Institute of Oceanology, Chinese Academy of Sciences
Article Information
- Qiang LIN(林强), Ningqiu LI(李宁求), Xiaozhe FU(付小哲), Qiandong HU(胡钱东), Ouqin CHANG(常藕琴), Lihui LIU(刘礼辉), Defeng ZHANG(张德锋), Guangjun WANG(王广军), Guibao SAN(伞桂宝), Shuqin WU(吴淑勤)
- An outbreak of granulomatous infl ammation associated with Francisella noatunensis subsp. orientalis in farmed tilapia (Oreochromis niloticus×O. aureus) in China
- Journal of Oceanology and Limnology, 34(3): 460-466
- http://dx.doi.org/10.1007/s00343-016-4311-2
Article History
- Received: Nov. 14, 2014
- Accepted: Feb. 6, 2015
2. Freshwater Aquaculture Collaborative Innovation Center of Hubei Province, Wuhan 430070, China;
3. Panyu District Agricultural Science Research Institute, Guangdong Province, Guangzhou 511450, China
Tilapia have become one of the most important farmed aquatic species throughout the world and are now produced in nearly 100 countries (Romana-Eguia et al.,2004) . China is the biggest tilapia producer in the world,with the annual production accounting for almost half the global harvest. However,the rapid expansion and high-intensity of tilapia farming has led to outbreaks of a range of diseases.
Between January and February 2013,there was a significant increase in morbidity and mortality in several freshwater tilapia ponds on a farm in Guangzhou,South China. Approximately 40%–50% of the tilapia ponds on the 200 acre farm were infected during this period. However,no apparent onset clinical signs were observed among fish in the infected ponds. Infection of fish was characterized by several stages,including anorexia,lethargy,and slow spiral or circular swimming behavior. Additionally,necropsy of infected fish revealed a high number of white granulomas of various sizes (1–5 mm) in the kidney,liver,heart,and spleen.
White granulomas or nodules in the internal organs have been documented during illness in many fishes,including tilapia (Kamaishi et al.,2005) ,salmonids (Aro et al.,2014) ,large yellow croaker (Zhang et al.,2014) ,Russian sturgeons (Antuofermo et al.,2014) ,mullet (Varello et al.,2014) ,Atlantic cod (Gudmundsdottir et al.,2014) ,sea breams (Schefzyk et al.,2012) ,burbot Lota lota (Zerihun et al.,2011) ,snakehead fish (Liu and Li,2012) ,large mouth bass (Goodwin et al.,2002) ,and other species (Hsieh et al.,2007; Vojtech et al.,2009; Kamaishi et al.,2010) .The pathological symptoms caused by Francisella (Kamaishi et al.,2005) are also observed in infections caused by Mycobacterium (Antuofermo et al.,2014) ,Yersinia ruckeri (Gudmundsdottir et al.,2014) ,Aeromonas schubertii (Liu and Li,2012) ,Pseudomonas plecoglossicida (Zhang et al.,2014) ,Nocardia (Wang et al.,2005) ,and Piscirickettsia (Fryer and Hedrick,2003) . Thus,it is very difficult to distinguish these pathogens based on clinical signs. To the best of our knowledge,the diseases caused by Francisella have not been reported in tilapia in China. We describe the histopathology,microbiology,and molecular characteristics of the granulomatous inflammation affecting tilapia aquaculture in Guangdong Province,China.
2 MATERIAL AND METHOD 2.1 Sample collectionWe obtained 10 tilapia from a tilapia farm in Guangdong Province,China in January and February 2013. The farm was experiencing morbidity and mortality associated with white granulomas on internal organs. The spleen,liver,gills,intestine,head kidney,and kidney were dissected and removed from each individual.
2.2 Histological analysisThe spleen,liver,gills,intestine,head kidney,and kidney were fixed in 10% neutral buffered formalin. The samples were embedded in paraffin wax using standard protocols. Sections were cut and mounted onto glass slides before staining with haematoxylin and eosin (H&E) ,and then examined by light microscopy following standard histological methods.
2.3 Isolation,media,and growth conditionsThe spleen,liver,and kidney from tilapia exhibiting signs of disease were aseptically collected and inoculated onto blood agar,brain-heart infusion agar (BHI with agar) ,BHI with 5% bovine blood,tryptic soy agar (TSA) ,TSA with 5% bovine blood,thiosulfate citrate bile salts sucrose agar (TCBS) ,cystine heart agar supplemented with bovine hemoglobin solution (CHAH) ,and Thayer-martin agar plates. Inoculated media were incubated at 20°C and 28°C for 10 d. Once single colonies were observed,the isolate was purified under the same conditions. A modified Mueller-Hinton II cation adjusted broth supplemented with 2% IsoVitaleX (BD BBL) and 0.1% glucose (MMH) was used to propagate isolates. The isolates were cultured in MMH to extract DNA. The liquid mediums were grown in a shaker at 175 rpm overnight at 20°C.
2.4 DNA extraction and 16S rRNA gene amplificationSpleen,liver,and kidney tissue (approximately 100 mg) from diseased fish were homogenized in sterile containers with 1 mL sterile water. We then transferred 500 μL of the suspension to a new sterile centrifuge tube to extract the DNA. DNA was extracted using a Bacterial DNA Kit (OMEGA) following the manufacturers protocol. To amplify the 16S rRNA gene,2 μL of the DNA was added to 18 μL of reaction mixture,which consisted of 10×PCR buffer with 1 mmol/L MgCl2,0.2μmol/L deoxynucleotide triphosphate,0.2 μmol/Luniversal forward primer 16sF (5' AGA GTT TGA TCC TGG TCA GAA CGA ACG CT 3') ,0.2 μmol/L universal reverse primer 16sR (5' TAC GGC TAC CTT GTT ACG ACT TCA CCC C 3') ,and 0.5 U TaqDNA polymerase (TaKaRa,Dalian,China) . PCR amplification was carried out using the following protocol: denaturing for 3 min at 94°C; 35 cycles of 94°C for 30 s,60°C for 45 s,and 72°C for 90 s; and an extension period at 72°C for 7 min.
2.5 DNA sequencing and phylogenetic analysisThe PCR products were purified using a Cycle Pure kit (Omega Bio-Tek,Norcross,GA,USA) following the manufacturers protocol. Sequencing was then performed by Shanghai Sangon Biological Engineering Technology & Services Co.,Ltd (Shanghai,China) . Sequences obtained in the present study were submitted to GenBank and compared with other sequences using the BLASTN program. The partial sequence of 16S rRNA of the genus Francisella was obtained and aligned using Clustal W. The phylogenetic tree was constructed using the neighbor-joining method in the Molecular Evolutionary Genetic Analysis (MEGA) package (version 5) .
2.6 Experimental challengesWe cultured Francisella sp. on MMH and supernatant that was filtered from tissue homogenate of tilapia with internal white granulomas in sterile water. The gz201301 strain isolated from tilapia tissues was suspended in saline solution (0.65%) at a concentration of 1.0×108 cfu/mL. Tilapia (average length 11.0 cm) were maintained in three different tanks (10 fish per tank) at 20–25°C. All fish included in this challenge experiment were acclimated for 7 d in the experimental tanks before being infected by intraperitoneal injection of Francisella. The fish in group A were challenged with 0.2 mL of the gz201301 strains. The fish in group B were injected with 0.2 mL supernatant filtered from tissue homogenate (liver) . The fish in group C received 0.2 mL saline solution (control group) . Mortality was recorded in all tanks every 12 h for 30 d. Dead fish were subjected to a complete clinical, histopathological, and bacteriological examination and PCR testing.
3 RESULT 3.1 Clinical signs and histopathologyA complete necropsy of the tilapia revealed large numbers of white granulomas of various sizes (1–5 mm) in the internal organs (including head kidney,kidney,liver,heart,and spleen) ,gastrointestinal walls,choroid gland,and mesenteric fat (Fig. 1) . The number of granulomas was higher in the spleen and head kidney than in the liver or trunk kidney. No granulomas were observed in the gills. In some cases,fish had hemorrhagic ascites. Most of the fish had marked renomegaly and splenomegaly (Fig. 2) . No parasites or other pathogens were identified.
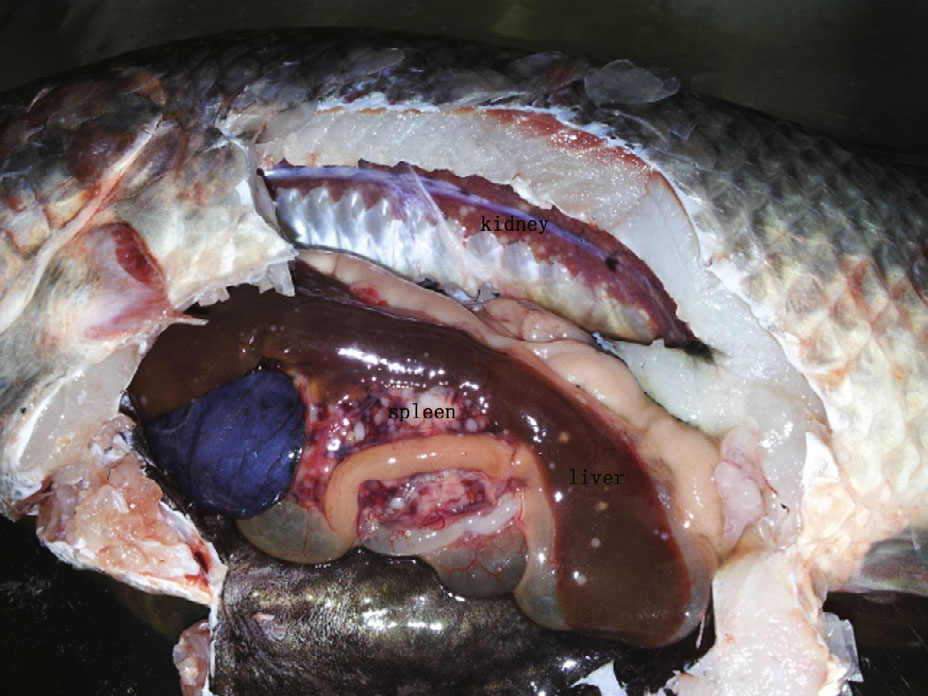
|
| Figure 1 Tilapia, from a fish farm, showing the typical signs of Francisella sp., including white granulomas on the spleen, kidney, and liver |

|
| Figure 2 Spleen A and B from tilapias infected with Francisella sp. |
Histopathologically,the spleens were significantly enlarged,with up to 90% parenchymal replacement by multiple and coalescing granulomatous lesions (Fig. 3) . Numerous granulomatous lesions were also observed in the kidney and spleen. The larger granulomas in the spleen contained a necrotic center of a small and dark nucleus and eosinophilic cytoplasm,encapsulated by numerous macrophages and foamy,vacuolated,and fibrous capsules (Fig. 4) . Multiple fluid-filled cavities and ectatic blood vessels were observed in the granulomatous splenitis. Occasionally,a fine,fibrous capsule encompassed the granulomas. The cytoplasmic vacuoles contained numerous (1–2 micron) pleomorphic coccoid bacteria. Additionally,the epithelioid macrophages and peripheral macrophages contained lesser numbers of bacteria.
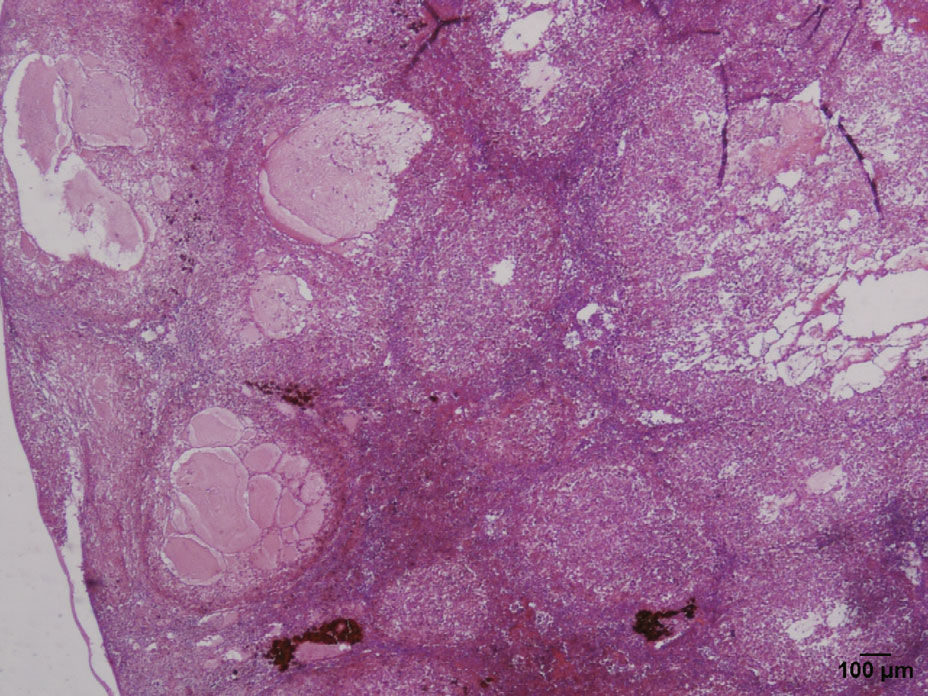
|
| Figure 3 Spleen from tilapia with multiple granulomas (haematoxylin and eosin, 40×) |
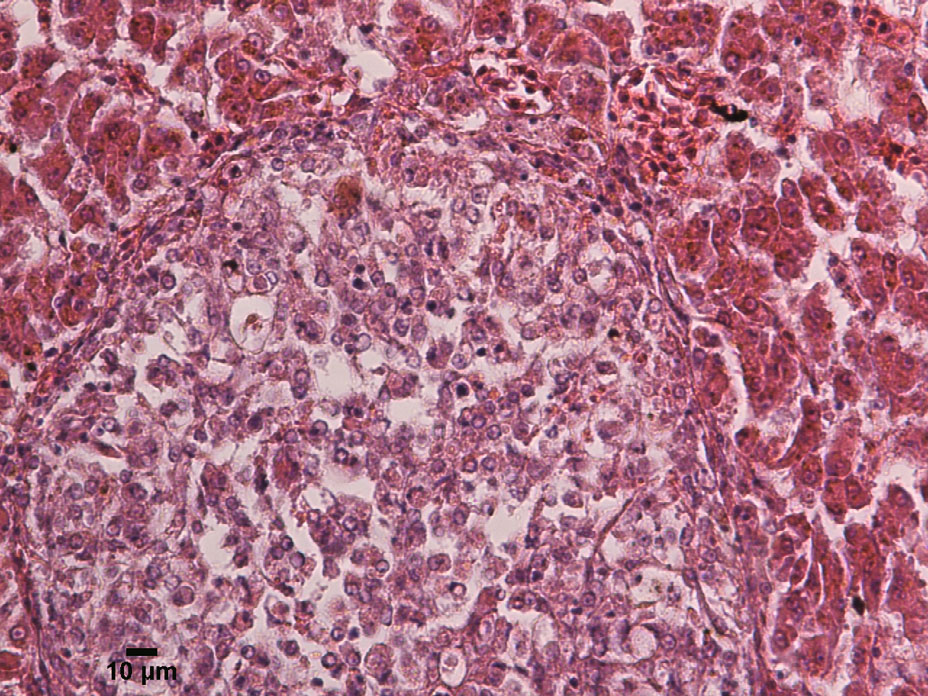
|
| Figure 4 Typical granuloma in the kidney, with a necrotic hyperchromatic core surrounded by epithelioid macrophages, and necrotic cells, HE 200× |
We obtained grayish-white,smooth,and convex colonies (1–2 mm) on CHAH after 5 d at 20°C. No isolates were observed on blood agar,BHI,BHI with 5% bovine blood,TSA,TSA with 5% bovine blood,or thiosulfate citrate bile salts sucrose agar (TCBS) . The isolate was designated as gz201301. Bacterial colonies did not propagate at 28°C.
The 16S rRNA gene of the gz201301 strain isolate was sequenced. No variation was observed between the 16S rRNA sequences. The 16S rRNA sequence of strain gz201301 was submitted to GenBank (No. KJ201765) and compared with other sequences in GenBank using the BLASTN program. The neighbor-joining tree based on the 16S rRNA gene revealed the phylogenetic relationship of the sequences with Francisella sp. (Fig. 5) . As expected,the 16S rRNA sequences of strain gz201301 showed 99.64%–100% identity to F.noatunensis subsp. orientalis,99%– 99.22% identity to the F.noatunensis subsp. noatunensis,98.93%–99.07% identity to F.philomiragia,and 97.72% identity to F.tularensis.
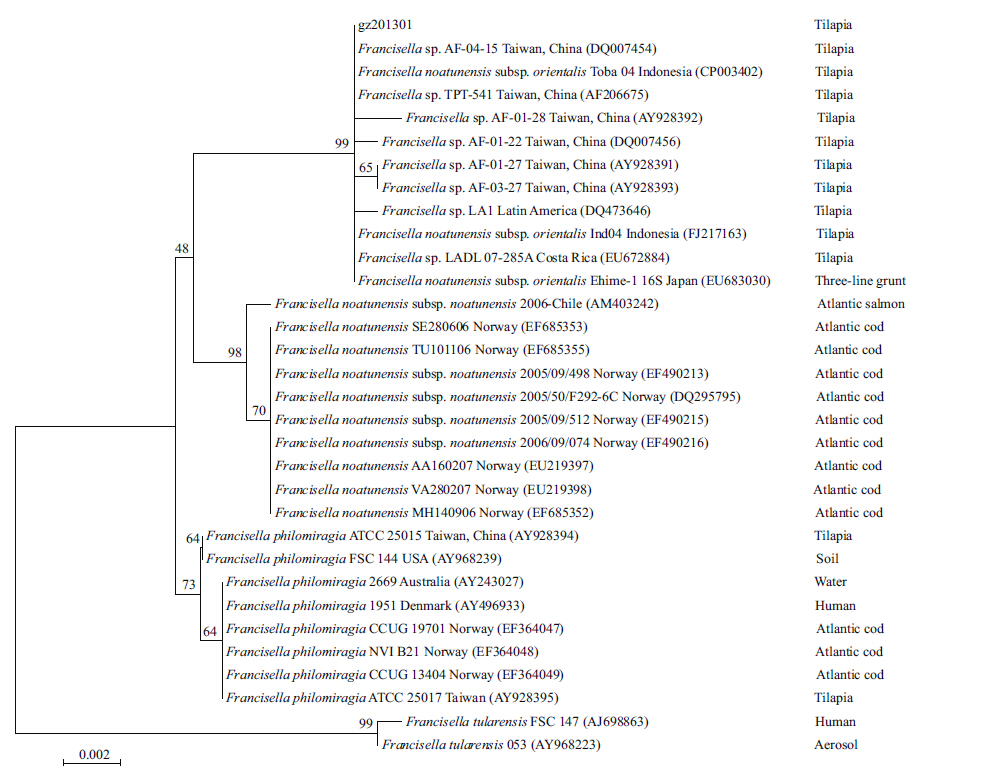
|
| Figure 5 Evolutionary relationship of 32 partial 16S rRNA sequences of members of the genus Francisella |
The cumulative mortality rate of tilapia in A group was 40% (4/10) during the 30 d after injection of Francisella sp. Many granulomas were observed in the spleen,liver,kidneys,and heart upon necropsy of both dead and surviving tilapia. Groups B and C had no mortality and all organs were normal in the postmortem examination. Francisella sp. was re-isolated from group A with CHAH. The 16S sequences of re-isolated colonies were the same as strain gz201301. No bacterium was detected from groups B and C by bacteriological,histopathological,or molecular analysis.
4 DISCUSSIONF.noatunensis subsp. orientalis is a Gram-negative,facultative intracellular bacterial pathogen that causes chronic disease in warm fish species. The granulomatous inflammation was initially found in Taiwan in the early 1990s; however,no pathogens were isolated and confirmed. Therefore,it was provisionally called a ‘Piscirickettsiacaea-like organism’ (Chen et al.,1994; Chern and Chao,1994) prior to the pathogen being identified as a Francisella sp. In 2002,a new Francisella was isolated from cultures of three-line Grunt (Kamaishi et al.,2005) . PCR was used to identity these pathogens as members of the genus Francisella (Hsieh et al.,2006; Nylund et al.,2006) . To date,F.noatunensis subsp. Noatunensis (Mauel et al.,2005) and F.noatunensis subsp. orientalis (Jeffery et al.,2010) have been isolated from fish. These bacteria can infect tilapia (Hsieh et al.,2006; Soto et al.,2011) ,hybrid striped bass (Ostland et al.,2006) ,Atlantic cod (Olsen et al.,2006; Mikalsen et al.,2007) ,salmons (Birkbeck et al.,2007) ,three line grunt (Kamaishi et al.,2005) ,and many other warm water fish species (Hsieh et al.,2007; Vojtech et al.,2009; Kamaishi et al.,2010) .
Francisella sp. does not grow on TSA (Mauel et al.,2005) ,MacConkey agar,BHI containing 5% sheep red blood cells,blood-cystine-glucose agar,or in 6% NaCl (Hsieh et al.,2006) . Conversely,Francisella sp. can grow on cystine heart agar supplemented with bovine hemoglobin solution or cystine heart agar supplemented with 5% sheep blood plate (Soto et al.,2011) . In our study,colonies were only observed on CHAH. The aquatic Francisella sp. can grow at 6–25°C,but does not grow at 30–37°C (Kamaishi et al.,2005; Ostland et al.,2006; Ottem et al.,2007) . Similarly,gz201301 cannot grow at 28°C. The prevalence of natural outbreaks of this disease in tilapia in Guangdong decreases significantly in April (water temperature: 24–29°C) with clinical disease essentially disappearing in May (when water temperature exceeds 30°C) . This illustrates that the Francisella-like disease is seasonal.
Based on phylogenetic analysis of 16S rRNA,the neighbor-joining tree indicated that gz201301 is most closely related to F.noatunensis with 99% similarity. The next closest relationship to gz201301 was F.philomiragia with 97% similarity. This strain was less closely related to F.tularensis. F.noatunensis consists of two subspecies adapted to different host temperatures. F.noatunensis subsp. noatunensis was isolated from fish living in colder waters (Nylund et al.,2006; Olsen et al.,2006; Birkbeck et al.,2007; Ottem et al.,2007) and F.noatunensis subsp. orientalis was isolated from fish living in warmer waters (Kamaishi et al.,2005; Mauel et al.,2005,2007; Jeffery et al.,2010; Soto et al.,2011) . Francisella sp. might have host specificity but further study is required to confirm this. (Birkbeck et al.,2011; Colquhoun and Duodu,2011) .
F.noatunensis subsp. orientalis is a recently recognized fish pathogen with highly morbidity rates that has disrupted tilapia farms in many countries. Identification of this disease is delayed because fish are typically asymptomatic,there are often multiple infectious agents,and there are difficulties isolating bacteria from previously antibiotic treated hosts (Soto et al.,2009) . The fish infected by this bacterium have no external clinical signs,though white granulomas can be observed in the internal organs of moribund fish. Therefore,there is a need to develop a rapid detection assay to identify the pathogen to allow for prevention and treatment. Once the pathogen is detected,infected fish can be isolated and treated to prevent an outbreak. It is unclear how these bacteria enter tilapia farms. The infection at the farm in this study was not introduced by purchase of fry as the farm maintains its own broodstock. We speculate that infectious bacteria spread through canals in the vicinity of farms. Large numbers of wild tilapia live in the surrounding canals and may be infected by F.noatunensis subsp. orientalis,though we did not detect its presence.
In conclusion,this is the first report of a Francisella infection caused by F.noatunensis subsp. orientalis in farmed tilapia in China. A rapid detection method should be developed for early diagnosis and treatment.
| Antuofermo E, Pais A, Nuvoli S, Hetzel U, Burrai G P, Rocca S, Caff ara M, Giorgi I, Pedron C, Prearo M, 2014. Mycobacterium chelonae associated with tumor-like skin and oral masses in farmed Russian sturgeons(Acipenser gueldenstaedtii). BMC Vet. Res., 10 (1) : 18 . Doi: 10.1186/1746-6148-10-18 |
| Aro L, Correa K, Martínez A, Ildefonso R, Yáñez J M, 2014. Characterization of Mycobacterium salmoniphilum as causal agent of mycobacteriosis in Atlantic salmon, Salmo salar L. , from a freshwater recirculation system. J. Fish Dis., 37 (4) : 341 –348. |
| Birkbeck T H, Bordevik M, Frøystad M K, Baklien Å, 2007. Identification of Francisella sp. from Atlantic salmon, Salmo salar L., in Chile. J. Fish Dis., 30 (8) : 505 –507. |
| Birkbeck T H, Feist S W, Verner-Jeff reys D W, 2011. Francisella infections in fish and shellfish. J. Fish Dis., 34 (3) : 173 –187. Doi: 10.1111/jfd.2011.34.issue-3 |
| Chen S C, Tung M C, Chen S P, Tsai J F, Wang P C, Chen R S, Lin S C, Adams A, 1994. Systematic granulomas caused by a rickettsia-like organism in Nile tilapia, Oreochronuis niloticus(L. ), from southern Taiwan. Journal of Fish Diseases, 17 (6) : 591 –599. |
| Chern R S, Chao C B, 1994. Outbreaks of a disease caused by rickettsia-like organism in cultured tilapias[Oreochromis spp. and Tilapia spp.] in Taiwan. Fish Pathology, 29 (2) : 61 –71. |
| Colquhoun D J, Duodu S, 2011. Francisella infections in farmed and wild aquatic organisms. Vet. Res., 42 (45) : 1 –15. |
| Fryer J L, Hedrick R P, 2003. Piscirickettsia salmonis:a Gramnegative intracellular bacterial pathogen of fish. J. Fish Dis., 26 (5) : 251 –262. Doi: 10.1046/j.1365-2761.2003.00460.x |
| Goodwin A E, Lochmann R T, Tieman D M, Mitchell A J, 2002. Massive hepatic necrosis and nodular regeneration in largemouth bass fed diets high in available carbohydrate. Journal of the World Aquaculture Society, 33 (4) : 466 –477. Doi: 10.1111/jwas.2002.33.issue-4 |
| Gudmundsdottir B K, Gudmundsdottir S, Gudmundsdottir S, Magnadottir B, 2014. Yersiniosis in Atlantic cod, Gadus morhua(L. ), characterization of the infective strain and host reactions. J. Fish Dis., 37 (6) : 511 –519. |
| Hsieh C Y, Tung M C, Tu C, Chang C D, Tsai S S, 2006. Enzootics of visceral granulomas associated with Francisella-like organism infection in tilapia(Oreochromis spp. ). Aquaculture, 254 (1-4) : 129 –138. Doi: 10.1016/j.aquaculture.2006.03.044 |
| Hsieh C Y, Wu Z B, Tung M C, Tsai S S, 2007. PCR and in situ hybridization for the detection and localization of a new pathogen Francisella-like bacterium(FLB)in ornamental cichlids. Dis. Aquat. Organ., 75 : 29 –36. Doi: 10.3354/dao075029 |
| Jeff ery K R, Stone D, Feist S W, Verner-Jeff reys D W, 2010. An outbreak of disease caused by Francisella sp. in Nile tilapia Oreochromis niloticus at a recirculation fish farm in the UK. Dis. Aquat. Organ., 91 (2) : 161 –165. |
| Kamaishi T, Fukuda Y, Nishiyama M, Kawakami H, Matsuyama T, Yoshinaga T, Oseko N, 2005. Identification and pathogenicity of intracellular Francisella bacterium in three-line grunt Parapristipoma trilineatum. Fish Pathology, 40 (2) : 67 –72. Doi: 10.3147/jsfp.40.67 |
| Kamaishi T, Miwa S, Goto E, Matsuyama T, Oseko N, 2010. Mass mortality of giant abalone Haliotis gigantea caused by a Francisella sp. bacterium. Dis. Aquat. Organ., 89 (2) : 145 –154. |
| Liu J Y, Li A H, 2012. First case of Aeromonas schubertii infection in the freshwater cultured snakehead fish, Ophiocephalus argus(Cantor), in China. J. Fish Dis., 35 (5) : 335 –342. Doi: 10.1111/jfd.2012.35.issue-5 |
| Mauel M J, Miller D L, Styer E, Pouder D B, Yanong R P E, Goodwin A E, Schwedler T E, 2005. Occurrence of Piscirickettsiosis-like syndrome in tilapia in the continental United States. Journal of Veterinary Diagnostic Investigation, 17 (6) : 601 –605. Doi: 10.1177/104063870501700616 |
| Mauel M J, Soto E, Moralis J A, Hawke J, 2007. A piscirickettsiosis-like syndrome in cultured Nile tilapia in Latin America with Francisella spp. as the pathogenic agent. J. Aquat. Anim. Health, 19 (1) : 27 –34. Doi: 10.1577/H06-025.1 |
| Mikalsen J, Olsen A B, Tengs T, Colquhoun D J, 2007. Francisella philomiragia subsp. noatunensis subsp. nov., isolated from farmed Atlantic cod(Gadus morhua L.). Int. J. Syst. Evol. Microbiol., 57 (9) : 1960 –1965. |
| Nylund A, Ottem K F, Watanabe K, Karlsbakk E, Krossoy B, 2006. Francisella sp. (Family Francisellaceae)causing mortality in Norwegian cod(Gadus morhua)farming. Arch. Microbiol., 185 (5) : 383 –392. |
| Olsen A, Mikalsen J, Rode M, Alfjorden A, Hoel E, Straum-Lie K, Haldorsen R, Colquhoun D, 2006. A novel systemic granulomatous infl ammatory disease in farmed Atlantic cod, Gadus morhua L. , associated with a bacterium belonging to the genus Francisella. Journal of Fish Diseases, 29 (5) : 307 –311. |
| Ostland V E, Stannard J A, Creek J J, Hedrick R P, Ferguson H W, Carlberg J M, Westerman M E, 2006. Aquatic Francisella-like bacterium associated with mortality of intensively cultured hybrid striped bass Morone chrysops×M. saxatilis. Diseases of Aquatic Organisms, 72 (2) : 135 –145. |
| Ottem K F, Nylund A, Karlsbakk E, Friis-Moller A, Krossoy B, 2007. Characterization of Francisella sp. , GM2212, the first Francisella isolate from marine fish, Atlantic cod(Gadus morhua). Arch. Microbiol., 187 (5) : 343 –350. |
| Romana-Eguia M R R, Ikeda M, Basiao Z U, Taniguchi N, 2004. Genetic diversity in farmed Asian Nile and red hybrid tilapia stocks evaluated from microsatellite and mitochondrial DNA analysis. Aquaculture, 236 (1-4) : 131 –150. Doi: 10.1016/j.aquaculture.2004.01.026 |
| Schefzyk M, Richter E, Röhrbein J H, Schaefer T, Wedi B, Raap U, 2012. The fate of 20 sea breams. Mycobacterium marinum infection. Hautarzt., 63 (9) : 716 –718. |
| Soto E, Baumgartner W, Wiles J, Hawke J P, 2011. Francisella asiatica as the causative agent of piscine francisellosis in cultured tilapia(Oreochromis sp. )in the United States. J. Vet. Diagn. Invest., 23 (4) : 821 –825. Doi: 10.1177/1040638711407058 |
| Soto E, Hawke J P, Fernandez D, Morales J A, 2009. Francisella sp. , an emerging pathogen of tilapia, Oreochromis niloticus(L.), in Costa Rica. J. Fish Dis., 32 (8) : 713 –722. |
| Varello K, Prearo M, Serracca L, Meloni D, Rossini I, Righetti M, Pezzolato M, Fioravanti M L, Ercolini C, Bozzetta E, 2014. Granulomatous lesions in a wild mullet population from the eastern Ligurian Sea(Italy):mycobacteriosis vs. pseudotuberculosis. J. Fish Dis., 37 (6) : 553 –558. Doi: 10.1111/jfd.2014.37.issue-6 |
| Vojtech L N, Sanders G E, Conway C, Ostland V, Hansen J D, 2009. Host immune response and acute disease in a zebrafish model of Francisella pathogenesis. Infect. Immun., 77 (2) : 914 –925. Doi: 10.1128/IAI.01201-08 |
| Wang G L, Yuan S P, Jin S, 2005. Nocardiosis in large yellow croaker, Larimichthys crocea(Richardson). J. Fish Dis., 28 (6) : 339 –345. Doi: 10.1111/jfd.2005.28.issue-6 |
| Zerihun M A, Berg V, Lyche J L, Colquhoun D J, Poppe T T, 2011. Mycobacterium salmoniphilum infection in burbot Lota lota. Dis. Aquat. Organ., 95 (1) : 57 –64. Doi: 10.3354/dao02347 |
| Zhang J T, Zhou S M, An S W, Chen L, Wang G L, 2014. Visceral granulomas in farmed large yellow croaker, Larimichthys crocea(Richardson), caused by a bacterial pathogen, Pseudomonas plecoglossicida. J. Fish Dis., 37 (2) : 113 –121. Doi: 10.1111/jfd.2014.37.issue-2 |
 2016, 34
2016, 34


