Institute of Oceanology, Chinese Academy of Sciences
Article Information
- Weihong ZHAO(赵卫红), Zisheng WANG(王资生), Yebing YU(於叶兵), Zhitao QI(齐志涛), Linlan LÜ(吕林兰), Yuxia ZHANG(张余霞), Fu LÜ(吕富)
- Growth and antioxidant status of oriental river prawn Macrobrachium nipponense fed with diets containing vitamin E
- Journal of Oceanology and Limnology, 34(3): 477-483
- http://dx.doi.org/10.1007/s00343-015-4396-z
Article History
- Received: Dec. 28, 2014
- Accepted: Feb. 25, 2015
2. Jiangsu Provincial Key Laboratory of Coastal Pond Aquaculture Ecology, Yancheng 224051, China
It is well known that vitamin E is essential to animals (McDowell and Cunha,1989) . And dietary supplement of vitamin E to aquatic organisms was first studied in fish,as reviewed by Hamre (2011) . With the rapid development of prawn aquaculture,the contribution of vitamin E to their production has also received increasing attention in recent several decades. Reports showed that addition of vitamin E to prawns diets improved their survival (Kanazawa,1985; He et al.,1992; Lee and Shiau,2004) . And vitamin E was proven to be indispensable to oocyte development of prawn,e.g. vitamin E was deposited in the oocyte (Cavalli et al.,2001; Wouters et al.,2001) ,promoted the development of oocyte and embryo (Luo et al.,2005) ,increased oocyte hatchery rate (Cahu et al.,1995; Luo et al.,2005) ,and advanced larva survival (Cavalli et al.,2003; Nguyen et al.,2012) . However,there is no reports about vitamin E dietary supplementation in the oriental river prawn,Macrobrachium nipponense,one of the most important aquaculture species in China, because of its refined taste and its rich nutrients.
Vitamin E has a 6-hydroxychromane ring which donates a hydrogen atom to chain propagating lipid peroxyl radical and gives rise to chromanoxyl radical (Hamre,2011) . So it plays an important role in protecting ployunsaturated fatty acids from peroxidation and scavenging from radicals (Evstigneeva et al.,1998) . Hence,vitamin E is a critical part of antioxidant defense systems,which include antioxidant enzymatic components,such as superoxide dismutase (SOD) and catalase (CAT) acting on O-2 and H2O2,respectively,and glutathione peroxidase (GSH-Px) ,which scavenges H2O2 and lipid hydroperoxides (Winston and Di Giulio,1991) . The health of aquatic organisms is linked to the balance between reactive oxygen species and antioxidants. Accordingly,SOD,CAT,and GSH-Px activities are widely used as simple and reliable biomarkers of oxidative stress in aquatic organisms (Regoli and Principato,1995; Chien et al.,2003; Wang et al.,2004; Wang et al.,2006; Liu et al.,2007; Pacheco et al.,2011) . In fish,the relationship between dietary vitamin E and antioxidant enzyme activities has been reported in several studies (Blazer and Wolke,1984; Hardie et al.,1990; Lygren et al.,2000; Ortuño et al.,2000) ,which showed that vitamin E could enhanced both humoral and cellular defenses,while vitamin E deficient diets have been were related to reduced immune responses. However,knowledge about prawns is scanty (Lee and Shiau,2004; Liu et al.,2007; Nguyen et al.,2012) . Therefore,in this paper the dietary vitamin E requirement of the oriental river prawn was determined by investigating its growth response and antioxidant status at different levels of dietary vitamin E supplementation.
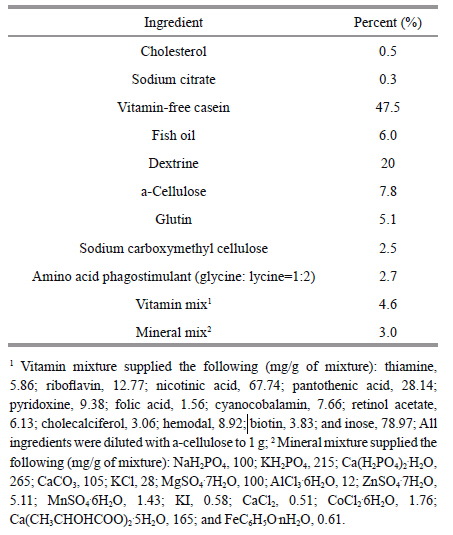
2 MATERIAL AND METHOD 2.1 Diet preparationThe composition of the basal diet formulation is given in Table 1 and was designed according to the references (Lee and Shiau,2004) . Vitamin-free casein,fish oil,and corn starch were used as dietary protein,lipid and carbohydrate sources,respectively. Vitamin E (DL-α-Tocopheryl acetate,DL-α-TOA) ,was added to the test diets at levels of 0,25,50,75,100,200,and 400 mg/kg.The vitamin E contents of the seven diets were determined using chemical chromatometry kits (Nanjing Jiancheng Bioengineering Institute, Nanjing, China) to be 2.42 (unsupplemented control) , 25.16 (25 mg/kg diet) ,48.87 (50 mg/kg diet) , 72.26 (75 mg/kg diet) , 97.58 (100 mg/kg diet) , 197.52 (200 mg/kg diet) , and 280.97 (400 mg/kg diet) . The diets were prepared by thoroughly mixing the dry ingredients, passing them through a mincer with a die, and drying the resulting strings in an oven at 45 ℃. The dried diets were stored at -20 ℃ after being broken up and sieved into pellets
Prawns (weight of 0.3–0.4 g) were obtained from Tongyu River (Yancheng city,Jiangsu province,China) . They were acclimated to laboratory conditions for 15 days in a plastic tank (76 cm L×57 cm H×50 cm W) . Then 50 prawns were randomly assigned to a tank as a group. Each group had 3 parallels. Water was continuously aerated under a photoperiod of 12 h light (09.00–21.00) and 12 h dark at room temperature. 25% water was exchanged daily. Groups of prawns were fed with 17 g minced earthworms experimental diets per 1 kg body weight at 9:00,14:00,and 20:00 h daily for 60 days. Uneaten feed,fecal pellets and dead prawns were siphoned off daily. And the dead prawns were not replaced.
At the end of the feeding trial,prawns were fasted for 24 h,then the prawns of each group were bulk weighed. Growth was measured by the percentage of body weight gain per surviving prawn in each aquarium. After being weighted,10 prawns were randomly taken from each group. And their muscle and hepatopancreas were removed and weighed,homogenized in nine volumes of saline,and centrifuged at 3 000 r/min for 10 min at 0°C to remove debris. The resultant supernatant fluid was obtained to measure SOD,CAT,and GSH-Px activities,and vitamin E contents.
2.3 Enzyme activity and vitamin E content assaysActivities of SOD,CAT,and GSH-Px,protein contents,and vitamin E contents were measured using kits (Nanjing Jiancheng Bioengineering Institute,China) according to the manufacturer’s protocols. The assays are briefly described as follows:
SOD activity was measured by its ability to inhibit superoxide anion generated by the xanthine and xanthine oxidase reaction system at 550 nm according to McCord and Fridovich (1984) and expressed as unit per milligram protein. One unit of SOD is defined as the amount of enzyme necessary to produce a 50% inhibition of the nitroblue tetrazolium reduction rate.
CAT activity was measured following hydrogen peroxide reduction at 405 nm according to the method of Aebi (1984) and expressed as unit per milligram protein. One unit of CAT is defined as the amount of enzyme necessary to reduce 1μmol H2O2 to H2O in 1 minute.
GSH-Px activity was measured following the method of Flohé and Günzler (1984) . After the addition of 1 mmol GSH (reduced glutathione) ,the NADPH-consumption rate was monitored at 412 nm and expressed as unit per milligram protein. One unit of GSH-Px activity is defined as the amount of enzyme necessary to decrease 1 μmol GSH per mg protein.
Total soluble protein contents were determined according to Lowry et al. (1951) ,using bovine serum albumin (BSA) as the standard.
Vitamin E was extracted by n-heptane and measured at 533 nm by the method of Desai (1984) ,where the ferric ions are reduced to ferrous ions in the presence of tocopherol and bathophenanthroline to form a pink colored substance.
2.4 Statistical analysisTo compare the effects of dietary treatments on all parameters measured,data were subjected to one-way analysis of variance (ANOVA) . Differences between means were compared by Duncan multiple range tests following a significant F-test. A significance level of P<0.05 was used for all statistical tests.
3 RESULT 3.1 Growth and survivalWeight gain was significantly higher in the vitamin E supplemented diet groups than in the unsupplemented one (P<0.05) ,and was highest in prawns fed with the 100 mg/kg vitamin E diet. The differences of the weight gain between the high group (200 and 400 mg) ,middle groups (75 mg) and low group (25 and 50 mg) were not significant (P>0.05) (Table 2) . The survival differences among all the tested groups were not significant (P>0.05) (Table 2) .

|
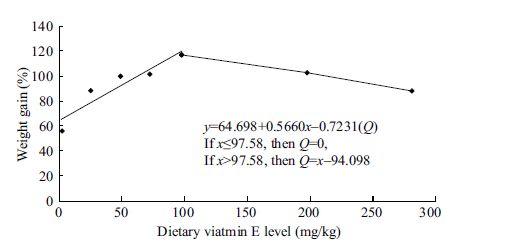
A broken line regression model (Robbins et al.,2006) was employed to express the relationship between weight gain and dietary vitamin E level (Fig. 1) ,and peak growth was reached when the dietary vitamin E level was 94.10 mg/kg.
|
| Figure 1 The Eff ect of dietary vitamin E on relative weight gain of Macrobrachium nipponense |
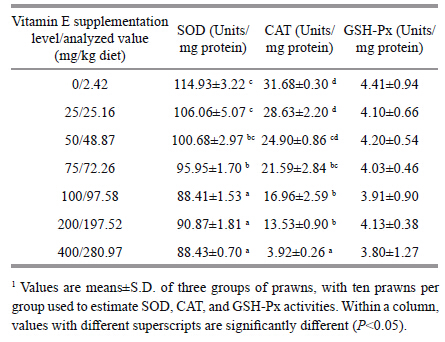
The activities of SOD and CAT were lower in the vitamin E supplemented diet groups than in the unsupplemented one (Table 3) . SOD activity decreased with vitamin E level increasing from 0 to 100 mg/kg diet. However,it kept steady when vitamin E increased from 100 to 400 mg/kg diet. And no significant differences were observed in SOD activity between groups fed with 100,200,and 400 mg/kg vitamin E diets (P>0.05) . CAT activity decreased with vitamin E increasing in diet. And it was highest in prawns fed with 0 and 25 mg/kg vitamin E diets,intermediate in prawns fed with 50,75,100,and 200 mg/kg vitamin E diets,and lowest in prawns fed with 400 mg/kg vitamin E diets. There was no significant difference in GSH-Px activity between all the test groups (P>0.05) .
|
The vitamin E contents in muscle and hepatopancreas increased with its level in diet (Table 4) . Vitamin E contents in the muscle were significantly lower than those in the hepatopancreas (P<0.05) . The differences between the high vitamin E group (400 mg/kg vitamin E diet) ,the middle groups (50,75,100,and 200 mg/kg vitamin E diets) and the low groups (0 and 25 mg/kg vitamin E diets) were significant in the muscle (P<0.05) . The difference in vitamin E contents between prawns fed with 0 and 25 mg/kg vitamin E diets was significant in the hepatopancreas (P<0.05) ,while there was no significant difference in the muscle (P>0.05) . Vitamin E contents were significantly different in the hepatopancreas between 50 and 75 mg/kg groups (P<0.05) . And significant difference was also observed between 100 and 200 mg/kg groups (P<0.05) . The linear correlations of vitamin E content between dietary level (x) and muscle content (y) ,and between dietary level (x) and hepatopancreas content (z) were y=0.1289x+2.8397 (R2=0.93) and z=1.5029x+58.439 (R2=0.93) ,respectively.

|
The results showed that dietary supplementation with vitamin E could enhance the growth of O. mykiss. After 60 days,the weight gains of prawns fed with vitamin E supplemented diets were higher than that of the unsupplemented one group. The result is in agreement with findings in M. japonicus (Kanazawa,1985) ,Litopenaeus setiferus (Kanazawa,1985) ,P. monodon (Lee and Shiau,2004) ,and Pleoticus mulleri ( FernándezGimenez et al.,2004) . The requirement for vitamin E in the oriental river prawn is 94.10 mg/kg,as estimated by the weight gain calculated with broken-line regression analysis (Robbins et al.,2006) . Similar results were found in L. vannamei (He et al.,1992) and P. monodon (Lee and Shiau,2004) ,where the requirements are 99 mg/kg and 85.89 mg/kg,respectively. Just like other nutrients,And the requirement for vitamin E also depends on physiological conditions,the interaction with other nutrition nutrients and the environment. For example,more vitamin E was required for fertilization than for growth (Wouters et al.,2001) . High levels of polyunsaturated fatty acids,and low levels of vitamin C,selenium and astaxanthin increased its requirement in fish (Hamre,2011) . Under adverse conditions,such as when acute changes in salinity occur,prawns required more vitamin E (Liu et al.,2007) .
The activity of SOD in the hepatopancreas of O. mykiss decreased with the increase of vitamin E supplementation in the diet. The activity of SOD in the hepatopancreas of O. mykiss gradually decreased when increasing concentrations of vitamin E (up to 100 mg/kg) . This is a positive sign of maintenance of good general health. Similar results were reported in Macrobrachium rosenbergii (Dandapat et al.,2000) and Sparus aurata (Mourente et al.,2002) . Vitamin E can lower SOD activity because vitamin E can decrease the production of superoxide free radicals (O-2) ,which can be is broken down by SOD through the process of dismutation to O2 and H2O2 (2O-2+H- -H2O2+O2) (Fang et al.,2002) .The data presented in this paper also showed thatCAT activity in the hepatopancreas of O. mykiss decreased significantly,while GSH-Px activity remained constant. Both of CAT and GSH-Px can neutralize H2O2 in cellular systems with different affnity. So intracellular H2O2 content is the main factor deciding which of them will be functional (Barman,1974) . In this trial,since vitamin E effectively eliminated SOD activity in O. mykiss,the product of SOD,H2O2 content lowered. The result that CAT activity decreased with H2O2 decreasing suggested that CAT was probably an ineffective scavenger of H2O2 at low contents in this prawn,like the report from S. aurata (Mourente et al.,2002) . Besides,CAT only removes H2O2 while GSH-Px is responsible for the neutralization of both organic and inorganic hydroperoxides (Mourente et al.,2002) . These are probable the reasons why CAT activity decreased sharply and GSH-Px activity remained constant in the trial.
Vitamin E contents in the hepatopancreas and muscle of O. mykiss were higher in the vitamin E supplemented groups than in the unsupplemented one group. This result is similar to the reports from juvenile hybrid tilapia (Huang and Huang,2004) ,O. mykiss (Kiron et al.,2004) ,and Marsupenaeus japonicus Bate (Nguyen et al.,2012) . Vitamin E contents in the hepatopancreas and muscle of O. mykiss showed linear correlations with those in diets. Similar results were obtained using diet supplementation of vitamin E from 0 to 500 mg/kg in African catfish,whose vitamin E levels in muscle,plasma,and liver were related to those in the diet (Baker and Davies,1996) . And the linear relationship was confirmed in S. schlegeli (Bai and Lee,1998) . However,when vitamin E in the diet reached 500 mg/ kg,vitamin E content in the tissue of S. schlegeli did not increase any further (Bai and Lee,1998) . All the above reports indicate that suitable dietary levels of vitamin E can be effectively absorbed by the tissues of fishes,shrimps and prawns.
Vitamin E content in hepatopancreas was higher than that in muscle in this trial because as a lipid- soluble substance,vitamin E is stored in lipid rich organs such as the hepatopancreas (Fang et al.,2002) . Interestingly,in our previous study,the hepatopancreas in O. mykiss was found to be the main nutrition source for ovary development (Zhao et al.,2011) . It is known that vitamin E is involved in oocyte development,as its content increases during oocyte development (Cavalli et al.,2001; Wouters et al.,2001) . On the contrary,deficiency of vitamin E retards oocyte development (Alava et al.,1993) . Vitamin E deposited in the hepatopancreas in O. mykiss is probably incorporated with lipids into the oocyte. The hypothesis was proved by Cavalli et al. (2001) who found a high correlation between the deposition of lipids and vitamin E content in the oocytes of M. Rosenbergii.
5 CONCLUSIONOverall,O. mykiss required about 94.10 mg/ kg vitamin E diet for optimal growth. And there was no difference in survival rate among all vitamin E supplement groups. The activities of hepatopancreas SOD and CAT decreased significantly when dietary vitamin E was more than 72.26 mg/kg diet while there was no significant change in GSH-Px activity among all the vitamin E supplement groups. Linear correlations were found between dietary vitamin E levels with vitamin E contents both in muscle and hepatopancreas in the prawn.
| Aebi H, 1984. Catalase in vitro. Methods Enzymol., 105 : 121 –126. Doi: 10.1016/S0076-6879(84)05016-3 |
| Alava V R, Kanazawa A, Teshima S, Koshio S, 1993. Effects of dietary vitamins A, E, and C on the ovarian development of Penaeus japonicus. Nippon Suisan Gakk, 59 (7) : 1235 –1241. Doi: 10.2331/suisan.59.1235 |
| Bai S C, Lee K J, 1998. Diff erent levels of dietary DL-non-tocopheryl acetate affect the vitamin E status of juvenile Korean rockfish, Sebastes schlege. Aquaculture, 161 (1-4) : 405 –414. Doi: 10.1016/S0044-8486(97)00288-3 |
| Baker R T M, Davies S J, 1996. Changes in tissue non-tocopherol status and degree of lipid peroxidation with varying non-tocopheryl acetate inclusion in diets for the African catfish. Aquacult. Nutr., 2 (2) : 71 –79. Doi: 10.1111/anu.1996.2.issue-2 |
| Barman T E, 1974. Enzyme Handbook. Springer Verlag, New York (suppl. 1) : 36 –37. |
| Blazer V S, Wolke R E, 1984. The effects of non-tocopherol on the immune response and non-specific resistance factors of rainbow trout. (Salmo gairdneri Richardson). Aquaculture, 37 (1) : 1 –9. |
| Cahu C L, Cuzon G, Quazuguel P, 1995. Eff ect of highly unsaturated fatty acids, non-tocopherol and ascorbic acid in broodstock diet on egg composition and development of Penaeus indicus. Comp. Biochem. Phys. A:Phys., 112 (3-4) : 417 –424. Doi: 10.1016/0300-9629(95)02009-8 |
| Cavalli R O, Batista F M M, Lavens P, Sorgeloos P, Nelis H J, De Leenheer A P, 2003. Eff ect of dietary supplementation of vitamins C and E on maternal performance and larval quality of the prawn Macrobrachium rosenbergii. Aquaculture, 227 (1-4) : 131 –146. Doi: 10.1016/S0044-8486(03)00499-X |
| Cavalli R O, Tamtin M, Lavens P, Sorgeloos P, Nelis H J, De Leenheer A, 2001. The content of ascorbic acid and tocopherol in the tissues and eggs of wild Macrobrachium rosenbergii during maturation. J. Shellfish Res., 20 (3) : 939 –943. |
| Chien Y H, Pan C H, Hunter B, 2003. The resistance to physical stresses by Penaeus monodon juveniles fed diets supplemented with astaxanthin. Aquaculture, 216 (1-4) : 177 –191. Doi: 10.1016/S0044-8486(02)00056-X |
| Dandapat J, Chainy G B N, Rao K J, 2000. Dietary vitamin-E modulates antioxidant defence system in giant freshwater prawn, Macrobrachium rosenbergii. Comp. Biochem. Phys. C:Pharmacol. Toxicol. Endocrinol., 127 (1) : 101 –115. Doi: 10.1016/S0742-8413(00)00132-8 |
| Desai I D, 1984. Vitamin E analysis method for animal tissues. Methods Enzymol, 105 : 138 –147. Doi: 10.1016/S0076-6879(84)05019-9 |
| Evstigneeva R P, Volkov I M, Chudinova V V, 1998. Vitamin E as a universal antioxidant and stabilizer of biological membranes. Mem. Cell. Bio l., 12 (2) : 151 –172. |
| Fang Y Z, Yang S, Wu G Y, 2002. Free radicals, antioxidants, and nutrition. Nutri tion, 18 (10) : 872 –879. |
| Fernández Gimenez A V, Fenucci J L, Petriella A M, 2004. The effect of Vitamin E on growth, survival and hepatopancreas structure of the Argentine red shrimp Pleoticus muelleri Bate(Crustacea, Penaeidea). Aquacu. Res., 35 (12) : 1172 –1178. Doi: 10.1111/are.2004.35.issue-12 |
| Flohé L, Günzler W A, 1984. Assays of glutathione peroxidase. Methods in Enzymology, 105 : 115 –121. |
| Hamre K, 2011. Metabolism, interactions, requirements and functions of vitamin E in fish. Aquacu. Nutr., 17 (1) : 98 –115. Doi: 10.1111/anu.2010.17.issue-1 |
| Hardie L J, Fletcher T C, Secombes C J, 1990. The effect of vitamin E on the immune response of the Atlantic salmon(Salmo salar L. ). Aquaculture, 87 (1) : 1 –13. Doi: 10.1016/0044-8486(90)90206-3 |
| He H Q, Lawrence A L, Liu R Y, 1992. Evaluation of dietary essentiality of fat-soluble vitamins, A, D, E and K for Penaeid shrimp(Penaeus vannamei). Aquaculture, 103 (2) : 177 –185. Doi: 10.1016/0044-8486(92)90411-D |
| Huang C H, Huang S L, 2004. Eff ect of dietary vitamin E on growth, tissue lipid peroxidation, and liver glutathione level of juvenile hybrid tilapia, Oreochromis niloticus×O. aureus, fed oxidized oil. Aquaculture, 237 (1-4) : 381 –389. |
| Kiron V, Puangkaew J, Ishizaka K, Satoh S, Watanabe T, 2004. Antioxidant status and nonspecific immune responses in rainbow trout(Oncorhynchus mykiss)fed two levels of vitamin E along with three lipid sources. Aquaculture, 234 (1-4) : 361 –379. Doi: 10.1016/j.aquaculture.2003.11.026 |
| Lee M H, Shiau S Y, 2004. Vitamin E requirements of juvenile grass shrimp, Penaeus monodon, and effects on nonspecific immune responses. Fish Shellfish Immun., 16 (4) : 475 –485. Doi: 10.1016/j.fsi.2003.08.005 |
| Liu Y, Wang W N, Wang A L, Wang J M, Sun R Y, 2007. Effects of dietary vitamin E supplementation on antioxidant enzyme activities in Litopenaeus vannamei(Boone, 1931)exposed to acute salinity changes. Aquaculture, 265 (1-4) : 351 –358. Doi: 10.1016/j.aquaculture.2007.02.010 |
| Lowry O H, Rosebrough N J, Farr A L, Randall R J, 1951. Protein measurement with the Folin phenol reagent. J. Bio. Chem., 193 : 265 –275. |
| Luo W, Wang Q, Zhao Y L, Gu Z M, Mi G Q, Huang X M, Liu Q W, 2005. Effects of dietary vitamin E on reproduction of redclaw crayfish Cherax quadricarinatus. Ocean. Limn. Sin., 36 (4) : 335 –342. |
| Lygren B, Hamre K, Waagbø R, 2000. Eff ect of induced hyperoxia on the antioxidant status of Atlantic salmon Salmo salar L. fed three different levels of dietary vitamin E. Aquac. Res., 31 (4) : 401 –407. |
| McCord J M, Fridovich I, 1984. Superoxide dismutase:an enzymac function for erythrocuprein(hemocuprein). J. Biol. Chem., 244 (22) : 6049 –6055. |
| McDowell L R, Cunha T J, 1989. Vitamins in Animal Nutrition:Comparative Aspects to Human Nutrition. Academic Press, San Diego, California : 101 –104. |
| Mourente G, Díaz-Salvago E, Bell J G, Tocher D R, 2002. Increased activities of hepatic antioxidant defence enzymes in juvenile gilthead sea bream(Sparus aurata L. )fed dietary oxidised oil:attenuation by dietary vitamin E. Aquaculture, 214 (1-4) : 343 –361. |
| Nguyen B T, Koshio S, Sakiyama K, Harakawa S, Gao J, Mamauag R E, Ishikawa M, Yokoyama S, 2012. Effects of dietary vitamins C and E and their interactions on reproductive performance, larval quality and tissue vitamin contents in kuruma shrimp, Marsupenaeus japonicus Bate. Aquaculture, 334-337 : 73 –81. Doi: 10.1016/j.aquaculture.2011.11.044 |
| Ortuño J, Esteban M A, Meseguer J, 2000. High dietary intake of non-tocopherol acetate enhances the non-specific immune response of gilthead seabream(Sparus aurata L. Fish Shellfish Immu., 10 (4) : 293 –307. Doi: 10.1006/fsim.1999.0238 |
| Pacheco R, Ascencio F, Zarain M, Gómez G, Campa Á, 2011. Enhancement of superoxide dismutase and catalase activity in juvenile brown shrimp, Farfantepenaeus californiensis(Holmes, 1900), fed 尾-1. 3 glucan vitamin E, and 尾-carotene and infected with white spot syndrome virus. Lat. Am. J. Aquat. Res., 39 (3) : 534 –543. |
| Regoli F, Principato G, 1995. Glutathione, glutathionedependent and antioxidant enzymes in mussel, Mytilus galloprovincialis, exposed to metals undert field and laboratory conditions:implications for the use of biochemical biomarkers. Aquat. Toxicol., 31 (2) : 143 –164. Doi: 10.1016/0166-445X(94)00064-W |
| Robbins K C, Saxton A M, Southern L L, 2006. Estimation of nutrient requirements using broken-line regression analysis. J. Anim. Sci., 84 (Suppl) : 155 –165. |
| Wang W N, Wang A L, Zhang Y J, Li Z H, Wang J X, Sun R Y, 2004. Effects of nitrite on lethal and immune response of Macrobrachium nipponense. Aquaculture, 232 (1-4) : 679 –686. Doi: 10.1016/j.aquaculture.2003.08.018 |
| Wang W N, Wang Y, Wang A L, 2006. Eff ect of supplemental L-ascorbyl-2-polyphosphate(APP)in enriched live food on the immune response of Penaeus vannamei exposed to ammonia-N. Aquaculture, 256 (1-4) : 552 –557. Doi: 10.1016/j.aquaculture.2006.02.017 |
| Winston G W, Di Giulio R T, 1991. Prooxidant and antioxidant mechanisms in aquatic organisms. Aquat. Toxicol., 19 (2) : 137 –161. Doi: 10.1016/0166-445X(91)90033-6 |
| Wouters R, Molina C, Lavens P, Calderón J, 2001. Lipid composition and vitamin content of wild female Litopenaeus vannamei in different stages of sexual maturation. Aquaculture, 198 (3-4) : 307 –323. Doi: 10.1016/S0044-8486(01)00522-1 |
| Zhao W H, Chen L Q, Qin J G, Wu P, Zhang F Y, Li E C, Tang B P, 2011. MnHSP90 cDNA characterization and its expression during the ovary development in oriental river prawn, Macrobrachium nipponense. Mol. Bio l. Rep., 38 (2) : 1399 –1406. Doi: 10.1007/s11033-010-0243-7 |
 2016, 34
2016, 34