, Gong Z B, 2011. Distribution and chemical composition of phthalate esters in seawater and sediments in Quanzhou Bay, China. J. Environ. Health, 28 (10) : 898 –902. |
 2016, 34
2016, 34