Institute of Oceanology, Chinese Academy of Sciences
Article Information
- Wei LIU(刘玮), Haiyi WU(吴海一), Dongmei ZHAN(詹冬梅), Delin DUAN(段德麟)
- Phenological study of Sargassum thunbergii (Fucales, Phaeophyta) in Lidao Bay, Rongcheng, China
- Journal of Oceanology and Limnology, 34(3): 498-506
- http://dx.doi.org/10.1007/s00343-016-5046-9
Article History
- Received: Mar. 5, 2015
- Accepted: Apr. 14, 2015
2. University of Chinese Academy of Sciences, Beijing 100049, China;
3. Marine Biology Institute of Shandong Province, Qingdao 266100, China
Sargassum thunbergii (Mert.) O. Kuntze is widely distributed throughout the rocky littoral regions of the Chinese coastline and is commonly used as raw material for alginate,fertilizer,and medicine (Tseng,1983) . This algae often forms seaweed beds that provide nurseries for invertebrates and preserves environmental conditions. Thus,S. thunbergii plays an important ecological role in maintaining the intertidal ecosystem. Additionally,because of its high nutritional value,S. thunbergii has economic potential as a food source for the sea cucumber Stichopus japonicus (Wu et al.,1995; Yan et al.,1998) . Recently,natural stocks of S. thunbergii have declined significantly as a result of overexploitation (Zhang et al.,2012) . Thus,there is a need for large-scale enhancement and cultivation of S. thunbergii to conserve this natural resource. However,attempts at large-scale cultivation have been unsuccessful because of a lack of understanding of their basic biology. The few studies of Sargassum to date have focused on its ecology and developmental biology (Umezaki,1974; Koh et al.,1993; Zheng and Chen,1993; Zhao et al.,2008; Wang et al.,2012; Liu et al.,2013; Rover et al.,2015) .
The phenology of S. thunbergii is not well documented,although annual variation in this species has been evaluated (Umezaki,1974; Koh et al.,1993; Zheng and Chen,1993) . S. thunbergii has an annual cycle of growth and reproduction. New seedlings are regenerated from the perennial holdfast and eggs are produced from the receptacles during the maturation season. After reproduction,mature thalli become senescent and are shed. Different geographical populations of S. thunbergii exhibit different patterns of thalli growth (Umezaki,1974; Koh et al.,1993; Zheng and Chen,1993) . The flexible life cycle and morphological variation of Sargassum populations is thought to be largely a function of environmental differences (Engelen et al.,2005; Yu et al.,2012; Endo et al.,2013) . However,there is little quantitative information about the relationship between the environment (e.g.,temperature,day length,and tidal cycles) ,variation in thalli,and the size distribution of individuals in populations of S. thunbergii.
To improve our understanding of the phenology of S. thunbergii,we evaluated the growth pattern of this species at Lidao Bay,a region that has an abundance of S. thunbergii. The objectives of this study were: (1) to evaluate the relationship between environmental variables and thallus length or biomass (air temperature,seawater temperature,and day length) ; (2) to evaluate the temporal shift in thallus length frequency distribution; (3) to determine whether location in the tidal zone influences thallus length and biomass.
2 MATERIAL AND METHODSurveys were conducted on the rocky shore of Lidao Bay (37°12′N,122°36′E) . Lidao Bay is located on the east coast of Shandong Peninsula,where hard substrata of stones and boulders are distributed on the littoral region. The intertidal zone slopes gently,over a vertical distance of about 3 m. There is relatively strong wave action and active water exchange throughout the year,with annual surface seawater temperature ranging from -1 to 26°C and salinity from 27 to 30. S. thunbergii is a relatively common seaweed in Lidao Bay,but it is patchily distributed on the hard substrata from the middle tidal zone to the low tide zone.
Algal samples were collected monthly from April 2011 to March 2012. During sample collection,seawater temperature and air temperature were measured using a mercury thermometer,and the day length was calculated based on the time of sunrise and sunset using the horizon for reference. The S. thunbergii population in the intertidal zone was divided into three treatment groups based on the location relative to the vertical tidal level in April,2011 (Fig. 1) . Survey sites for the collection of algal samples were determined by GPS (Garmin GPSMAP 76CSX,USA) within the 0.5-m wide Sargassum tidal zones along the coast. At each site,three patches were surveyed with a sampling frame (the sampling frame size: 10 cm×10 cm) . Within the sampling frame,the Sargassum samples including the intact holdfast were scraped from rocks to ensure that the branches were complete. Approximately 400-600 thalli were collected at each sampling time. Algal samples were then stored in sealed bags and transported to the laboratory under cool conditions. Prior to measurement,algal samples were rinsed in filtered seawater and fouling organisms were eliminated. The lengths of the primary branches were measured,excluding the length of holdfast. The biomass was calculated by weighing the dry mass in the sampling area (0.01 m2) .
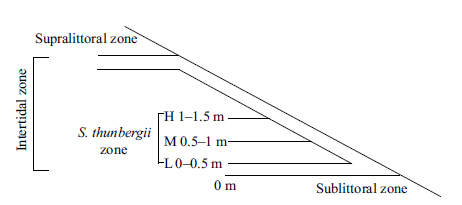
|
| Figure 1 Schematic of the distribution of Sargassum in the intertidal zone of Lidao Bay |
A total of nine patches were surveyed during each sampling period. All the thalli in each patch were measured and this data was used to estimate the thallus length for the local population in each month. The total dry mass of thalli in each patch was weighed and the biomass was calculated by measuring the dry mass in the sampling frame (0.01 m2) .
2.2 Effect of tidal influence on thalliThe thallus length for a tidal treatment group was represented by the average length and standard deviation. The dry mass from different tidal patches and the sampling area (0.01 m2) were used to calculate biomass for the different tidal treatment groups.
2.3 Thalli analysis of size distributionAll thalli from each monthly collection were classified into eight groups based on length (<10.0,10.0-19.9,20.0-29.9,30.0-39.9,40.0-49.9,50.0- 59.9,60.0-69.9,and ≥70.0 cm) . The frequency for each length class of thalli was estimated as a percent of the total. The biomass for each length class was calculated by measuring the total dry mass of thalli in a length class in the total patch area (0.09 m2) . Additionally,mature thalli that had megascopic receptacles were selected during maturity. The frequency of mature thalli in each length class was calculated as a percent of the total number of mature thalli.
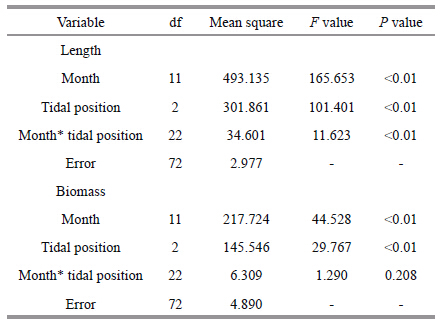
2.4 Data analysisRegression analysis was used to evaluate the correlation between the algal traits (thallus length and biomass) and environmental factors (air temperature,seawater temperature,and day length) . Two-way ANOVA was used to test the efforts of tidal position and temporal variation on the thallus length and biomass,respectively. Differences between groups were evaluated using Tukey’s test. Differences were considered significant at P<0.01. All analyses were performed in SPSS for Windows 13.0.
3 RESULTThe peak seawater temperature was 25.1°C (August 2011) and the minimum was -1.2°C (February 2012) . Annual air temperature fluctuated from -3.0 to 30.4°C. Generally,the pattern of change in air and seawater temperature was similar,though the magnitude of change in air temperature was greater than for seawater temperature. The change in thallus length and biomass of S. thunbergii was correlated to the change in temperature from April 2011 to March 2012 (Fig. 3) . Regression analysis revealed that there was a significant correlation between thallus length and temperature (Table 1) . The coeffcient of determination (R2) for the correlation between air temperature (AT) and thallus length (TL) was higher than for the correlation between seawater temperature (ST) and thallus length (TL) . Additionally,there was a significant correlation between air temperature (AT) and biomass (B) ,but not between seawater temperature (ST) and biomass (B) . The thallus length,the biomass,and the temperature peaked in the same month (August 2011) ,whereas the minimum thallus length and the biomass was measured 3 months prior to the minimum temperature (February 2012) .

|
| Figure 2 S. thunbergii in the intertidal zone at Lidao Bay |

|
| Figure 3 Seasonal variation in S. thunbergii thallus length and biomass with air temperature and seawater temperatur |

|
The day length varied from 9.6 to 14.7 h during the study,and the temporal pattern of changes in the thallus length and biomass was similar to that for day length (Fig. 4) . There was a significant correlation (R2=0.716,P<0.01) between thallus length (TL) and day length (DL) ,and between biomass (B) and day length (DL) (R2=0.739,P<0.01) . However,there were some differences in the pattern of change. The thallus length and the biomass peaked after the peak in day length,but before the shortest day length.
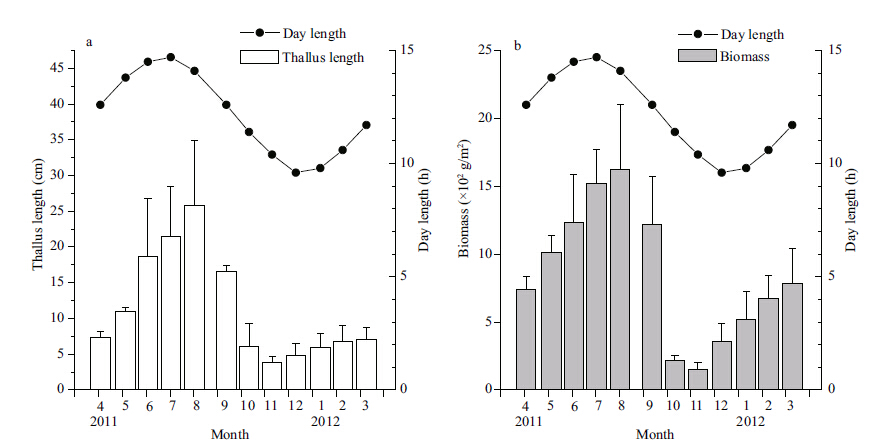
|
| Figure 4 Seasonal changes in S. thunbergii thallus length and biomass with day length |
The thallus length varied both temporally and between different tidal zones (Fig. 5) . The low tidal zone group had longer thalli (P<0.01) than the high and mid tidal zone groups. Additionally,the elongation of thallus length was higher in the low tidal group than in the remaining two groups. Thallus length was significantly affected by tidal zone location (Two-way ANOVA; P<0.01) and month (P<0.01; Table 2) . Similarly,the biomass also varied with time and tidal zone location. There were significant differences in biomass relative to the tidal zone location and the time (tidal zone location,P<0.01; month,P<0.01; Table 2) . The peak biomass in the high and mid-tidal zone groups was in July 2011,whereas the biomass in the low tidal zone group peaked the following month. Biomass was lowest in all three groups in November 2011.
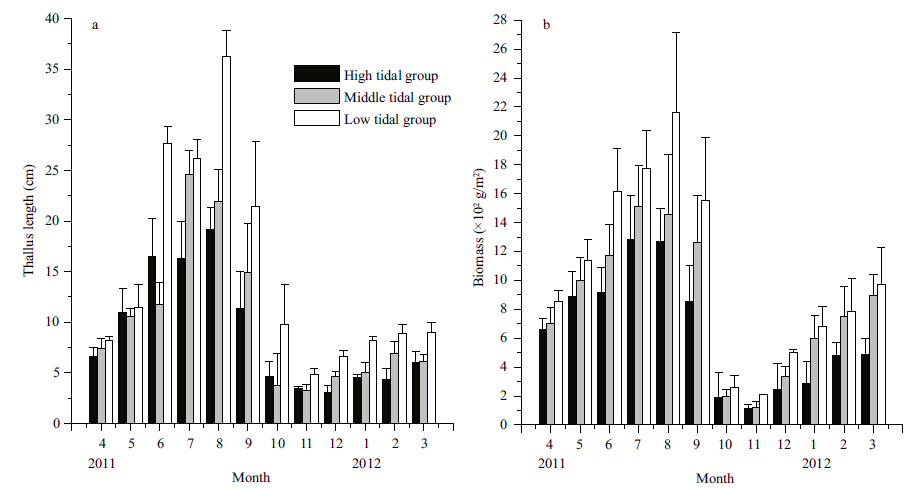
|
| Figure 5 Seasonal changes in thallus length and biomass of S. thunbergii collected in the low, mid, and high tidal zone |
|
The majority (>50%) of thalli were <10 cm in length before June with a maximum length of <40 cm (Fig. 6) . During the maturation stage from June to August,the frequency of the larger size classes increased steadily. In August,>60% of thalli were >20 cm in length. Thereafter,the frequency of the larger size classes decreased rapidly from September to October,and most thalli were <10 cm in November.From November 2011 to March 2012,the frequency of the smallest size class was >80% and the small size classes were dominant in the S. thunbergii population.
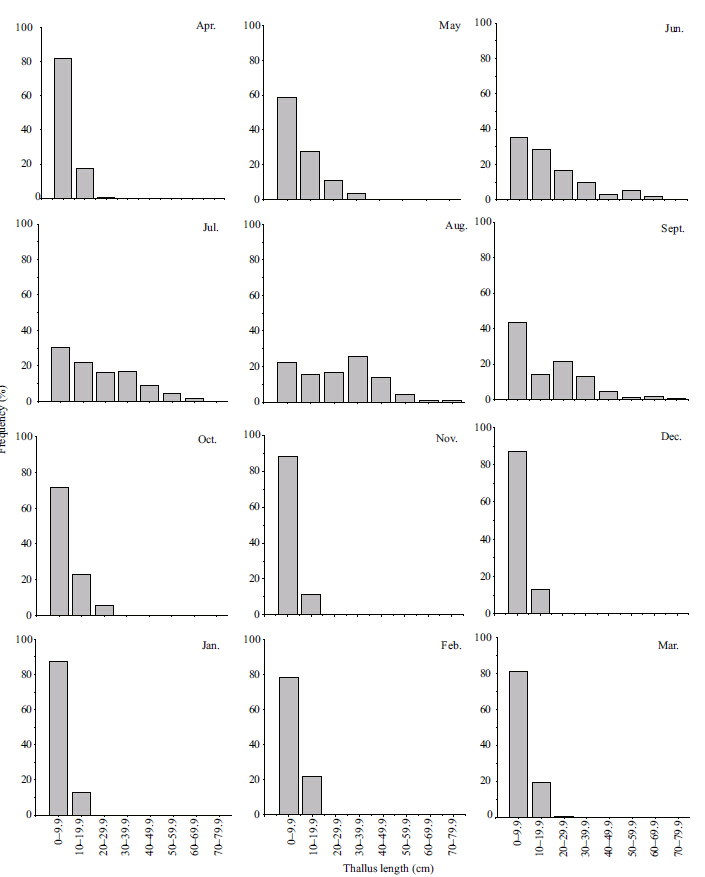
|
| Figure 6 Frequency distributions of thallus length in the S. thunbergii population |
The distribution of biomass among length classes varied with time (Fig. 7) ,particularly from June to September. Before June,the small size classes (<20 cm) contributed 80% to the biomass. The proportion of biomass contributed by the larger size classes (20-39.9 cm) increased gradually from June to September. After September,the proportion of biomass contributed by the larger size classes decreased rapidly as a result of massive shedding of lateral branches,and the small size classes (<20 cm) again contributed most to the biomass. From November to the following April,the smallest size class (<10 cm) accounted for >50% of the biomass.
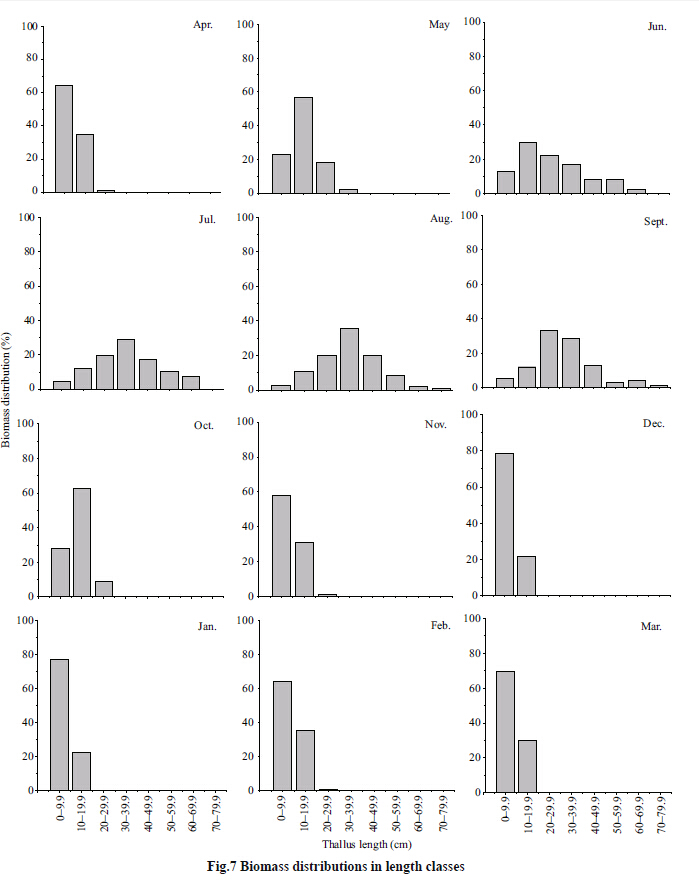
|
| Figure 7 Biomass distributions in length classes |
Receptacles of S. thunbergii were observed from June to August 2011,when seawater temperature was in the range of 14.4-25.1°C and the day length was 13.6-14.7 h. The frequency of mature thalli differed between the different length classes (Fig. 8) . The smallest length class had no mature thalli whereas the larger length classes (20-39.9 cm) had a significant portion of the mature thalli. Because there were very low numbers of long thalli (40-79.9 cm) ,the frequency of mature thalli was also low for these length classes.
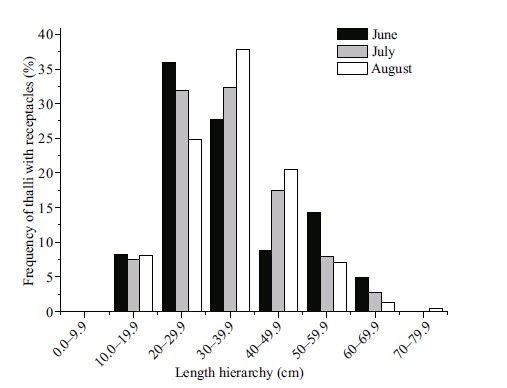
|
| Figure 8 Length frequency distribution of mature thalli |
The thalli of S. thunbergii exhibited an annual cycle,corresponding with changes in temperature and day length. The thalli grew and matured during the period of increasing temperature,suggesting that temperature influences maturation in S. thunbergii thalli. This is consistent previous reports (Yu et al.,2012) . The S. thunbergii in Lidao Bay (37°12′N,122°36′E) matured at a later date than those at Dongtou (27°54′N,121°09′E) in Zhejiang and Pingtan (25°30′N,119°46′E) in Fujian (Zheng and Chen,1993; Zhang et al.,2012) ,likely because of lower temperatures in Lidao Bay. Indeed,S. thunbergii populations from similar latitudes reached maturity at a similar time,likely because of the similarity in temperatures (Umezaki,1974; Zhang et al.,2009) . Moreover,we found that the temperature values differed at the time different geographical populations initiated to mature. This suggests that the maturation of S. thunbergii is independent of a temperature threshold. We propose that the growth and maturity of thalli is associated with the pattern of variation in temperature,consistent with the work of Deysher (1984) . The growth and maturity of thalli were also related to day length. The S. thunbergii thalli began to mature in early summer and receptacles appeared as day length increased. Our observations were consistent with work by Uchida (1993) and Zou et al. (2006) ,who showed that long days initiated maturation of some Sargassum species. Although there is evidence that receptacles form under shorter photoperiod conditions in some Sargassum species (De Wreede,1976; Yoshida et al.,2001) ,gametes are not released until the photoperiod increases (Yoshida et al.,1998) .
Our results also suggest that location in the tidal zone influences the length and biomass of thalli. The low tidal zone group had higher length and biomass than the mid and upper tidal zone groups (Fig. 5) . Compared with the low tidal group,inhabitants from higher tidal zones experience greater stress in the coastal environment. This can result in decreased growth of some Sargassum species,as noted in other studies (Davison et al.,1993; Chu et al.,2012) . Additionally,the reduced seawater exchange in the high tidal zone reduces nutrient supply and limits seaweed growth (Wheeler,1988; Norton,1991; Engelen et al.,2005) .
The length size class distribution and their contribution to biomass switched between the small size classes (<20 cm) and the large size classes (20-79.9 cm) coincident with thalli maturation (Figs. 6,7) . The mature thalli with receptacles were represented in the large length size classes (Fig. 8) . This suggests that long thalli are important for sexual reproduction,which is consistent with previous studies on some Sargassum species (Ang Jr,1992; Rivera and Scrosati,2006) . The thalli represented in the short length size classes had a low proportion of mature individuals (Fig. 8) ,but always occupied a certain proportion of the whole population throughout the year (Figs. 5,6) . We conclude that the short thalli are important for maintenance of vegetative regeneration in S. thunbergii,consistent with prior studies (Umezaki,1974; Koh et al.,1993; Zheng and Chen,1993) .
The transition between the short and long length size classes suggests that there is a trade-off between sexual reproduction and vegetative regeneration,as noted by other authors (McCourt,1984; Chu et al.,2011) .
5 CONCLUSIONWe found a significant linear relationship between algal traits (thallus length and biomass) and environmental factors (air temperature,seawater temperature,and day length) ,except between biomass and seawater temperature. Additionally,location in the tidal zone had a significant effect on thallus length and biomass. Thalli from the low tidal zone had higher length and biomass than those in the mid and high tidal zones. Additionally,the local S. thunbergii population matured from early June to mid-August and the mature thalli represented a large proportion of the 20-39.9 cm length size class. The length frequency distribution and contribution of length size classes to biomass changed significantly at the time of maturation.
6 ACKNOWLEDGEMENTWe sincerely thank Dr. J Y Li for critical comments and suggestions for the manuscript’s preparation and Mr. Donald Wade Sturge for reviewing the English of themanuscript.
| Ang P O Jr, 1992. Cost of reproduction in Fucus distichus. Mar. Ecol. Prog. Ser., 89 : 25 –35. Doi: 10.3354/meps089025 |
| Chu S H, Zhang Q S, Liu S K, Tang Y Z, Zhang S B, Lu Z C, Yu Y Q, 2012. Tolerance of Sargassum thunbergii germlings to thermal, osmotic and desiccation stress. Aquat. Bot., 96 (1) : 1 –6. Doi: 10.1016/j.aquabot.2011.09.002 |
| Chu S H, Zhang Q S, Liu S K, Zhang S B, Tang Y Z, Lu Z C, Yu Y Q, 2011. Trade-off between vegetative regeneration and sexual reproduction of Sargassum thunbergii. Hydrobiologia, 678 (1) : 127 –135. Doi: 10.1007/s10750-011-0835-9 |
| Davison I R, Johnson L E, Brawley S H, 1993. Sublethal stress in the intertidal zone:tidal emersion inhibits photosynthesis and retards development in embryos of the brown alga Pelvetia fastigiata. Oecologia, 96 (4) : 483 –492. Doi: 10.1007/BF00320505 |
| De Wreede R E, 1976. The phenology of three species of Sargassum(Sargassaceae, Phaeophyta)in Hawaii. Phycologia, 15 (2) : 175 –183. Doi: 10.2216/i0031-8884-15-2-175.1 |
| Deysher L E, 1984. Reproductive phenology of newly introduced populations of the brown alga, Sargassum muticum(Yendo)Fensholt. Hydrobiologia, 116-117 (1) : 403 –407. Doi: 10.1007/BF00027710 |
| Endo H, Nishigaki T, Yamamoto K, Takeno K, 2013. Age- and size-based morphological comparison between the brown alga Sargassum macrocarpum(Heterokonta; Fucales)from different depths at an exposed coast in northern Kyoto, Japan. J. Appl. Phycol., 25 (6) : 1815 –1822. Doi: 10.1007/s10811-013-0002-y |
| Engelen A H, Åberg P, Olsen J L, Stam W T, Breeman A M, 2005. Effects of wave exposure and depth on biomass, density and fertility of the fucoid seaweed Sargassum polyceratium(Phaeophyta, Sargassaceae). Eur. J. Phycol., 40 (2) : 149 –158. Doi: 10.1080/09670260500109210 |
| Koh C H, Kim Y, Kang S G, 1993. Size distribution, growth and production of Sargassum thunbergii in an intertidal zone of Padori, west coast of Korea. Hydrobiologia, 260-261 (1) : 207 –214. Doi: 10.1007/BF00049021 |
| Liu F, Pang S J, Gao S Q, Shan T F, 2013. Intraspecific genetic analysis, gamete release performance, and growth of Sargassum muticum(Fucales, Phaeophyta)from China. Chin. J. Ocean. Limnol., 31 (6) : 1268 –1275. Doi: 10.1007/s00343-013-2314-9 |
| McCourt R M, 1984. Seasonal patterns of abundance, distributions, and phenology in relation to growth strategies of three Sargassum species. J. Exp. Mar. Bio. Ecol., 74 (2) : 141 –156. Doi: 10.1016/0022-0981(84)90082-0 |
| Norton T A, 1991. Confl icting constraints on the form of intertidal algae. Brit. Phycol. J., 26 (3) : 203 –218. Doi: 10.1080/00071619100650191 |
| Rivera M, Scrosati R, 2006. Population dynamics of Sargassum lapazeanum(Fucales, Phaeophyta)from the Gulf of California, Mexico. Phycologia, 45 (2) : 178 –189. Doi: 10.2216/05-47.1 |
| Rover T, Simioni C, Hable W, Bouzon Z L, 2015. Ultrastructural and structural characterization of zygotes and embryos during development in Sargassum cymosum(Phaeophyceae, Fucales). Protoplasma, 252 (2) : 505 –518. Doi: 10.1007/s00709-014-0696-y |
| Tseng C K, 1983. Common Seaweeds of China. Beijing, China: Science Press. |
| Uchida T, 1993. The life cycle of Sargassum horneri(Phaeophyta)in laboratory culture. J. Phycol., 29 (2) : 231 –235. Doi: 10.1111/j.0022-3646.1993.00231.x |
| Umezaki I, 1974. Ecological studies of Sargassum thunbergii(Mertens)O. Kuntze in Maizuru Bay, Japan Sea. Bot. Mag. Tokyo, 87 (4) : 285 –292. |
| Wang R J, Wang Y, Tang X X, 2012. Identification of the toxic compounds produced by Sargassum thunbergii to red tide microalgae. Chin. J. Ocean. Limnol., 30 (5) : 778 –785. Doi: 10.1007/s00343-012-1294-5 |
| Wheeler W N, 1988. Algal productivity and hydrodynamics-a synthesis. Prog. Phycol. Res., 6 : 23 –58. |
| Wu X C, Lu B R, Tseng C K, 1995. Comparative fatty acid composition of four Sargassum species(Fucales, Phaeophyta). Chin. J. Ocean. Limnol., 13 (4) : 370 –373. Doi: 10.1007/BF02889473 |
| Yan X J, Hou X L, Sun B, Fan X, Han L J, 1998. Element composition of Sargassum thunbergii. Chin. J. Ocean. Limnol., 16 (2) : 189 –192. Doi: 10.1007/BF02845187 |
| Yoshida G, Arima S, Terawaki T, 1998. Growth and maturation of the 鈥榓utumn-fruiting type鈥? of Sargassum horneri(Fucales, Phaeophyta)and comparisons with the 鈥榮pringfruiting type鈥?. Phycol. Res., 46 (3) : 183 –189. Doi: 10.1111/pre.1998.46.issue-3 |
| Yoshida G, Yoshikawa K, Terawaki T, 2001. Growth and maturation of two populations of Sargassum horneri(Fucales, Pheaophyta)in Hiroshima Bay, the Seto Inland Sea. Fisheries Sci., 67 (6) : 1023 –1029. Doi: 10.1046/j.1444-2906.2001.00357.x |
| Yu Y Q, Zhang Q S, Lu Z C, Tang Y Z, Zhang S B, Chu S H, 2012. Small-scale spatial and temporal reproductive variability of the brown macroalga Sargassum thunbergii in contrasting habitats:a study on the island of Xiaoheishan, Changdao archipelago, China. Estuar. Coast. Shelf S ci., 112 : 280 –286. Doi: 10.1016/j.ecss.2012.08.001 |
| Zhang Q S, Li W, Liu S, Pan J H, 2009. Size-dependence of reproductive allocation of Sargassum thunbergii(Sargassaceae, Phaeophyta)in Bohai Bay, China. Aquat. Bot., 91 (3) : 194 –198. Doi: 10.1016/j.aquabot.2009.06.003 |
| Zhang Q S, Tang Y Z, Liu S K, Zhang S B, Lu Z C, Cu S H, Yu Y Q, 2012. Zygote-derived seedling production of Sargassum thunbergii:focus on two frequently experienced constraints in tank culture of seaweed. J. Appl. Phycol., 24 (4) : 707 –714. Doi: 10.1007/s10811-011-9689-9 |
| Zhao Z G, Zhao F J, Yao J T, Lu J M, Ang P O Jr, Duan D L, 2008. Early development of germlings of Sargassum thunbergii(Fucales, Phaeophyta)under laboratory conditions. J. Appl. Phycol., 20 (5) : 925 –931. Doi: 10.1007/s10811-008-9311-y |
| Zheng Y, Chen Z H, 1993. The seasonal growth and reproduction of Sargassum thunbergii(Pheaophyta)in Pingtan island, Fujian province. J. Fujian Norm. U niv., 9 (1) : 81 –85. |
| Zou D H, Gao K S, Ruan Z X, 2006. Seasonal pattern of reproduction of Hizikia Fusiformis(Sargassaceae, Phaeophyta)from Nanao island, Shantou, China. J. Appl. Phycol., 18 (2) : 195 –201. Doi: 10.1007/s10811-006-9097-8 |
 2016, 34
2016, 34


