Institute of Oceanology, Chinese Academy of Sciences
Article Information
- Aiyou HUANG(黄爱优), Guangce WANG(王广策)
- Identification and bioinformatics analysis of microRNAs from the sporophyte and gametophyte of Pyropia haitanensis
- Journal of Oceanology and Limnology, 34(3): 451-459
- http://dx.doi.org/10.1007/s00343-015-4235-2
Article History
- Received: Aug. 29, 2014
- Accepted: Jan. 15, 2015
Pyropia is an intertidal marine alga that belongs to the most genetically diverse order of the red algae,the Bangiales (Ragan et al.,1994) . Pyropia has an important role in biological research and agronomic industry; therefore,it has been proposed as a model organism for physiological and genetic studies of seaweed (Saga and Kitade,2002) . The life cycle of Pyropia comprises two different generations: a microscopic diploid filamentous sporophyte and a macroscopic haploid foliate gametophyte (Iwasaki, 1961) . The two generations are different in many aspects,including structural features,living environments and gene expression profiles (Yamazaki et al.,1998; Kitade et al.,1999; Sahoo et al.,2002; Asamizu et al.,2003) .
Pyropia haitanensis is an economically important genus that is cultured widely in China. The mature thallus of P.haitanensis is much larger than that of P.yezoensis. In addition,the thallus of P.haitanensis comprises more than two cell layers and is very resistant to drought (Ma and Cai,1996) . In addition, the filament of P.haitanensis releases their conchospores earlier and the blades grow much faster than those of P.yezoensis (Li and Zheng,1992) . This makes the collection of seedlings more effective. These factors have led to P.haitanensis being cultured on a larger scale than P.yezoensis,making up an important part of the total production of cultivated Pyropia in China (Blouin et al.,2011) . In spite of this, the majority of molecular mechanisms underlying the physiological processes of P.haitanensis remain virtually unknown. It is reported that P.haitanensis can utilize inorganic carbon and the sporophyte of P.haitanensis might possess a PCK-type C 4 -like carbon-fixation pathway (Luo et al.,2002; Fan et al., 2007) . This prompted us to the hypothesis that gene regulators,e.g.,microRNAs (miRNAs) ,may show some differences between the two generations.
MiRNAs are small non-coding RNAs that play an important role in post-transcriptional gene regulation (Lagos-Quintana et al.,2001; Lau et al.,2001; Lee and Ambros,2001) . They are thought to exist widely in plants,animals and virus,with high conservation within each kingdom (Reinhart et al.,2002; Bartel, 2004) . The expression of miRNAs has a spatiotemporal pattern and can influence the level of gene products. MiRNAs play important roles in various processes (Pasquinelli et al.,2000; Lagos-Quintana et al.,2001; Lau et al.,2001; Bashirullah et al.,2003; Lim et al.,2003) . MiRNAs have been studied widely in higher plants,but relative rarely in algae.
Previous studies have reported the existence of miRNAs in P.yezoensis (Liang et al.,2010; He et al.,2012) . In this study,we constructed and sequenced small RNA libraries from sporophytes (PHF) and gametophytes (PHL) of P.haitanensis. This study provides insights into the expression and function of small RNAs in P.haitanensis and even in P.yezoensis.
2 MATERIAL AND METHOD 2.1 Strains and culture conditionsThe sporophytes and gametophytes were cultured in PES medium (Provasoli,1963) made with steamsterilized local seawater and a 12-h photoperiod, using constant aeration and renewing the culture medium every week. The sporophytes were grown at 20°C under 40 μmol photons/ (m2∙s) illumination intensity. The gametophytes were preserved at -20°C after air drying in the shade. The dried blades were resuscitated in PES medium at 10°C under cool-white fluorescent light of about 30 μmol photons/ (m2∙s) for about a week before use. They were collected,frozen in liquid nitrogen and then stored at -80°C before RNA extraction.
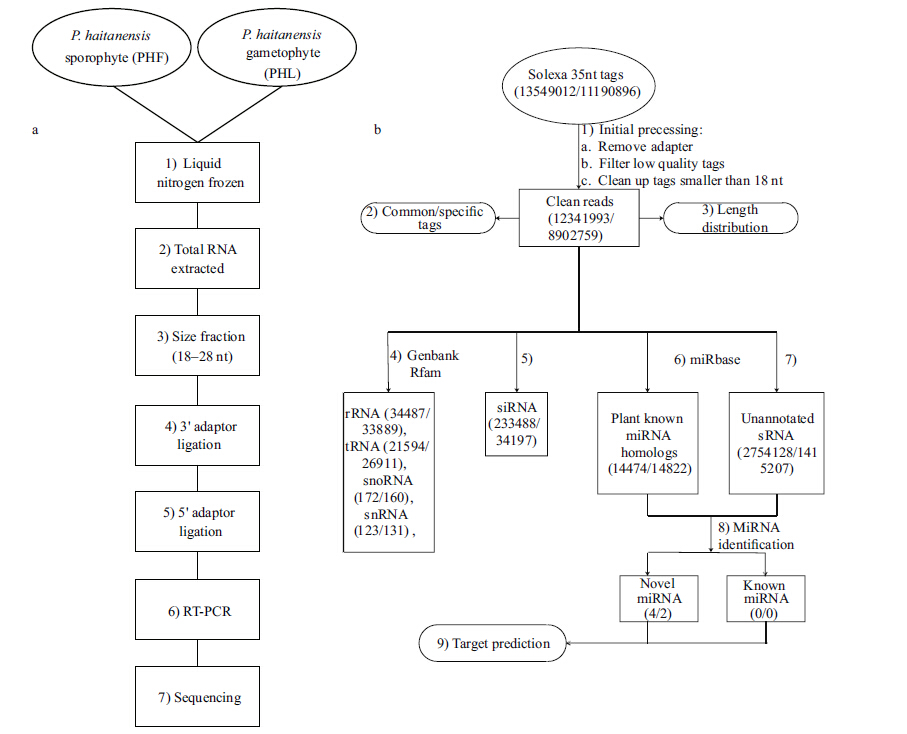
2.2 RNA extraction,small RNA library construction and sequencingTotal RNA was extracted using the Trizol reagent (Invitrogen,USA) ,according to manufacturers’ protocol. Fragments of 18-28 nt were recovered by gel purification. A pair of adaptors was ligated to the 5' and 3 ' ends of the small RNAs (sRNA) . The ligated sRNAs were then used as templates for reverse transcription-polymerase chain reaction (RT-PCR) amplification. The PCR products were then sequenced using a Solexa 1G Genome Analyzer,according to the manufacturer’s protocols (Fig. 1a) .
|
| Figure 1 Flow chart of the procedure for sample preparation, sequencing and the processing of reads |
After filtering out low quality tags,removing adapter contaminants and sequences smaller than 18 nt,the remaining clean reads were mapped to noncoding sRNAs downloaded from Rfam (http://www.sanger.ac.uk/software/Rfam) to identify non-coding sRNA degradation fragments using blastn (Altschul et al.,1997) with an e-value of 0.01 as the cutoff. Homologs of known miRNAs were identified by comparing all clean reads with all plant miRNAs downloaded from miRBase (miRBase Sequence Database version 19; http://www.mirbase.org/) . Short interfering RNAs (siRNAs) were identified by comparing all clean reads with each other: a pair of perfectly complementary sRNAs with 2 nt overhanging on the 3 ' end were considered as potential siRNAs. All clean reads were then annotated according to their identities with the sRNA categories mentioned above. In the case where an RNA matched with more than one small RNA category,a priority rule was adopted: ribosomal RNA (rRNA) etc. (in which GenBank > Rfam) > known miRNA > siRNA (Calabrese et al.,2007) . Small RNAs that were mapped to none of sRNA categories were termed unannotated small RNAs (Fig. 1b) .
2.4 MiRNA identificationPlant miRNA homologs and non-annotated sRNAs were used to identify potential known and novel miRNAs in P.haitanensis. As the genome of P.haitanensis has not been sequenced,we searched P.haitanensis and P.yezoensis expressed sequence tags (ESTs) for potential miRNA precursors. Basically,sRNAs that mapped to ESTs and their surrounding sequences (300 nt upstream and downstream) were extracted and folded conceptually to determine if they could form a hairpin structure that met the criteria used for precursor filtering (Allen et al.,2005) . The criteria applied were as follows: minimum free energy (MFE) ≤-18 kcal/mol,as determined by by Mfold (Mathews et al.,1999,2004) ; ≥16 bp and ≤4 bulges or asymmetries between the miRNA and miRNA* (Meyers et al.,2008) ; space between miRNA and miRNA* ≤300 nt; mature sequence length between 18-25 nt; and the 5 ' homogeneity ≥0.5 (Kozomara and Griffiths-Jones,2011) . The 5 ' homogeneity was defined as the total number of reads that had the same 5 ' end as the mature miRNA divided by the total number reads mapped to the precursor (Kozomara and Griffiths-Jones,2011) .
2.5 Novel miRNA target predictionGiven the lack of a sequenced genome of P.haitanensis,we searched P.haitanensis EST sequences for potential miRNA targets,which were identified according to criteria developed for plant miRNA target prediction (Allen et al.,2005; Schwab et al.,2005) . The criteria comprised: ≤4 mismatches between the small RNA and the target; ≤2.5 mismatches in positions 1-12; no mismatches in positions 10-11; and ≤2 adjacent mismatches,and no adjacent mismatches in positions 2-12 (counting from the 5 ' -end of the miRNAs and G-U bases as 0.5 mismatches) . Additionally,the minimum free energy (MFE) of the miRNA/target duplex should ≥74% of the MFE of their perfect complement.
2.6 Experimental verification of the expression of P.haitanensis miRNAsThe expression of P.haitanensis miRNAs was detected by RT-PCR as described previously (He et al.,2012) . CDNAs were synthesized and used for PCR amplification using the NCode™ VILO™ miRNA cDNA Synthesis Kit (Invitrogen,USA) . The sense primers comprised the sequence of each miRNA (Table 1) and the antisense primer was the universal primer supplied in the cDNA synthesis kit.
To verify the expression of miR2119 and its probable role in the drought response,P.haitanensis blades were dehydrated in air and collected at 0,10, 20,and 60 min before being re-hydrated for 15 min. To verify the influence of abscisic acid (ABA) ,the blades were treated with 100 μmol/L ABA in seawater. Total RNA was extracted using the Trizol reagent (Invitrogen) ,according to manufacturers’ protocol. The expression of miR2119 was verified by northern blot hybridization using the High Sensitive MiRNA Northern Blot Assay kit (Signosis,USA) ,as described previously (Huang et al.,2011a) . Briefly,5 μg total RNA (or 5 μL marker) was loaded into each well and run at 60V with 0.5× TBE until bromophenol blue reached approximately 3 cm from the bottom of the gel. The gel was transferred to a glass tray filled with 0.5× TBE buffer,assembled into the transfer unit and transferred at 60V at for l h in a cold room. After transfer,the membrane was exposed with a Stratagene UV cross-linker for 45s to immobilize the RNA, dried at 42°C for 15 min,hybridized with 10 μL of biotin labeled High Sensitive probe overnight in 4 mL NB hybridization solution,and then blocked and detected according to the manufactures’ protocol. The biotin labeled High Sensitive probe was designed according to the complementary sequence of miR2119.
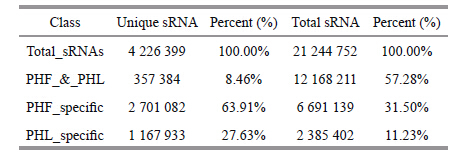
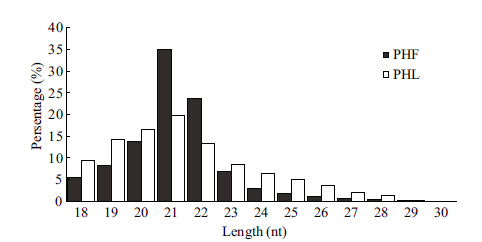
3 RESULT 3.1 Small RNAs in P.haitanensisThe two runs yielded 13 549 012 and 11 190 896 total reads from PHF and PHL,respectively. After removing low quality sequences,adaptor sequences and sequences smaller than 18 nt,we obtained 12 341 993 and 8 902 759 total reads,representing 3058 466 and 1 525 317 unique reads,with distribution of lengths ranging from 18 nt to 44 nt,of which sequences of 21 nt represented the most abundant component (Fig. 2) ,from PHF and PHL,respectively. All clean reads were annotated according to their identities; i.e.,rRNA etc.,miRNA and siRNA (Table 2) . Of the sRNAs annotated,siRNAs represented a major part in both the total and unique small RNA pool in PHF,while homologs of known miRNAs represented a significant part in the total RNA pool in PHL. These were followed by rRNAs and transfer RNAs (tRNAs) ,which made up of 1.13% and 0.71% in the unique pool of PHF,while the data were 2.22% and 1.76% in that of PHL. Small nuclear RNAs (snRNAs) and small nucleolar RNAs (snoRNAs) represented only small parts in both the total and unique data set in both libraries.
|
| Figure 2 Length distributions of unique small RNA sequencesin P.haitanensis |
Of the unique sequences,approximately 70% were sequenced once. Only 8.46% of sRNAs were shared by PHF and PHL (Table 3) ,indicating a diverse sRNA data set in P.haitanensis.
Using criteria developed for plant miRNA identification,five miRNAs were identified in P.haitanensis (Table 4) . They showed no sequence homology with known miRNAs in other organisms. The length of pre-miRNAs ranged from 47 to 189 nt, with a mean of 115 nt. The MFE ranged from -28.8 to -87.8 kcal/mol,with a mean of -46 kcal/mol. These miRNAs were designated as Pha-miR 1-5, respectively. Sequence terminal variation analysis showed that the percentage of length heterogeneity for 5 ' end of P.haitanensis miRNAs was about 4%, while for the 3 ' end it was 52%; variations were either a 3-nt deletion or extension (Fig. 3) . Of the five miRNAs identified,only one was shared by PHF and PHL,three were sequenced exclusively from PHF and one was sequenced exclusively from PHL.
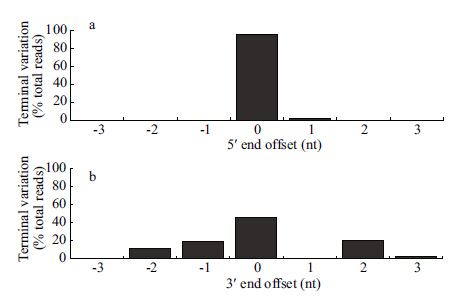
|
| Figure 3 P. haitanensis miRNA Sequence terminal variation analysis |
We compared all clean reads with all known plant miRNAs and found a number of homologs; however, these homologs did not pass the precursor filtering, probably because of the lack of P.haitanensis genome sequences,although the scenarios that P.haitanensis lacks homologous miRNAs with other organisms cannot be ruled out. Among these homologs,some were particularly highly expressed.
3.4 Potential miRNA targetsTo determine the probable roles of the five miRNAs,we predicted their targets for these miRNAs,using the rules for plant miRNA target prediction suggested by Allen et al. (2005) . Targets for Pha-miR1,Pha-miR3, and Pha-miR 4 were suggested. However,as the genome of P.haitanensis has not been sequenced and the EST sequences were limited,it is difficult to determine whether these potential targets have any functional bias.
3.5 siRNA in P.haitanensisIn addition to miRNA,plants also produced a diverse set of siRNAs,such as trans-acting siRNAs (ta-siRNAs) and natural antisense siRNAs (natsiRNAs) . We queried if P.haitanensis produced siRNAs. We aligned tags from the clean reads against each other to identify potential siRNA candidates; the two perfectly complementary sRNAs with 2 nt hanging at the 3'-end were considered as siRNA.2055 768 (16.66%) and 242 913 (2.73%) total sequences,representing 233 488 (7.63%) and 34 197 (2.24%) unique sequences were annotated as siRNAs in PHF and PHL,respectively. We could not determine their location or whether they were phased relative to each other because of the lack of genome information. In addition,we could not determine if they play a role in silencing of repetitive sequences in P.haitanensis, as repetitive sequences of P.haitanensis are limited.
3.6 Validation of P.haitanensis miRNAsWe used RT-PCR to detect the expression of P.haitanensis miRNAs. The mature sequence of phamiR5 was validated by PCR amplification. PCR products of the expected sizes (60-80 bp) were amplified (Fig. 4) ,increasing our confidence in its expression. Some larger PCR products might result from precursors.
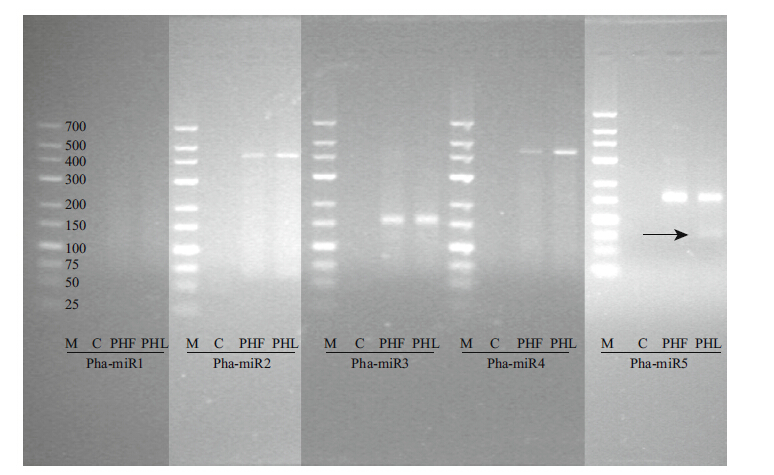
|
| Figure 4 Experimental validation of P. haitanensis miRNAs by PCR amplification |
Among the miRNAs that were homologs of known plant miRNAs,those homologous to miR2119 were present in PHL with 1 479 099 copies; therefore,we selected miR2119 for further study. To verify the probable roles of miR2119 in P.haitanensis,total RNA was extracted from samples with different dehydration levels and ABA treatment. Northern blotting was used to detect the initial expression of miR2119 and its precursors in P.haitanensis. Northern blot hybridization detected no mature miR2119,but two precursors,in all the samples (Fig. 5a) . The precursor showed a moderate increase in accumulation upon drought treatment,but not by ABA treatment. In addition,PCR products of the expected sizes (60-80 bp) for mature miR2119 and some larger PCR products resulting from precursors were amplified (Fig. 5b) . These provided strong evidence of its expression.
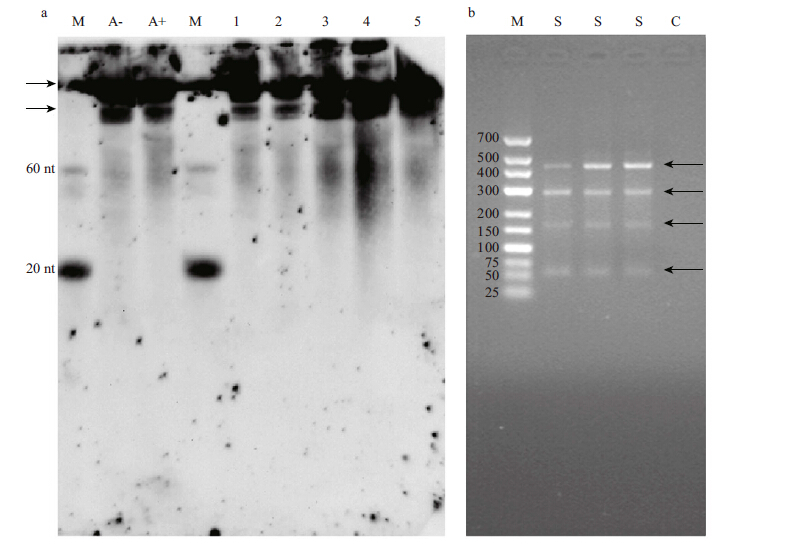
|
| Figure 5 Experimental validation of the expression of miR2119 in P. haitanensis by northern blot analysis and PCR amplification |
In both PHF and PHL,sRNAs of 21 nt in size were the most abundant,which is the case in the moss Physcomitrella patens and the unicellular green alga Chlamydomonas reinhardtii (Arazi et al.,2005; Axtell et al.,2007; Zhao et al.,2007) . In our previously study of Phaeodactylum tricornutum,the most abundant sRNAs were 22 nt in size (Huang et al.,2011a) ,and in the green algae Ulva prolifera they were 25 nt in size (Huang et al.,2011b) . This was not the case in Arabidopsis thaliana,where sRNAs of 24 nt were the most abundant (Xie et al.,2005; Rajagopalan et al.,2006) . The length of an sRNA is determined by the types of enzymes that take part in its processing (Zhao et al.,2007) ; therefore,we hypothesized that these lower photosynthetic organisms might have RNA processing complexes that are different from A. thaliana.
We obtained 12 341 993 and 8 902 759 total reads, representing 3 058 466 and 1 525 317 unique reads, from PHF and PHL,respectively. The reasons for the large difference in the numbers of unique reads between the two libraries might be: 1) RNA degradation occurred in the sample with fewer unique reads (PHL) ; 2) there is large difference in gene expression (e.g. different RNA processing complexes) between PHF and PHL. Since the RNA sample had been detected to be intact for all the samples,it is less possible that RNA degradation occurred only in PHL. In addition,the two generations are different in many aspects,including structural features,living environments and gene expression profiles (Yamazaki et al.,1998; Kitade et al.,1999; Sahoo et al.,2002; Asamizu et al.,2003) . Thus,it is more likely that different gene expressions between PHF and PHL resulted in different numbers of unique reads between the two libraries.
It is suggested that miRNAs might take part in sexual differentiation in P yropia (Blouin et al.,2011) . In this study,we identified five miRNAs from P.haitanensis,of which only one was shared by PHF and PHL,indicating that miRNAs might play different roles in different generations.
Many of homologs of known plant miRNAs were identified in P. haitanensis , and the percentage was particularly high in PHL. This was because homologs of miR2119 were present in PHL with 1 479 099 copies, indicating an important role in the gametophyte of P. haitanensis. In a study of the legume Phaseolusvulgaris,pvu-miR2119 showed a moderate but clear increase in accumulation upon drought and ABA treatments (Arenas-Huertero et al.,2009) . The gametophytes of P yropia live on static substrates of the intertidal zone,experiencing strong light,high temperature and desiccation stresses during low tide (Yamazaki et al.,1998; Kitade et al.,1999; Sahoo et al.,2002) . We hypothesized that homologs of miR2119 might play an important role in the drought response in gametophytes of P.haitanensis. We used northern blotting to detect the expression of miR2119 in samples with different dehydration levels and ABA treatment. No mature miR2119,but two precursors, were detected in all the samples (Fig. 5a) . It was probable that the expression of mature miR2119 was too low to be detected by northern blotting in these samples. However,using PCR amplification,a product of the expected size (60-80 bp) for mature miR2119 was detected (Fig. 5b) . The precursor showed a moderate increase in accumulation upon drought treatment,but not under ABA treatment, indicating that miR2119 might play a role in the dehydration response in P.haitanensis.
SiRNAs play important roles in gene silencing in many organisms. In this study,siRNA was differently expressed between the two samples,with up to 16.6% of total reads in PHF and only 2.73% in PHL. This indicated that siRNA might play an important role in sporophyte development of P.haitanensis.
5 CONCLUSIONP. haitanensis has a complex sRNA processing system. It contains novel miRNAs that have no identifiable homologs with other organisms, which might play important regulatory roles in P. haitanensis development.
| Allen E, Xie Z X, Gustafson A M, Carrington J C, 2005. microRNA-directed phasing during trans-acting siRNA biogenesis in plants. Cell, 121 (2) : 207 –221. Doi: 10.1016/j.cell.2005.04.004 |
| Altschul S F, Madden T L, Schäff er A A, Zhang J H, Zhang Z, Miller W, Lipman D J, 1997. Gapped BLAST and PSIBLAST:a new generation of protein database search programs. N ucleic A cids R es., 25 (17) : 3389 –3402. |
| Arazi T, Talmor-Neiman M, Stav R, Riese M, Huijser P, Baulcombe D C, 2005. Cloning and characterization of micro-RNAs from moss. The Plant Journal, 43 (6) : 837 –848. Doi: 10.1111/tpj.2005.43.issue-6 |
| Arenas-Huertero C, Pérez B, Rabanal F, Blanco-Melo D, De la Rosa C, Estrada-Navarrete G, Sanchez F, Covarrubias A A, Reyes J L, 2009. Conserved and novel miRNAs in the legume Phaseolus vulgaris in response to stress. Plant Mol. Biol., 70 (4) : 385 –401. Doi: 10.1007/s11103-009-9480-3 |
| Asamizu E, Nakajima M, Kitade Y, Saga N, Nakamura Y, Tabata S, 2003. Comparison of RNA expression profiles between the two generations of Porphyra yezoensis(Rhodophyta), based on expressed sequence tag frequency analysis. J. Phycol., 39 (5) : 923 –930. Doi: 10.1046/j.1529-8817.2003.03003.x |
| Axtell M J, Snyder J A, Bartel D P, 2007. Common functions for diverse small RNAs of land plants. The Plant Cell Online, 19 (6) : 1750 –1769. Doi: 10.1105/tpc.107.051706 |
| Bartel D P, 2004. MicroRNAs genomics, biogenesis, mechanism, and function. Cell, 116 (2) : 281 –297. Doi: 10.1016/S0092-8674(04)00045-5 |
| Bashirullah A, Pasquinelli A E, Kiger A A, Perrimon N, Ruvkun G, Thummel C S, 2003. Coordinate regulation of small temporal RNAs at the onset of Drosophila metamorphosis. Dev. Biol., 259 (1) : 1 –8. Doi: 10.1016/S0012-1606(03)00063-0 |
| Blouin N A, Brodie J A, Grossman A C, Xu P, Brawley S H, 2011. Porphyra:a marine crop shaped by stress. Trends Plant Sci., 16 (1) : 29 –37. Doi: 10.1016/j.tplants.2010.10.004 |
| Calabrese J M, Seila A C, Yeo G W, Sharp P A, 2007. RNA sequence analysis defines Dicer's role in mouse embryonic stem cells. Proc. Natl. Acad. Sci. U. S. A., 104 (46) : 18097 –18102. Doi: 10.1073/pnas.0709193104 |
| Fan X L, Fang Y J, Hu S N, Wang G C, 2007. Generation and analysis of 5318 expressed sequence tags from the filamentous sporophyte of Porphyra haitanensis(Rhodophyta). J. Phycol., 43 (6) : 1287 –1294. Doi: 10.1111/jpy.2007.43.issue-6 |
| He L W, Huang A Y, Shen S D, Niu J F, Wang G C, 2012. Comparative analysis of microRNAs between sporophyte and gametophyte of Porphyra yezoensis. Comp. Funct. Genomics, 2012 : 912843 . |
| Huang A Y, He L W, Wang G C, 2011a. Identification and characterization of microRNAs from Phaeodactylum tricornutum by high-throughput sequencing and bioinformatics analysis. BMC Genomics, 12 (1) : 337 . Doi: 10.1186/1471-2164-12-337 |
| Huang A Y, Wang G C, He L W, Niu J F, Zhang B Y, 2011b. Characterization of small RNAs from Ulva prolifera by high-throughput sequencing and bioinformatics analysis. Chin. Sci. Bull., 56 (27) : 2916 –2921. Doi: 10.1007/s11434-011-4678-6 |
| Iwasaki H, 1961. The life-cycle of Porphyra tenera in vitro. The Biological Bulletin, 121 (1) : 173 –187. Doi: 10.2307/1539469 |
| Kitade Y, Fukuda S, Saga N, 1999. Preliminary study on early development of monospore of Porphyra yezoensis(Bangiales, Rhodophyta). Fish Genet. Breed. Sci., 28 : 27 –34. |
| Kozomara A, Griffiths-Jones S, 2011. miRBase:integrating microRNA annotation and deep-sequencing data. N ucleic A cids R es., 39 (Suppl. 1) : D152 –D157. |
| Lagos-Quintana M, Rauhut R, Lendeckel W, Tuschl T, 2001. Identification of novel genes coding for small expressed RNAs. Sci ence, 294 (5543) : 853 –858. Doi: 10.1126/science.1064921 |
| Lau N C, Lim L P, Weinstein E G, Bartel D P, 2001. An abundant class of tiny RNAs with probable regulatory roles in Caenorhabditis elegans. Science, 294 (5543) : 858 –862. Doi: 10.1126/science.1065062 |
| Lee R C, Ambros V, 2001. An extensive class of small RNAs in Caenorhabditis elegans. Sci ence, 294 (5543) : 862 –864. Doi: 10.1126/science.1065329 |
| Li S Y, Zheng B F, 1992. Studies on the rotation culture of Porphyra haitanensis T. J. Chang et B. F. Zheng and Porphyra yezoensis Ueda. Oceanol. Limnol. Sin., 23 (5) : 537 –540. |
| Liang C W, Zhang X W, Zou J, Xu D, Su F, Ye N H, 2010. Identification of miRNA from Porphyra yezoensis by high-throughput sequencing and bioinformatics analysis. PLoS One, 5 (5) : e10698 . Doi: 10.1371/journal.pone.0010698 |
| Lim L P, Lau N C, Weinstein E G, Abdelhakim A, Yekta S, Rhoades M W, Burge C B, Bartel D P, 2003. The microRNAs of Caenorhabditis elegans. Genes Dev., 17 (8) : 991 –1008. Doi: 10.1101/gad.1074403 |
| Luo Q J, Pei L Q, Pan S Y, Wang Y, Fei Z Q, 2002. Utilization of inorganic carbon in free-living conchocelis of Porphyra haitanensis. J. Fish. China, 26 (5) : 477 –480. |
| Ma J H, Cai S Q, 1996. The Cultivation and Processing of Porphyra yezoensis Ueda. Science Press, Beijing. |
| Mathews D H, Disney M D, Childs J L, Schroeder S L, Zuker M, Turner D H, 2004. Incorporating chemical modification constraints into a dynamic programming algorithm for prediction of RNA secondary structure. Proc. Natl. Acad. Sci. U. S. A., 101 (19) : 7287 –7292. Doi: 10.1073/pnas.0401799101 |
| Mathews D H, Sabina J, Zuker M, Turner D H, 1999. Expanded sequence dependence of thermodynamic parameters improves prediction of RNA secondary structure. J. Mol. Biol., 288 (5) : 911 –940. Doi: 10.1006/jmbi.1999.2700 |
| Meyers B C, Axtell M J, Bartel B, Bartel D P, Baulcombe D, Bowman J L, Cao X F, Carrington J C, Chen X M, Green P J, Griffiths-Jones S, Jacobsen S E, Mallory A C, Martienssen R A, Poethig R S, Qi Y J, Vaucheret H, Voinnet O, Watanabe Y, Weigel D, Zhu J K, 2008. Criteria for annotation of plant microRNAs. Plant Cell, 20 (12) : 3186 –3190. Doi: 10.1105/tpc.108.064311 |
| Pasquinelli A E, Reinhart B J, Slack F, Martindale M Q, Kuroda M I, Maller B, Hayward D C, Ball E E, Degnan B, Müller P, Spring J, Srinivasan A, Fishman M, Finnerty J, Corbo J, Levine M, Leahy P, Davidson E, Ruvkun G, 2000. Conservation of the sequence and temporal expression of let-7 heterochronic regulatory RNA. Nature, 408 (6808) : 86 –89. Doi: 10.1038/35040556 |
| Provasoli L, 1963. Growing Marine Seaweeds. In: Proceedings of the 4th International Seaweed Symposium. Pergamon Press, New York9-17. |
| Ragan M A, Bird C J, Rice E L, Gutell R R, Murphy C A, Singh R K, 1994. A molecular phylogeny of the marine red algae(Rhodophyta)based on the nuclear smallsubunit rRNA gene. Proc. Natl. Acad. Sci. U. S. A., 91 (15) : 7276 –7280. Doi: 10.1073/pnas.91.15.7276 |
| Rajagopalan R, Vaucheret H, Trejo J, Bartel D P, 2006. A diverse and evolutionarily fl uid set of microRNAs in Arabidopsis thaliana. Genes Dev., 20 (24) : 3407 –3425. Doi: 10.1101/gad.1476406 |
| Reinhart B J, Weinstein E G, Rhoades M W, Bartel B, Bartel D P, 2002. MicroRNAs in plants. Genes Dev., 16 (13) : 1616 –1626. Doi: 10.1101/gad.1004402 |
| Saga N, Kitade Y, 2002. Porphyra:a model plant in marine sciences. Fisheries Science-Tokyo, 68 (Suppl. 2) : 1075 –1078. |
| Sahoo D, Tang X R, Yarish C, 2002. Porphyra:the economic seaweed as a new experimental system. Curr. Sci., 83 (11) : 1313 –1316. |
| Schwab R, Palatnik J F, Riester M, Schommer C, Schmid M, Weigel D, 2005. Specific effects of microRNAs on the plant transcriptome. Dev. Cell, 8 (4) : 517 –527. Doi: 10.1016/j.devcel.2005.01.018 |
| Xie Z X, Allen E, Fahlgren N, Calamar A, Givan S A, Carrington J C, 2005. Expression of Arabidopsis miRNA genes. Plant Physiol., 138 (4) : 2145 –2154. Doi: 10.1104/pp.105.062943 |
| Yamazaki A, Nakanishi K, Saga N, 1998. Axenic tissue culture and morphogenesis in Porphyra yezoensis(Bangiales, Rhodophyta). J. Phycol., 34 (6) : 1082 –1087. Doi: 10.1046/j.1529-8817.1998.341082.x |
| Zhao T, Li G L, Mi S J, Li S, Hannon G J, Wang X J, Qi Y J, 2007. A complex system of small RNAs in the unicellular green alga Chlamydomonas reinhardtii. Genes Dev., 21 (10) : 1190 –1203. Doi: 10.1101/gad.1543507 |
 2016, 34
2016, 34