Institute of Oceanology, Chinese Academy of Sciences
Article Information
- Anguo ZHANG(张安国), Xiutang YUAN(袁秀堂), Wenjiu HOU(侯文久), Xiaodong LI(李晓东), Kai ZHAO(赵凯), Weixin CHEN(陈卫新), Xiurong SU(苏秀榕)
- Biodeposition, respiration, and excretion rates of an introduced clam Mercenaria mercenaria in ponds with implications for potential competition with the native clam Meretrix meretrix in Shuangtaizi estuary, China
- Journal of Oceanology and Limnology, 34(3): 467-476
- http://dx.doi.org/10.1007/s00343-016-4344-6
Article History
- Received: Nov. 30, 2014
- Accepted: Mar. 12, 2015
2. National Marine Environmental Monitoring Center, State Oceanic Administration (SOA) , Dalian 116023, China;
3. College of Animal Husbandry and Veterinary, Liaoning Medical University, Jinzhou 121001, China;
4. Panjin Guanghe Aquaculture Company, Panjin 124200, China;
5. Panshan Marine Fisheries Bureau, Panjin 124200, China
Bivalve introductions as culture species (often referred to as non-indigenous,non-native,and invasive) for hundreds of years by humans to regions outside their native ranges have been considered as one of the greatest modes of introduced marine species (Mann,1983; Chew,1990; Padilla et al.,2011) . Some practices are the introduction of the Bay scallop Argopecten irradians from America to China in 1982 (Zhang et al.,1986) ,the Pacific oyster Crassostrea gigas from Japan to the Pacific coast of North America in 1903 (Chew,1990,2001) ,and the northern quahogs Mercenaria mercenaria from America to China in 1997 (Zhang et al.,2003) . The introduced bivalves may escape from aquaculture operations and establish feral populations that can impact other ecological and commercial important species in the areas where they have invaded. For example,the Asian clam Corbicula fluminea modify invaded habitats to the point where indigenous species can no longer survive (Gonzalez et al.,2008; Sousa et al.,2009; Simard et al.,2012) . The zebra mussel Dreissena polymorpha have caused reductions in native filter-feeders (Naddafi et al.,2007; van Appledorn et al.,2007) ,and the hard clam M. mercenaria have a substantial and long-lasting influence on the genotype composition of the hard clam populations in Gulf of Mexico waters (Arnold et al.,2009) . Surprisingly,only a few quantitative or experimental studies have examined the impacts of introduced bivalves on the native ecologically and commercially important species. Therefore,this is clearly an area where more studies are needed.
The bivalve Mercenaria mercenaria is known as northern quahogs,cherrystones,and hard clams in the commercial trade and is indigenous to the Atlantic coast of North America,from the Gulf of St. Lawrence to the Gulf of Mexico (Harte,2001; Hiwatari et al.,2006) . The northern quahogs Mercenaria mercenaria was first introduced for commercial cultivation in China in the mid-1990s and has been gradually cultivated along the coastal and estuarine areas in the Bohai Sea and Yellow Sea of China with the development of artificial breeding and cultivation (Zhang et al.,2003) ,especially in ponds beside Shuangtaizi estuary. The introduced clam M. mercenaria fishery gradually predominates the local aquaculture industry,and becomes the main economic source for local fishermen,with about 1.5×104 hm2 in terms of culture areas and approximately 15 t/hm2 in terms of production in Shuangtaizi estuary (data from Panjin Guanghe Aquaculture Company of China) . Therefore,the role of the introduced clam M. mercenaria in pond ecosystem should be investigated to address its ecological roles.
A survey shows that the hard clam M. mercenaria has been cultivated in ponds of Shuangtaizi estuary,as resources of the native clam Meretrix meretrix decline because of overharvest and disease breakout (Zhang et al.,2014) . The introduced clam M. mercenaria may establish feral populations through the dispersal of adults or larvae in Shuangtaizi estuary. Therefore,the new feral populations of M. mercenaria may have large impacts on the ecosystems and other ecological and commercial important species. Furthermore,the introduced clam M. mercenaria resembles the native clam Meretrix meretrix in many biological and ecological aspects. Both of them belong to the Veneridae in systematic taxonomy (Harte,2001; Zhang et al.,2012) ,and have similar morphology (Fig. 1) ,distribute in similar latitudes areas,and inhabit in the estuarine lower intertidal and subtidal areas (Grizzle et al.,2001; Zhang et al.,2012) . Additionally,they are both infaunal bivalves,filtering phytoplankton and other organic seston. These biological and ecological similarities suggest that the introduced clam M. mercenaria may directly compete with the native clam M. meretrix for suspended food and space in estuarine ecosystems. Meanwhile,local communities and fisheries are still concerned about the potential competition with the native clam Meretrix meretrix in estuaries. Therefore,it is necessary to document the potential competitions of M. mercenaria feral population with the native species M. meretrix under identical field environmental conditions on a basis of physiological ecology.

|
| Figure 1 Morphological characteristics of Mercenaria mercenaria (a) and Meretrix meretrix (b) |
Several studies have concentrated on the seeding breeding,cultivation,and growth performance of M. mercenaria (Weiss et al.,2002; Zhang et al.,2003; Chung et al.,2007; Weiss et al.,2007; Zarnoch and Schreibman,2008; Arnold et al.,2009; Wall et al.,2011,2013) ,but only a few have investigated its physiological ecology (Sma and Baggaley,1976; Grizzle et al.,2001; Wen et al.,2004; Parent et al.,2008) . In addition,suspension-feeding bivalves are well known for their active biodeposition capacity. For instance,they release amounts of inorganic nutrients into the water column through excretion and decomposition of biodeposits,thereby strongly enhancing pelagic-benthic coupling (Dame 1996; Zhou et al.,2006a,b) . Therefore,biodeposition and excretion rates are probably the best parameters for quantifying the impact of filter-feeding bivalves on ecosystems. To date,the comprehensive biodepositon,respiration,and excretion processes of M. mercenaria especially those based on in situ/field data are rarely known. Therefore,the key physiological parameters,such as biodeposition,respiration,ammonium,and phosphate excretion rates of the introduced clam M. mercenaria,are measured by field experiments to determine the role of M. mercenaria in pond ecosystem,and implications for the niche competition with the native clam Meretrix meretrix are also discussed. Furthermore,All of these results could be helpful for the shellfish aquaculture management and species-introduction policy strategies.
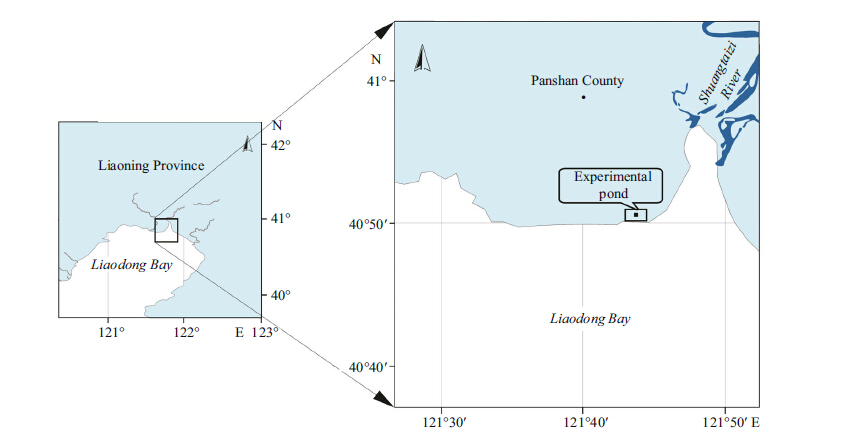
2 MATERIAL AND METHOD 2.1 Study locationThe Shuangtaizi estuary,located in the northern Bohai Sea of China,is one of the few remaining intertidal sand flats and an important clam fishery area (Fig. 2) . The sediment in the intertidal zone of the estuary consists principally of clayey silt and fine-grained sand,which is a suitable habitat and breeding ground of many benthic bivalves such as Meretrix meretrix. The northern quahogs M. mercenaria has been introduced in Shuangtaizi estuary for pond culture since 2003 to replace the fishery of the native clam Meretrix meretrix which is largely depleted because of overfishing and disease breakout.
|
| Figure 2 Geography of Shuangtaizi estuary and the experimental site |
The introduced clam M. mercenaria species (shell height,36.61±0.34 mm) were collected from culture ponds of the Panjin Guanghe Aquaculture Company in July,October,and December of 2010 and April of 2011. The clams were all transported into a 2 m-deep 6 hm2 pond with a sandy substrate. The experimental pond was connected to the estuary and continuously supplied with seawater according to the local estuarine tidal cycle throughout the year (Fig. 2) . The introduced clam M. mercenaria individuals were acclimated for approximately 24 h,and only clams that actively burrowed into sediment during the acclimation period were selected for the experiments.
2.3 Environmental factors and water column characteristics in experimental pondWater temperature,salinity,and chlorophyll a (Chl a,μg/L) of the experimental pond were measured using a YSI6600V2 Handheld Multi-parameter Instrument (YSI Incorporated,Ohio,USA) . Seawater samples were collected from the pond every 1 d to 2 d for a period of 5 d to 7 d to determine the total particulate matter (TPM,mg/L) ,particulate organic matter (POM,mg/L) ,particulate organic carbon (POC,mg/L) ,particulate organic nitrogen (PON,mg/L) ,and particulate organic phosphorus (POP,μg/L) in water column. The water samples were carried to the laboratory immediately,and 250 mL to 500 mL of each water sample was filtered through pre-burned (450°C for 6 h) and pre-weighed filters with a 47-mm diameter (Whatman GF/F,0.7 μm) for TPM and POM determination. The filters were rinsed with approximately 15 mL of distilled water to remove salts,and then dried at 65°C for 48 h before reweighing for TPM calculation. Then,the filters were combusted at 450°C for 6 h prior to final weighing for the POM calculation. For POC and PON assay,250 mL to 500 mL of each water sample was also filtered through pre-burned and pre-weighed filters (Whatman GF/F,0.7 μm) . The filters were dried at 65°C for 48 h and analyzed with a Vario Macro CHN element analyzer (Elementar Analysensysteme GmbH,Hanau,Germany) . POP was calculated by total phosphorus (TP) minus inorganic phosphorus (IP) . TP and IP were analyzed according to the modified method by Zhou et al. (2003) .
2.4 Biodeposition rate measurementBiodeposition rates (Rb) of M. mercenaria were measured using sediment traps made up of 19-cm polyvinyl chloride cylindrical tubes (Yuan et al.,2010,2011) . The trap comprised A and B sections with heights of 13 cm and 60 cm,respectively (Fig. 3) . Section A contained four circular tubes with 8 cm in diameter and 5 cm in height distributed uniformly and placed vertically. The length between the top of the circular tube and the rim of section A was 5 cm. Sea sand (grain size: 250 μm to 500 μm) was collected from the intertidal zone of Shuangtaizi estuary and rinsed with seawater,dried at 65°C,and then combusted in a muffle furnace at 550°C for 12 h to remove the OM. Before performing the experiment,each circular tube was filled with a sandy substrate up to 3 cm to provide refuge for the buried clams. In each season,biodeposition experiment was conducted by randomly placing six sediment traps in three stainless steel cages (45 cm×45 cm×80 cm) in the experimental pond. Three traps contained clams (four clams per trap) ,whereas the other three traps without clams served as controls. The traps were immersed in the pond at 1.5 m depth to maintain a constant ambient water temperature. The traps were removed and transported to the laboratory after 5 to 7 consecutive days for collection of the test clams. The sediment in the traps was allowed to settle before the overlying water was carefully siphoned and collected in a glass beaker. The deposited sediment was measured after drying to a constant weight at 65°C for at least 48 h. After completing the experiments,the shell heights (H,mm) of test clams were measured with vernier calipers,and the soft tissue from each clam was excised and dried at 65°C for 24 h to 48 h to a constant weight for dry weight (DW,g) determination.

|
| Figure 3 The sketch of a biodeposits trap |
The biodeposition rates were determined from the difference between the weights of the dry matter collected in the traps containing clams and the control traps using the following equation: Rb= (Dt -Dc) /T/N,where Rb is the biodeposition rate (g dry material/ ind./d) ,Dt is the weight of the biodeposits collected in the traps containing clams (g) ,Dc is the weight of the sediment collected in the traps without clams (control) (g) ,T is the duration of the measurement period (d) ,and N is the number of clams in the traps.
2.5 Respiration and excretion rate measurementThe respiration rate (RO) ,ammonium and phosphate excretion rates (RN and RP) of M. mercenaria were measured with 6.4 L respiration chambers. Each chamber was sealed with a rubber plug and contained 5 cm-depth sandy substrate. The sources and treatment methods of the sandy substrate were similar to the biodeposition rate experiment. Two to six clams,depending on the field temperature in different seasons,were placed into each chamber,which was immersed in the pond at 1.5 m depth to maintain a constant ambient water temperature. The experiments were conducted by maintaining the chambers at a high concentration of at least 60% of the initial concentration (by the number of clams based on pre- experiments) to avoid an effect on the normal physiological activity of the clams. Three replicates were set up for each treatment,and three chambers without clams were used as the controls for each run. To record the stable oxygen consumption,the clams were acclimated in the chambers sealed with rubber plugs until their valves opened. The chambers were removed from the seawater after 24 h to avoid circadian rhythm effects on the rates. Subsequently,the rubber plugs from the chambers were removed,and a YSI6600V2 Handheld Multiparameter Instrument (YSI Incorporated,Ohio,USA) was inserted to determine the oxygen concentration after mixing water using a magnetic stirrer. DO concentrations of experimental and control chambers were used to determine the respiration rate based on the following equation: RO=[ (Ct0-Ct1) ×Vr×24]/ [N× (t1-t0) ],where RO is the respiration rate (mg/ (ind.·d) ) ,and t0 as well as t1 are the beginning and end times (h) of the measurement period. Ct0 and Ct1 are the oxygen concentrations in the water (mg/L) at t0 and t1. V is the volume of the respiration chambers (excluding clam volume) ,and N is the number of clams in the chamber.
The ammonium and phosphate excretion rates of M. mercenaria were simultaneously measured in the experiment of the respiration rate. In a typical procedure,a 500-mL water sample was siphoned from each respiration chamber after being removed from the seawater and then immediately carried to the field laboratory (approximately 5 min) . Ammonium and phosphate analyses were conducted according to the method by Grasshoff et al. (1983) . The excretion rates were calculated by subtracting the ammonium (or phosphate) concentration in the control chambers from those of the experimental chambers,according to the following formula: RN (or RP) =[ (Ct1-Ct0) ×Vr×24]/[N× (t1-t0) ],where RN and RP are the ammonium and phosphate rates (mg/ (ind.·d) ) ,t0 and t1 are the beginning and end times (h) of the measurement period; Ct1 and Ct0 are the ammonium and phosphate concentrations in the water (mg/L) at t1 and t0,respectively,V is the volume of the respiration chambers (excluding the clam volume) ; and N is the number of clams in the chamber.
2.6 Statistical analysisThe physiological rates (Rb,RO,RN,and RP) were standardized to an equivalent individual by the following formula: Ys= (Ws/We) b×Ye (Zhou et al.,2006a) ,where Ys represents the value for the standard individual,Ye is the experimental physiological rate,Ws stands for standard weight (0.72 g for M. mercenaria) ,We is the experimental dry tissue weight of an individual,and b is the weight exponent that functions physiological rate to dry tissue weight through the allometric equation: Rx (Rb,RO,RN and RP) =a×Web.
One-way analysis of variance (ANOVA) with post-hoc tests were performed using SPSS software (version 13.0) . The t-test statistics analysis was used to determine the differences in the R values between the introduced clam M. mercenaria and the native clam Meretrix meretrix in the same season. Partial correlation analysis was used to test relationships among the physiological rates of M. mercenaria and the correlative parameters. A statistical significance level of 0.05 was used.
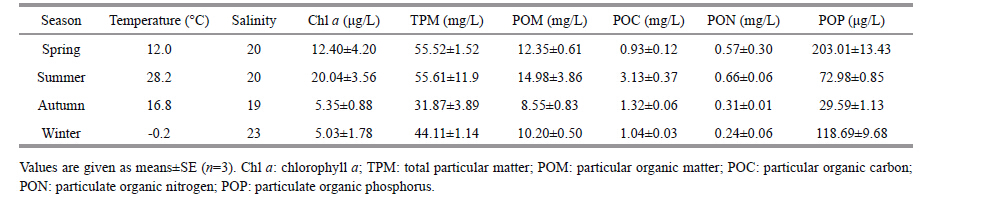
3 RESULT 3.1 Water column characteristics and environmental factors in experimental pondDuring the experimental period,seawater temperature in the experimental pond varied from -0.2°C to 28.2°C,and the salinity ranged seasonally from 19 to 23 (Table 1) . The chlorophyll a (Chl a) concentrations changed from 5.03 μg/L to 20.04 μg/L. The TPM concentrations in water column were high,fluctuating between 31.87 mg/L and 55.61 mg/L,which probably resulted from the resuspension events caused by strong winds. POC concentrations of the pond water reached a maximum of 3.13 mg/L in summer and did fluctuate significantly between summer and other three seasons. PON concentrations of the pond water reached high values in spring and summer,but low values in autumn and winter. POP concentrations of the pond water reached a maximum of 203.01 μg/L in spring and a minimum of 29.59 μg/L in autumn.
|
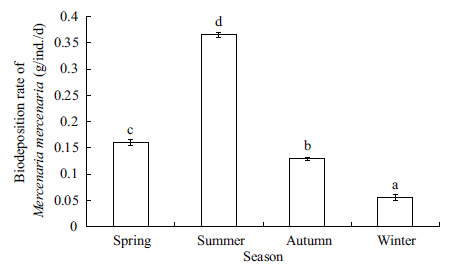
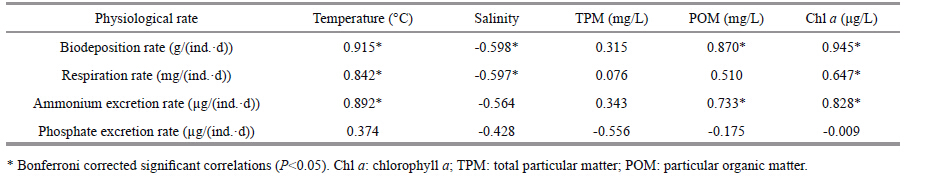
The biodeposition rates of M. mercenaria fluctuated significantly throughout the year (ANOVA,F=1 195.78,P=0.000) ,with the highest value (0.37 g/ (ind.·d) ) in summer and the lowest value (0.06 g/ (ind.·d) ) in winter (Fig. 4) . The correlation analysis shows that the Rb of M. mercenaria was positively correlated with water temperature,POM concentrations,and Chl a concentrations,however,negatively correlated with water salinity (Table 2) .
|
| Figure 4 Seasonal variation in biodeposition rate of the introduced clam Mercenaria mercenaria |
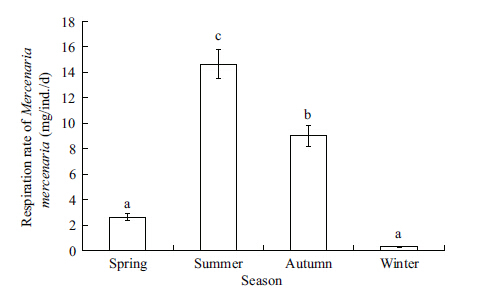
The RO of M. mercenaria showed a distinct pattern among seasons (ANOVA,F=79.990,P=0.000) . It increased in spring,peaked in summer (14.66 mg/ (ind.·d) ) ,declined in autumn,and reached the lowest values in winter (0.31 mg/ (ind.·d) ) (Fig. 5) . The correlation analysis shows that the RO of M. mercenaria was positively correlated with the water temperature and Chl a concentrations,nevertheless,negatively correlated with water salinity (Table 2) .
|
| Figure 5 Seasonal variation in respiration rate of the introduced clam Mercenaria mercenaria |
|
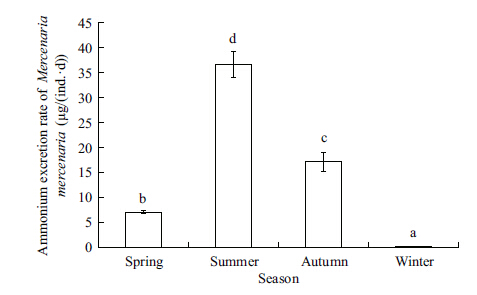
The RN of M. mercenaria varied distinctly (ANOVA,F=287.459,P=0.000) ,with the maximum value (36.70 μg/ (ind.·d) ) in summer and the minimum value (0.18 μg/ (ind.·d) ) in winter (Fig. 6) . The correlation analysis shows that the RN of M. mercenaria was positively correlated with water temperature,Chl a concentrations,and POM concentrations (Table 2) .
|
| Figure 6 Seasonal variation in ammonium excretion rate of the introduced clam Mercenaria mercenaria |
Suspension-feeding bivalves are well-known for their active biodeposition capacity. For instance,they discharge biodeposits (feces and pseudofeces) to the sediment surface,which can significantly modify nutrient cycles in ecosystems (Norkko et al.,2001) . The current study showed that average biodeposition rate of M. mercenaria was 0.18 g/ (ind.·d) . The culture pond is ca. 6 hm2,and the number of M. mercenaria species in each pond is approximately 1.8×106 individuals. It was surveyed that there are about 1 820 ponds in Shuangtaizi estuary. Thus,we estimated that M. mercenaria populations may discharge dry biodeposits up to 2.1×105 t in ponds in Shuangtaizi estuary annually. In short,like other benthic suspension-feeding bivalves such as Ruditapes philippinarum (Yuan et al.,2011) ,the introduced clam M. mercenaria populations could influence the sediment quality of the pond through biodeposition and decomposition of biodeposits.
The suspension-feeding bivalves release amounts of inorganic nutrients back into the water column through excretion,and the excreted inorganic NH4+-N and PO43--P play a role in sustaining primary productivity and marine nutrient cycling in ecosystems (Smaal and Vonck,1997; Zhou et al.,2006a) . Therefore,suspension-feeding bivalves strongly affect pelagic-benthic coupling near the sediment- water interface. The average respiration,ammonium excretion,and phosphate excretion rates of the introduced clam M. mercenaria in this study was 6.65 mg/ (ind.·d) ,15.28 μg/ (ind.·d) ,and 7.48 μg/ (ind.·d) ,respectively. Based on the data obtained in this study,we estimated that the clams M. mercenaria may contribute to 18.3 t ammonia and 9.0 t phosphate to ponds and consume 7.9×103 t O2 from the ponds in Shuangtaizi estuary annually. Thus,the introduced clam M. mercenaria should be considered as an important component in the pond’s ecological processes.
4.2 Potential ecological competition of Mercenaria mercenaria with Meretrix meretrixThe introduced clam M. mercenaria resembles the native clam Meretrix meretrix in ecological aspects. Both are benthic suspension-feeding bivalves,inhabit in the estuarine lower intertidal and subtidal areas (Grizzle et al.,2001; Zhang et al.,2012) ,and feeds on suspended particulate matters and phytoplankton (Zhuang and Wang,2004; Chung et al.,2007) . Meanwhile,We measured biodeposition,respiration,and excretion rates of the native clam Meretrix meretrix (shell height,36.36±0.23 mm) in Shuangtaizi estuary (Zhang et al.,2014) ,which under identical environmental conditions with physiological rates measurement experiments of the introduced clam M. mercenaria. Therefore,based on the results of Zhang et al (2014) ,we compared the key physiological rates (e.g. biodeposition,respiration,and excretion) of two species to elucidate the potential competition between M. mercenaria and Meretrix meretrix in estuarine ecosystem.
The introduced clam M. mercenaria is a very effective suspension-feeder compared with other benthic suspension-feeding bivalves,such as Meretrix meretrix (Zhang et al.,2014) and Ruditapes philippinarum (Han et al.,2001; Yuan et al.,2011) . Therefore,the introduced clam M. mercenaria may potentially compete with the native clam Meretrix meretrix for suspended food in Shuangtaizi estuary. The biodeposition rate is an important indicator for the metabolic activity of bivalves. The biodeposition rate of M. mercenaria was higher than that of M. meretrix (0.30 g/ (ind.·d) ; 0.02 g/ (ind.·d) ) (Zhang et al.,2014) in the highest temperature season (summer) and the lowest temperature season (winter) ,but it was lower than that of Meretrix meretrix (0.28 g/ (ind.·d) ; 0.15 g/ (ind.·d) ) (Zhang et al.,2014) in appropriate temperature seasons (spring and autumn) . Moreover,besides autumn,there existed significant differences among two species for biodeposition rate in spring,summer,and winter (t-test,P<0.05) ,respectively. Hence,the introduced clam M. mercenaria may have broader environmental tolerances and could facilitate its dispersal potential when it escaped from aquaculture operations or larvae transport.
Respiration and excretion rates are also important indicators for the metabolic activity of bivalves. There was no significant difference in the respiration rates between two species in summer,autumn and winter (t-test,P>0.05) ,respectively. However,there was distinct difference between two species in spring (t- test,P<0.05) . Furthermore,the introduced clam M. mercenaria consumed 2.74 times less oxygen than Meretrix meretrix (7.16 mg/ (ind.·d) ) (Zhang et al.,2014) in spring. The temperature (28°C) in which the respiration rate of M. mercenaria (14.66 mg/ (ind.·d) ) and Meretrix meretrix (15.88 mg/ (ind.·d) ) (Zhang et al.,2014) reached the maximum value is higher than that of other benthic bivalves that inhabit in intertidal areas,such as Coelomactra antiquata (Meng et al.,2005) ; the two species are verified to inhabit in the estuarine lower intertidal and shallow subtidal areas (Grizzle et al.,2001; Zhuang and Wang,2004; Chung et al.,2007) .
The results demonstrated that the introduced clam M. mercenaria showed a similar seasonal variation of ammonia and phosphate excretion rates,with the maximum value in summer and the minimum value in winter. A comparison of the ammonia and phosphate excretion rates of M. mercenaria and Meretrix meretrix suggested that both are classified as ammonotelic organisms. There was significant difference in ammonia excretion rates between two species in spring,summer,and autumn (t-test,P<0.05) ,respectively,but no distinct difference in winter (t-test,P>0.05) . Furthermore,the introduced clam M. mercenaria excreted 6.8,3.4,and 1.9 times less ammonia than Meretrix meretrix (48.52 μg/ (ind.·d) ; 122.65 μg/ (ind.·d) ; 27.60 μg/ (ind.·d) ) (Zhang et al.,2014) in spring,summer and autumn,respectively. Comparison results indicated that there was distinct difference in phosphate excretion rates between two species in spring and summer (t-test,P<0.05) ,respectively,but no difference in autumn and winter (t-test,P>0.05) ,respectively. Moreover,the introduced clam M. mercenaria excreted 12.1 and 14.6 times less phosphate than Meretrix meretrix (33.72 μg/ (ind.·d) ; 156.29 μg/ (ind.·d) ) (Zhang et al.,2014) in spring and summer,respectively. The considerable scatter that is evident in the measured ammonia and phosphate excretion by Meretrix meretrix appears to be characteristic of these species when the data are compared with those obtained from M. mercenaria in this study.
The hard clam M. mercenaria has been cultivated in ponds of the estuarine areas,it may establish feral populations through the dispersal of adults or larvae transport in Shuangtaizi estuary. These new feral populations of M. mercenaria may have large impacts on other ecological and commercial important species such as Meretrix meretrix in Shuangtaizi estuary. The feral populations of M. mercenaria may directly compete for space and food,furthermore,the introduced clams might modify invaded habitats to the point where indigenous species can no longer survive and cause reductions in native filter-feeding bivalves such as Meretrix meretrix.
5 CONCLUSIONIn summary,we quantified the biodeposition,respiration,and excretion rates of the introduced clam M. mercenaria during field experiments in Shuangtaizi estuary,Bohai Sea of China. The results suggested that like other suspension-feeding bivalves,the introduced clam M. mercenaria populations played an important part in the pelagic-benthic coupling in pond ecosystem through excretion and biodeposition. Moreover,the introduced species occupied an environmental niche similar to that of the native counterpart and may compete with it for space and food. Hence,the introduced clam M. mercenaria might have a strong competition with the native clam Meretrix meretrix by establishing feral populations through dispersal of adults or larvae in Shuangtaizi estuary.
6 ACKNOWLEDGMENTWe thank Martin J. Booth for his assistance with the language of the paper,and several anonymous referees for professional comments on this manuscript.
| Arnold W S, Geiger S P, Stephenson S P, 2009. Mercenaria mercenaria introductions into Florida, USA, waters:duration, not size of introduction, infl uences genetic outcomes. Aquat. Biol., 5 : 49 –62. Doi: 10.3354/ab00137 |
| Chew K K, 1990. Global bivalve shellfish introductions. World Aquacult., 21 (1) : 9 –22. |
| Chew K K. 2001. Introduction of hard clam(Mercenaria mercenaria)to the Pacific coast of North America with notes on its introduction to Puerto Rico, England, and France. In:Kraeuter J N, Castagna M eds. Biology of the Hard Clam. Elsevier, Amsterdam. p.701-709. |
| Chung K W, Fulton M H, Scott G I, 2007. Use of the juvenile clam, Mercenaria mercenaria, as a sensitive indicator of aqueous and sediment toxicity. Ecotox. Environ. Safe., 67 (3) : 333 –340. Doi: 10.1016/j.ecoenv.2006.10.009 |
| Dame R F, 1996. Ecology of Marine Bivalves:An Ecosystem Approach. CRC Press, Boca Raton. |
| Gonzalez A, Lamberti A, Ricciardi A, 2008. When does ecosystem engineering cause invasion and species replacement?. Oikos, 117 (8) : 1247 –1257. Doi: 10.1111/oik.2008.117.issue-8 |
| Grasshoff K, Kremling K, Erhardt M, 1983. Methods of Seawater Analysis. Verlag Chemie, Weiheim, Germany: . |
| Grizzle R E, Bricelj V M, Shumway S E. 2001. Physiological Ecology of Mercenaria mercenaria. In:Kraeuter J N, Castagna M eds. Biology of the Hard Clam. Elsevier, Amsterdam. p.305-382. |
| Han J, Zhang Z N, Yu Z S, Widdows J, 2001. Differences in the benthic-pelagic particle fl ux(biodeposition and sediment erosion)at intertidal sites with and without clam(Ruditapes philippinarum)cultivation in eastern China. J. Exp. Mar. Bio. Ecol., 261 (2) : 245 –261. Doi: 10.1016/S0022-0981(01)00278-7 |
| Harte M E. 2001. Systematics and taxonomy. In:Kraeuter J N, Castagna M eds. Biology of the Hard Clam. Elsevier, Amsterdam. p.3-51. |
| Hiwatari T, Shinotsuka Y, Kohata K, Watanabe M, 2006. Exotic hard clam in Tokyo Bay identified as Mercenaria mercenaria by genetic analysis. Fisheries Sci., 72 (3) : 578 –584. Doi: 10.1111/fis.2006.72.issue-3 |
| Mann R, 1983. The role of introduced bivalve mollusc species in mariculture. J. World Maricul. Soc., 14 (1-4) : 546 –559. |
| Meng X P, Dong Z G, Cheng H L, Li X Y, Li J L, 2005. Oxygen consumption and ammonia-N excretion rates of Coelomactra antiquata. Chin. J. Appl. Ecol., 16 (12) : 2435 –2438. |
| Naddafi R, Pettersson K, Eklöv P, 2007. The effect of seasonal variation in selective feeding by zebra mussels(Dreissena polymorpha)on phytoplankton community composition. Freshwater Biol., 52 (5) : 823 –842. Doi: 10.1111/fwb.2007.52.issue-5 |
| Norkko A, Hewitt J E, Thrush S F, Funnell T, 2001. Benthicpelagic coupling and suspension-feeding bivalves:linking site specific-sediment flux and biodeposition to benthic community structure. Limnol. Oceanogr., 46 (8) : 2067 –2072. Doi: 10.4319/lo.2001.46.8.2067 |
| Parent G J, Pernet F, Tremblay R, Sévigny J-M, Ouellette M, 2008. Remodeling of membrane lipids in gills of adult hard clam Mercenaria mercenaria during declining temperature. Aquat. Biol., 3 : 101 –109. Doi: 10.3354/ab00073 |
| Simard M A, Paquet A, Jutras C, Robitaille Y, Blier P U, Courtois R, Martel A L, 2012. North American range extension of the invasive Asian clam in a St. Lawrence River power station thermal plume. Aquat. Invasions, 7 (1) : 81 –89. |
| Sma R F, Baggaley A, 1976. Rate of excretion of ammonia by the hard clam Mercenaria mercenaria and the American oyster Crassostrea virginica. Mar. Bio l., 36 (3) : 251 –258. Doi: 10.1007/BF00389286 |
| Smaal A C, Vonck A P M A, 1997. Seasonal variation in C, N and P budgets and tissue composition of the mussel Mytilus edulis. Mar. Eco. l Prog. Ser., 153 : 167 –179. Doi: 10.3354/meps153167 |
| Sousa R, Gutiérrez J L, Aldridge D C, 2009. Non-indigenous invasive bivalves as ecosystem engineers. Biol. Invasions, 11 (10) : 2367 –2385. Doi: 10.1007/s10530-009-9422-7 |
| van Appledorn M, Lamb D A, Albalak K, Bach C E, 2007. Zebra mussels decrease burrowing ability and growth of a native snail, Campeloma decisum. Hydrobiologia, 575 (1) : 441 –445. Doi: 10.1007/s10750-006-0280-3 |
| Wall C C, Gobler C J, Peterson B J, Ward J E, 2013. Contrasting growth patterns of suspension-feeding molluscs(Mercenaria mercenaria, Crassostrea virginica, Argopecten irradians, and Crepidula fornicata)across a eutrophication gradient in the Peconic Estuary, NY, USA. Estuar. Coast., 36 (6) : 1274 –1291. Doi: 10.1007/s12237-013-9632-1 |
| Wall C C, Peterson B J, Gobler C J, 2011. The growth of estuarine resources(Zostera marina, Mercenaria mercenaria, Crassostrea virginica, Argopecten irradians, Cyprinodon variegatus)in response to nutrient loading and enhanced suspension feeding by adult shellfish. Estuar. Coast., 34 (6) : 1262 –1277. Doi: 10.1007/s12237-011-9377-7 |
| Weiss E T, Carmichael R H, Valiela I, 2002. The effect of nitrogen loading on the growth rates of quahogs(Mercenaria mercenaria)and soft-shell clams(Mya arenaria)through changes in food supply. Aquaculture, 211 (1-4) : 275 –289. Doi: 10.1016/S0044-8486(02)00018-2 |
| Weiss M B, Curran P B, Peterson B J, Gobler C J, 2007. The infl uence of plankton composition and water quality on hard clam(Mercenaria mercenaria L. )populations across Long Island's south shore lagoon estuaries(New York, USA). J. Exp. Mar. Bio l. Ecol., 345 (1) : 12 –25. |
| Wen H X, Zhang T, Yang H S, Liu B Z, Zhou Y, Mao Y Z, Zhang F S, 2004. Effect of temperature on respiration and excretion of hard clam(Mercenaria mercenaria Linnaeus,1758). Oceanologia Limnol. Sin., 35 (6) : 549 –554. |
| Yuan X T, Zhang M J, Liang Y B, Liu D, Guan D M, 2010. Self-pollutant loading from a suspension aquaculture system of Japanese scallop(Patinopecten yessoensis)in the Changhai sea area, Northern Yellow Sea of China. Aquaculture, 304 (1-4) : 79 –87. Doi: 10.1016/j.aquaculture.2010.03.026 |
| Yuan X T, Zhang S L, Liu S X, Liang B, Liang Y B, Zhang G F, 2011. Self-pollution in Ruditapes philippinarum bottom-cultured area of Zhuanghe coast. Chin. J. Appl. Ecol., 22 (3) : 785 –792. |
| Zarnoch C B, Schreibman M P, 2008. Infl uence of temperature and food availability on the biochemical composition and mortality of juvenile Mercenaria mercenaria(L. )during the over-winter period. Aquaculture, 274 (2-4) : 281 –291. |
| Zhang A G, Yuan X T, Hou W J, Chen W X, Zhao K, Ba F Y, Zhang Z Z, 2014. Infl uence of biodeposition, respiration, and excretion of the buried clam Meretrix meretrix on the pelagic-benthic coupling in Shuangtaizi Estuary. Acta Ecol. Sin., 34 (22) : 6573 –6582. |
| Zhang F S, He Y Z, Liu X S, Ma J H, Li S Y, Qi L X, 1986. A report on the introduction, spat-rearing and experimental culture of Bay scallop Argopecten irradians Lamarck. Oceanologia Limnol. Sin., 17 (5) : 367 –374. |
| Zhang S P, Wang H X, Xu F S, 2012. Taxonomic study on Meretrix(Bivalvia, veneridae)from China seas. Acta Zootaxonomica Sinica, 37 (3) : 473 –479. |
| Zhang T, Yang H S, Liu B Z, Li B Q, Liu S L, Wen H X, He Y C, Zhang F S, 2003. Effects of environmental factors on the survival and growth of juvenile hard clam Mercenaria mercenaria(Linnaeus, 1758). Oceanologia Limnol. Sin., 34 (2) : 142 –149. |
| Zhou Y, Yang H S, Zhang T, Liu S L, Zhang S M, Liu Q, Xiang J H, Zhang F S, 2006a. Infl uence of filtering and biodeposition by the cultured scallop Chlamys farreri on benthic-pelagic coupling in a eutrophic bay in China. Mar. Ecol. Prog. Ser., 317 : 127 –141. Doi: 10.3354/meps317127 |
| Zhou Y, Yang H S, Zhang T, Qin P B, Xu X L, Zhang F S, 2006b. Density-dependent effects on seston dynamics and rates of filtering and biodeposition of the suspensioncultured scallop Chlamys farreri in a eutrophic bay(northern China):an experimental study in semi-in situ flow-through systems. J. Mar. Syst., 59 (1-2) : 143 –158. Doi: 10.1016/j.jmarsys.2005.11.002 |
| Zhou Y, Zhang F S, Yang H S, Zhang S M, Ma X N, 2003. Comparison of effectiveness of different ashing auxiliaries for determination of phosphorus in natural waters, aquatic organism and sediments by ignition method. Water Res., 37 (16) : 3875 –3882. Doi: 10.1016/S0043-1354(03)00267-7 |
| Zhuang S H, Wang Z Q, 2004. Infl uence of size, habitat and food concentration on the feeding ecology of the bivalve, Meretrix meretrix Linnaeus. Aquaculture, 241 (1-4) : 689 –699. Doi: 10.1016/j.aquaculture.2004.09.005 |
 2016, 34
2016, 34


