Institute of Oceanology, Chinese Academy of Sciences
Article Information
- P. KUKLIN Alexey, Ts. TSYBEKMITOVA Gazhit, P. GORLACHEVA Evgenia, B. BAZAROVA Balzhit, V. AFONIN Alexey
- The ecosystem of Lake Kenon: past and present (Transbaikal Territory, Russia)
- Journal of Oceanology and Limnology, 34(3): 507-516
- http://dx.doi.org/10.1007/s00343-016-4285-0
Article History
- Received: Oct. 29, 2014
- Accepted: Jan. 22, 2015
Research is done on Aquatic ecosystems of different kinds: e.g. natural (Alimov et al.,2003) and artificial (Andrianova et al.,2006) ecosystems. Such water basin ecosystems can be subject to different anthropogenic factors,such as transport,agriculture,industry (chemical,metallurgical,power) . Economic development is demanding more and more power capacity,which furthermore leads to construction of new thermal and nuclear power stations. As a result,natural reservoirs are being increasingly transformed. Support and mitigation of problems in water quality combined heat and power plants (CHPs) .
Of themselves,hydrobionts cannot be used to classify the substances,the energy or the information in the whole system. Studying the ecosystem condition,we assess the results of organisms’ vital functions under the influence of complex factors. Location of water ecosystem in contrast to climatic conditions (cold winter to hot summer,different rainfall amount) promotes better understanding of such processes in the ecosystem.
The aim of the research has been to assess longstanding consequences of the complex factors due to technological processes are very urgent. Hence,ecosystem elements (including hydrobiont communities) are the main indicators of water quality,and they and their dynamics are being increasingly studied in natural reservoirs under the influence of influencing the environment and to separate the different biological components in the Lake Kenon ecosystem.
Different factors (rainfall amount,heat and the supply of chemical substances,fish introductions) have influenced the Lake Kenon ecosystem during the period covered by the present paper. Longstanding influence of this complex of factors on the water ecosystem in general is reflected in the change in hydrobiont composition and distribution.
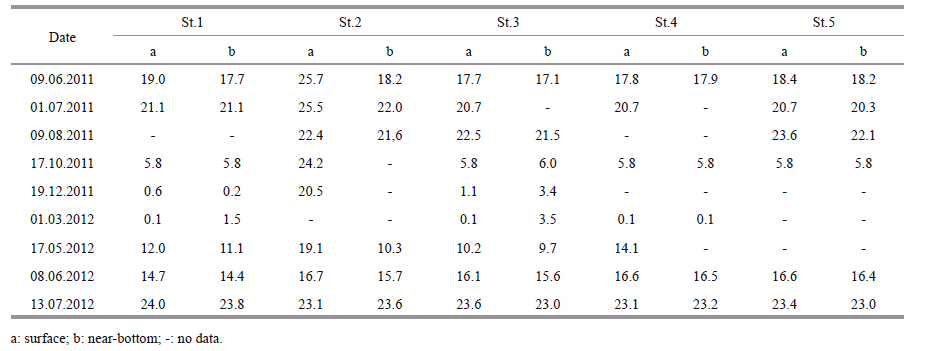
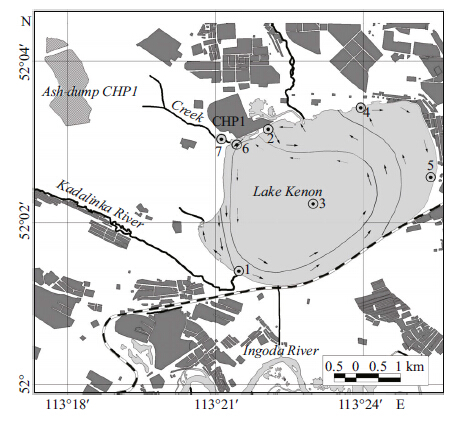
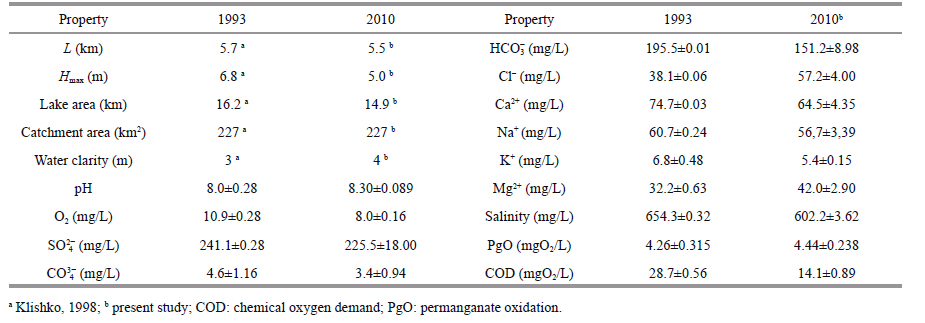
2 MATERIAL AND METHODThe rainfall data presented were provided by the Chita regional center for hydrometeorology and environmental monitoring. Hydrochemical observations at Lake Kenon were made in 2010-2014 at seven stations (Fig. 1) ,monthly during the period of open water and once in winter period. At the third station,three horizons were sampled: the surface,the transparency zone,and the bottom. At other stations,two horizons were sampled: the surface and the bottom. Hydrochemical materials were collected using the technique suggested by Semyonov (Semyonov,1977) . Water samples were analyzed by atomic absorption,photometry and titrimetry according to the State Standard 4389-72,4192-82,3351-74,Federal nature protection normative documents 14.1:2:4.138-98,14.1:2:4.139-98,and Scientific council on analytical methods 335-g. Data presented in Table 1 are averaged at station 5,the farthest from CHP-1.
|
| Figure 1 Index-map of sampling location |
|
Hydrobiological observations at Lake Kenon were made monthly in 2010-2014 at seven stations (Fig. 1) during the open water period using some techniques (Chugunova,1959; Pravdin,1966; Kachaeva,1976; Katanskaya,1981) . Algae were gathered from rocky substrates,projecting cover and epibioses area were taken into account. There were washout calculations of phytomass per vegetation weight from aquatic vegetation. Aquatic vegetation was studied by the profile method. We caught the fish by gill nets (14-50 mm mesh) at stations 4 and 6. Juvenile fish were caught by fry net.
We studied the qualitative composition of organic substance in water by fixing extracellular enzymatic processes assessing proteolytic and amylolytic water activity and determining the peculiarities of their changes in different periods. Enzymatic water activity was determined using Korneeva’s technique (Korneeva,1993) .
Plant and animal samples for testing the chemical elements were collected in August 2014 at the same area as water samples (Fig. 1) . Metal concentrations in hydrobionts were tested by mass spectral (PQ-2,VG Elemental,UK) and atomic emission (ICAP-61,Thermo Jarrell Ash,USA) analysis methods in Analytical Certification Testing Center at the Institute of Microelectronics Technology and High Purity Materials,Russian Academy of Sciences. Accreditation certificate is ROSS.0001.513800,given on August 10,2009. Samples were decomposed in autoclave Teflon reactor pots adding concentrated nitric acid (HNO3) and hydrogen peroxide (H2O2) . Measurement accuracy was tested using a standard sample of Elodea canadensis Michx. (1803) (SRM,EK-1,registration number COOMET 0065-2008-RU) and Perca fluviatilisi (Linnaeus,1758) muscle (SRM,Baikal perch tissue,BOk-2,registration number COOMET CRM 0068-2009-Ru) . The data were processed statistically using Statistica 10 for Windows (StatSoft,Inc) .
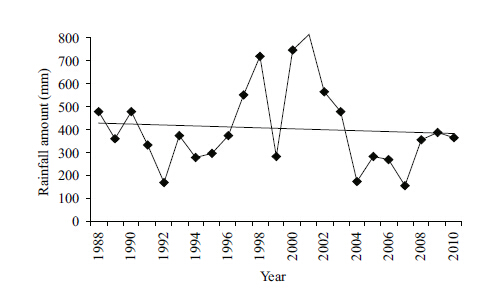
3 RESULT 3.1 Characteristic of water basin and surrounding catchment area locationLake Kenon is a part of the Amur River basin. It is a natural reservoir located in the Chita-Ingoda depression. The catchment area is characterized by the sequence of moistening and desiccation periods (Obyazov,2013) (Fig. 2) determining water ecosystems condition (Kuklin et al.,2013) . The Kadalinka and the Ivanovka Rivers flow into the lake. The rivers’ catchment areas in their higher reaches drain forests influenced by fires,in their lower reaches the rivers flow over steppe. Because of the regulated run-off,the Ivanovka River has been getting dry,while the Kadalinka River often flows due to ash-disposal area drainage waters in summer. Lake Kenon has been used as a water reservoir-cooler at CHP-1. The station reconstruction in the 2000s resulted in lowering of thermal load at Lake Kenon. The discharge of heated water decreased from 491.6 million m3/a in the early 1990s to 109.3 million m3/a in 2010. Compensating evaporation from the open water areas in winter,replenishment of the water reservoir from the River Ingoda occurs during the open water period. Storage of ash waste had been produced near the oxbow backwater of Lake Kenon before the 1970s,later the hydro ash-disposal area was built without any infiltration screen 3 km to the north-west of CHP-1. In the north,a creek formed by draining ash-disposal area waters of CHP-1 flows into the lake. A city beach is located on the northwestern coast.
|
| Figure 2 Rainfall amount |
Morphometric and physical-chemical characteristics of the water reservoir are presented in Table 1. The lake extends from the southwest to the northeast along the main wind direction. The lake shores are steep and do not cause flooding of the large areas during the water level increase. The lakeshores are mainly sandy. Fortification of railroad bed by large fragmental rocks along the southeastern coast resulted in rocky littoral areas. The depths more than 3.5m occur in most lake areas,bed silt includes organic mud with a proportion of different mineral particles. Natural fluctuations in water levels are currently smoothed by regulating the volumes of water transported from the Ingoda River.
3.2.2 Thermal regimeTwo zones in the water basin are not subjected to the impact of heated water (a bay at the Kadalinka River inflow and the northeastern coast of the lake) (Fig. 1) . Table 2 shows the distribution of water temperature in the basin during different seasons. The temperature distribution in 2013-2014 is shown in Table 2. The impact of warm-water flows on the southwestern coast is decreased by mixing of cold water from the Ingoda River. The greatest impact of warm water occurs near CHP-1 during the cold period. There is no ice cover from October until May.
During our research,water dissolved inorganic matter averaged 530 mg/L,and it was alkaline. The order of anion concentration was: SO42-> (НCO3-+CO32-) >Cl-. Their minimum concen-trations occur in open water. The order of cation concentrations in Lake Kenon water was: Ca2+>Na+>Mg2+>K+. Although there is an excess of Na+ over Ca2+ in summer,Ca2+ prevails in other seasons. The concentration of K+ was small relative of the total cations. In the high-water period of 1993 the concentration of Mg2+ was 1.3 times and that of Cl-41.5 times the corresponding concentrations in the low-water period of 2010,and other ions are characterized by a corresponding decrease of their content. Nevertheless,anion-cation composition of the waters did not change. The lake water composition is of a sulfate-hydrocarbonate-chloride calcium-sodic-magnesium character. SO42- content in Lake Kenon water is twice the maximum permissible concentration in fishery waters (FAF,2010) .
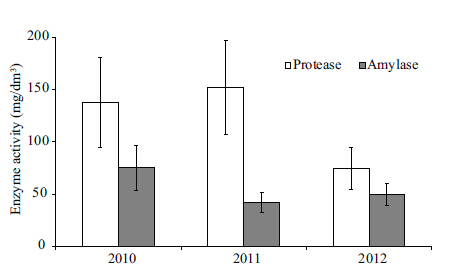
The composition of organic substances in the water basin was measured using the activity of proteolytic and amylolytic enzymes. Such studies had not been done before. The data presented characterize a low-water period of 2010-2012 (Fig. 3) .
|
| Figure 3 Water enzymatic activity in summer and autumn (M±SE, P<0.05) |
Proteolytic enzyme activity in 2010-2012 varied on average from 75 to 152 mg/L,and amylase activity from 42 to 75 mg/L. In summer and autumn enzyme activity increased in near-bottom water layers at almost all stations,and they indicate the decomposition of organic substances. Increase enzyme activity in surface waters was observed more rarely,indicating high densities of phyto-bacterioplankton caused by wind-wave accumulation of plankton.
The magnitude of water oxidizability is related to the amount of oxidizable organic substances. Oxidation by aquatic organisms was observed to be continuously carried out throughout the entire thickness from the basin surface to the bottom and in the top 10-20 cm layer of sediments. The ratio of permanganate oxidation to the bichromate indicates the proportion of autochthonous substance in the total organic matter. This ratio in Lake Kenon did not exceed 50%. In 2010 it amounted to 23% of the average data,and in 2011-2012 to 31%.
3.3 Hydrobiological characteristic of the lakeIchthyological research of Lake Kenon started in the late 1930s (Taranets,1937) ,followed by the Amur ichthyological expedition studies (Nikolsky,1956) . Basic hydrobionts research was done at the lake during the construction of a combined heat and power plant,and during the first ten years of its operation (Thermal,1972; Klishko,1998) . Current research mostly deals with low-water period (Bazarova et al.,2013; Bazarova,2013; Kuklin,2014; Tashlykova,2013; Tsybekmitova,2014) .
3.3.1 AlgaeThe current species composition of phytoplankton microalgae is presented in N.A. Tashlykova’s study (Tashlykova,2012) . The species composition of phytoplankton in Lake Kenon includes 68 species and algae forms. The following species dominate in terms of number: Gomphospheria lacustris Chod.,Tetraedron minimum (A. Br.) Hansg.,Scenedesmus quadricauda Chod.,Tetrastrum komarekii Hind.,Asterionella formosa Hass.,Puncticulata radiosa (Lemmermann) Hảkansson. The following are dominant n terms of biomass—G. lacustris,Peridinium sp. and Ceratium hirundinella (O.F.M.) Bergh. (Tashlykova,2012) .
Macroalgae (15 species of 4 systematic divisions) occur about the basin foreshore as well as on vegetation. In the periphyton,Cladophora fracta (Mühl. ex Vahl.) Kütz dominates. The highest biomasses of filamentous algae (up to 4.5 kg in wet weight per m2) are formed in the warm waters of the discharge canals of CHP-1 where water is 4-20°C higher than that in the basin,depending on the season. In August 2014,Spirogyra sp1 greatly formed in the area of water inflow from the Ingoda River. Currently,the dominant flora of Lake Kenon comprises Charophytes,growing from the water line to depths of 3.5-4.0 m. At the same time,species of the Charophyte family are presented in separate clumps in most deep-central (4.0-5.0 m) zones of the lake. Charophytes are the most abundant aquatic vegetation in terms of phytobiomass and currently dominate the lake vegetation cover.
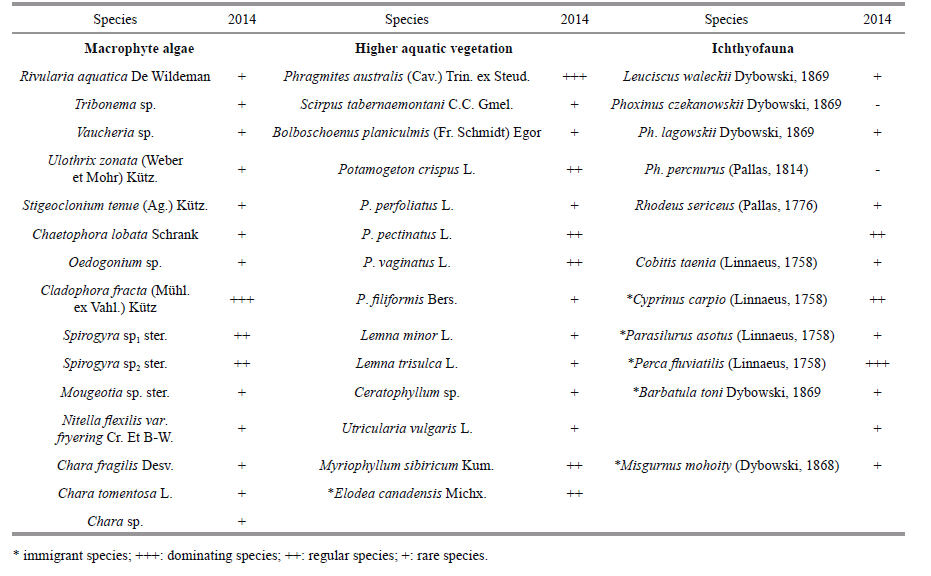
3.3.2 Aquatic higher plantsThe hydrophyte flora in Lake Kenon includes 40 species (Table 3) . The lake vegetation can be divided into three zones. The helophytes are Phragmites australis reed tangle with Scirpus sp. cenosis inclusions along the western and the northern lake shores. Neustophyte cenoses had been earlier represented by Persicaria amphibia (L.) S.F. Gray and Numphoides peltata (S.G. Gmel.) O. Kuntze,they were not found in 2010-2011. Some species of Sagittaria sp. occurred in the southwestern part of the lake. At present,charophytes (depth 0.2-4.8 m) along the western and the northern shores are dominating in hydrophytecenoses. Pomatogeton crispus and Myriophyllum sibiricum cenoses occur as well. In 2009,an alien species E. canadensis was found. It occurred on the western and northern shores in 2010 and on the western shore in 2011 (depth 1.0-3.0 m) (Bazarova,2013) .
High anthropogenic load and human activity have had a considerable impact on fish species diversity and ichthyocenosis structure (Table 3) . The fish fauna in Lake Kenon is represented by 13 species from 5 families where the families Percidae and Siluridae are immigrants. The most numerous species are the Cyprinidae family (7 species) comprising the 2/3 of the whole species diversity. Perca fluviatilisi and Cyprinus carpio dominate in number and biomass. Parasilurus asotus albino was caught in 1997,being very rare among the fish (Gorlacheva and Afonin,2008) . The example of P. asotus caught had a color body with yellowish tints,and all its fins were the same color. Although albinism of fish is rare,P. asotus occurs in the Mordovinsky Gulf of the Kuibyshev reservoir (Elkin and Sukhikh,1978) .
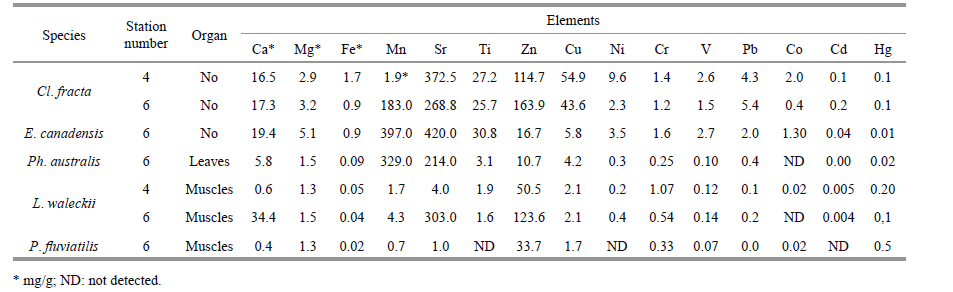
3.4 Toxic elements in water and hydrobiontsDue to the use of Lake Kenon as a recreational reservoir (swimming,amateur fishing) and a place where drainage discharge from ash disposal area and rainfall happen,we assessed the content of chemical elements in hydrobionts including heavy metals (Table 4) . The findings show that greater numbers of the chemical elements occur in plants than in fish. The highest concentrations of toxic elements were found in Cladophora fracta. Elodea canadensis is among the aquatic plants most sensitive to heavy metals (Küpper et al.,1996) . In our study,there were more chemical elements in the muscle of L. waleckii (Dybowski,1869) than in that of P. fluviatilis (L.) muscle.
The authors understand that not all relations in any ecosystem studied are persuasively proved (they might be debatable) ,nevertheless,their inclusion in our hypothesis is the basis for our future studies and analysis.
The complex of natural and anthropogenic factors determines the ecosystem of Lake Kenon. At any time,the ecosystem tends to equilibrium,supported its individual elements as a result of rearrangements that occur in communities of living organisms. For instance,change in the number of nutrients and their proportion causes reorganization in plant communities. Biogenic compounds can occur in the water basin,originating from the catchment area,from bed silt and by anthropogenic influence (recreation,warm waters from CHP,fishing) (Klishko,1998) .
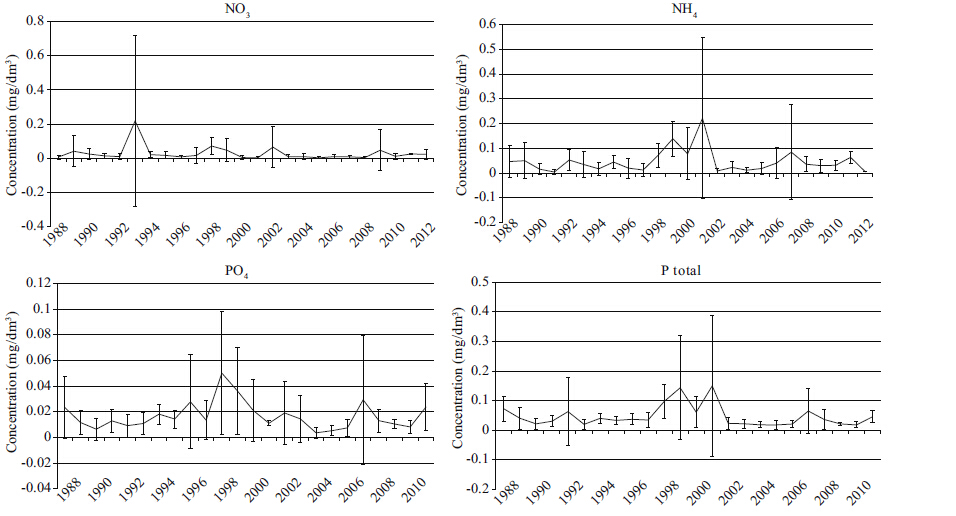
4.1 Precipitation and biogenic elementsDifferent biogenic compounds occur in the water basin depending on rainfall periods. During the time when precipitation exceeds the average annual norm,inflow of nutrients is higher. For example,the studies made in the 1970s (Shishkin and Lokot’,1973) showed twice as high a predominance of organic phosphorus over the inorganic form (their concentration ratio was 1.9) . Our results show that the greatest contents of phosphorus and orthophosphates (Fig. 4) occur in the periods of heavy precipitation. The correlation coeffcient between precipitation and general phosphorus content was 0.45 (P<0.05) . In inorganic nitrogen compounds,ammonium ions prevail and its correlation with atmospheric precipitation was 0.38 (P<0.1) . Nitrate concentration in wet years increases 1.2 times (Fig. 4) ,ammonium ions 3.8 times (Fig. 4) . The increase in nitrogen and phosphorus compounds in the water basin causes the increase in phytoplankton biomass (Shishkin and Lokot’,1973; Abdrakhmanov et al,2014) .
|
| Figure 4 Dynamics of biogenic substance content in Lake Kenon water (M±SD) |
According to the research findings in 2010-2012 (water-short periods) ,the ratio of between organic and inorganic phosphorus was 1.02. In the periods of low precipitation (2008-2014) ,flow of biogenic elements was not as great,which is evident from the ratio between organic and inorganic phosphorus (1.02) . Thus,in dry years production-destruction processes in the lake Kenon occur more evenly.
4.2 Temperature effect on hydrobiontsResearch on the effects of temperature on hydrobionts was done in the 1970s (Thermal,1972) . The greatest changes in hydrobiocenosis structure occur in the zone of heated-water influence,which is limited in area (Fig. 1) . In this zone,changes in the seasonal growth of P. crispus have happened and it has become an autumn-winter-spring species. Growth of its turions starts in August-September; plants with sprout length up to 1.5 m grow from the ice and die in June or the beginning of July. Thermal pollution has affected the biological characteristics of fish. Distortion of their sexual cycle has occurred. For example,for Leuciscus waleckii and Perca fluviatilis sexual maturation under the influence of high temperatures occurs 2-3 weeks earlier. The lack of full spawning grounds during this period leads to the fact that hard roe of P. fluviatilis or L. waleckii is not laid,and its reabsorption is not further observed. All these phenomena lead to the loss of reproduction ability. However,lengthening of the warm period for some fish species,for example,Carassius auratus gibelio,generate favorable year-round feeding.
4.3 Mineralization impact on hydrobiontsThe increase in average annual water mineralization from 420 mg/L to 530.0 mg/L since the 1950s has been determined by the heat and chemical impact of CHP-1. Drainage waters from the ash dump (Fig. 1) flow into the lake,increasing water basin chemical load. Usmanova (2012) states that the hydrocarbonate-sodium water composition (before CHP-1 operation) transformed into a ternary composition: sulfate-hydrocarbonate-chloride sodium-calcium-magnesium. We found that in the water composition from January to December 1993 and in 2010,Ca2+ prevails over Na+,resulting in sulfate-hydrocarbonate-chloride calcium-sodium-magnesium composition in Lake Kenon water. In summer,calcium is intensively absorbed by vegetation,while in winter it releases and influences the hydrochemical water type. Consequently,Usmanova (2012) shows only the composition of the summer characteristics water of the Lake Kenon. We assessed the organic substances in the water basin using enzyme activity (proteolytic and amylolytic) and biochemical oxygen demand (permanganate and bichromate) . Enzyme activity level indicates the rate of organic substance biochemical decomposition. In 2010-2012,proteolytic activity increased from 60 to 130 mg/L. In summer and autumn,at almost all stations it was higher in bed water layers than at the surface. Increase in proteolytic activity in surface layers was observed under conditions of the highest density of phyto-bacterioplankton caused by wind-wave plankton accumulation. There were no significant changes in amylolytic enzyme activity from 2010 to 2012. We determined that amylase activity was higher in the water basin areas overgrown with aquatic vegetation.
As mentioned earlier,change in hydrochemical water type caused the extinction of Esox Reichertii Dybowski,1869 from the ichthyocenosis. However,some other factors should also be considered,for example,intensive fishing in the 1970s,poaching in the 1990s,and decrease in the number of prey (Phoxinus,Rhodeus. sericeus,P. fluviatilis and L. waleckii) .
4.4 Hydrobiocenosis dynamicsChanges in the physical and chemical parameters of Lake Kenon ecosystem occasionally caused water blooms and increasing contents of toxic substances,as well as mass fish kills (Karasev,1968,1970; Klishko,1998; Ogly and Anisimova,2005) . Our findings now show that changes in the water chemical composition during a 50-year period have not caused any significant changes in aquatic vegetation. There are occasional intensive phytoplankton blooms in Lake Kenon in periods of high-water (Ogly,2008) . At the same time,in 1966-2010 the changes mainly had to do with a subdominant complex (Tashlykova,2012) .
The spread of filamentous algae spread is influenced by change in vegetation groups about the foreshore,warm-water discharge and biogens from fish-breeding farms at the headrace. Change in vegetation groups has resulted in habitat decrease for genus Mougeotia,Oedogonium,Zygnema. An area with open warm moving water in the water basin promotes year-round mass growth of C. fracta. Then the alga moves to the whole basin. Fish-breeding farms at the headrace have been a source of pollution in Lake Kenon (Klishko,1998) and have led to Spirogyra sp2 ster. phytobiomass decrease (Kuklin,2014) .
A comparative analysis of many years of research data has allowed us to find an increase in Charophytes phytobiomass values. In 1976 the average Charophyte phytomass in the lake was 393 g/m2 (Vladimirova,1979) ,in 1986 it had grown to 598 g/m2 (Zolotareva,1998) ,and in 2011 it had reached 893 g/m2. At the same time,there was an expansion of the general lake overgrowth area: in the 1970s,it comprised 44%; in the 1980s,it had grown to 50%,and currently vegetation overgrowth comprises 70% of the lake area. Charophytes dominate in lake bottom vegetation cenosis.
Besides the thermal effect,vegetation increase has also been influenced by water-level rise as well as by the appearance of phytophagous fish. For example,in the 1960s the lake was completely overgrown,dominated by Charophytes P. crispus and M. sibiricum. In the 1970s,vegetation in the reservoir disappeared because of a 1-m water rise. At that time a P. crispus cenosis formed intensively,especially in the high-temperature zone. To control P. crispus,Ctenopharingodon idella Valenciennes in Cuvier and Valenciennes,1844 and Hypophthalmichthys nobilis (Richardson,1845) were introduced (Gurova et al.,1972) . In 1986-1991,the role of P. crispus was less important,as Batracium sp. M. sibiricum increased,Charophytes occurred at 5-7 m depth. Now,E. canadensis occupies the western and northern shores together with P. crispus and M. sibiricum groups.
The lake ichthyocenosis is most affected by the human activity. In 1919,P. fluviatilis,an aggressive predatory species was introduced into the lake and competed with E. reichertii and P. asotus (Gorlacheva et al.,2011) . Before the 1960s the Lake Kenon ichthyocenosis had been intensively exploited (600-800 kg of big perch,carp,pike,and dace in one ice fishery) (Karasev,1968) . By 1968,commercial fishing had been discontinued. Fish were caught only by sport fishermen,amounting to 35-40 tons per year (Karasev,1968) . The intensive use of fish in the water basin at that time ignored the considerable reduction in spawning areas. The construction of Combined Heat and Power Plant (CHP-1) used the Malyi Kenon gulf that was the main place of fish spawning. In spring,spawning areas along the Kadalinka and the Ingoda rivers disappeared because of pollution and water-shortage period. In the 1980s,the lake was characterized as a basin with predominance of P. fluviatilis and L. waleckii. Currently,there is a decrease in species diversity,fish kills and reduction in fish production from 55 kg/ha to 15.1 kg/ha in the lake ichthyocenosis. Fish capacity in the water basin is supported artificially by introduction of Cyprinus carpio larvae into the lake. Experiments in fish capacity growth using Coregonus autumnalis Pallas,1776,Coregonus lavaretus (Linnaeus,1758) ,Coregonus peled (Gmelin,1788) did not have any positive effect (Biological engineering,1983) . Species including M. mohoity,G. strigatus (Regan,1908) were also introduced with C. carpio larvae. In the current ichthyocenosis composition there has been a decrease in predatory species and an increase in the number of euryphagous fish. P. fluviatilis in the water basin becomes predatory in later age. At present the most part of the fish are benthophage,but the young fish,including predators at the earlier stages of life,feed on zooplankton.
4.5 Heavy metalsHeavy metals in ashes and slag can penetrate into living organisms’ tissues in water and accumulate in them in higher concentrations than in the water environment. Algae are very susceptible to the content of heavy metals (Fargasova,1999; Lamai et al.,2005; Arunakumara and Zhang,2009) and can be used to indicate the water quality of the basin. It was found that C. fracta in respect to E. canadensis and N. flexilis accumulates several times more: Zn (12 times) ,Fe (7.0) ,Cu (6.0) ,Cr (5.0) ,Co (2.6) ,Ni (2.3) ,Mn (1.5) (Kuklin,2014) . The highest concentrations of the heavy metals in C. fracta in 2012 were recorded in CHP-1 area (station 2) and were similar to the concentrations in water basins having chronic pollution (Kuklin,2014) . According to the 2014 data,there was increase in Pb,Hg,Cu,Zn and Sr concentrations in C. fracta. Comparison of different lake areas in 2014 showed the highest concentrations of chemical elements in C. fracta to have been at the northern foreshore of the lake (Table 4) .
In the CHP-1 area,there was growth only in Ca,Fe and Sr concentrations in E. canadensis,but a decrease in concentrations of other elements. Assessment of the heavy-metal content in E. canadensis from different lake areas shows the largest concentrations of the heavy metals to have been near CHP-1.
The results of fish investigations on the heavy-metal content show the biggest concentrations in muscle of P. fluviatilis,with Hg up to 0.69 μg/g and Zn from 98.3 μg/g in liver to 105.0 μg/g in bone. In the body of C. aerates,Zn measured 78.8 μg/g in muscles to 344 μg/g in liver,while Cu measured 22.3 μg/g in liver. Earlier (Tsybekmitova,1998) the predominant accumulation of Fe,Zn and Mn in organs and tissues of C. auratus (Fe and Zn) ,and P. fluviatilis (Zn and Mn) was found. As compared to the earlier research,we have found that there has been no increase in Hg concentration in P. fluviatilis muscles.
5 CONCLUSIONThe research carried out shows that the ecosystem change in the water reservoir used as a cooler of the Combined Heat and Power Plant (CHP-1) occurs not only under the influence of the plant’s heat. Changes in physical and chemical features of the habitat have an effect also on the structure and functional characteristics of the ecosystem. Composition changes are most marked in fish as the highest trophic link in the ecosystem. The most dangerous effect has been the accumulation of different metals in toxic concentrations in hydrobionts. Diverse anthropogenic impacts make it diffcult to differentiate distinctly between the effect of the natural fluctuations and changes caused by the influence of the CHP-1. Correct understanding of the ecosystem changes in varying climatic situation will allow us to forecast the consequences of the operation of new combined heat and power plants.
| Abdrakhmanov R F, Poleva A O, Shkundina F B, 2014. Hydrochemical and hydrobioloigical regime of the Pavlovskoe Reservoir. Water Resources, 41 (1) : 87 –96. Doi: 10.1134/S0097807814010023 |
| Alimov A F, 2003. Towards A Theory of the Functioning of Aquatic Ecosystems. Leiden: Backhuys Publishers. |
| Andrianova A V, Zavorueva V V, Zadelenov V A, Lopatin V N, Mikhaleva T V, Shchur L A, 2006. Assessment of the present-day state of cooling-basin ecosystem at the Berezovskaya state regional power plant, Krasnoyarsk Territory. Water Resources, 33 (2) : 176 –186. Doi: 10.1134/S0097807806020072 |
| Arunakumara K K I U, Zhang X C, 2009. Effects of heavy metals(Pb2+ and Cd2+)on the ultrastructure, growth and pigment contents of the unicellular cyanobacterium Synechocystis sp. PCC6803. Chinese Journal of Oceanology and Limnology, 27 (2) : 383 –388. Doi: 10.1007/s00343-009-9123-1 |
| Bazarova B B, Gorlacheva E P, Matafonov P V, 2013. Alien species of Lake Kenon(Transbaikal region). Russian Journal of Biological Invasions, 4 (1) : 12 –16. Doi: 10.1134/S2075111713010037 |
| Bazarova B B, 2012. Long-term changes in the vegetation of Lake Kenon. Izv Irkutsk Gos. Univ. Ser. Biology. Ecology, 4 (5) : 18 –23. |
| Bazarova B B, 2013. Elodea canadensis Michx. and Charocea from Lake Kenon(Zabaikalsky Krai). Russian Journal of Biological Invasions (3) : 7 –15. |
| Chugunova N I. 1959. Guide to Study of Fish Age and Size. Publishing House of the AS of USSR, Moscow. 64p.(in Russian) |
| Klishko O K, 1998. Ecology of Urban Reservoir. Publishing House of the SB RAS, Novosibirsk. 258p.(in Russian)Elkin M, Sukhikh S. 1978. Catfish Albino. Fishery and Fish Farming, 3 : 23 . |
| FAF. 2010. On approval of water quality standards in fishery water bodies, including those of the maximum permissible concentrations of harmful substances in the waters of fishery water bodies. Federal Agency for Fisheries. http://www.bestpravo.ru/federalnoje/hj-gosudarstvo/o5g.htm.(in Russian) |
| Fargasova A, 1999. The green alga Scenedesmus quadricauda-a subject for the study of inhibitory effects of Cd, Cu, Zn, Pb and Fe. Biologia, 54 (3) : 393 –398. |
| Gorlacheva E P, Afonin A V, 2008. A catch Parasilurus asotus(Linnaeus, 1758)-an albino in Lake Kenon. Fish ery and Fish farm ing, 9 : 24 –25. |
| Gorlacheva E P, Afonin A V, 2012. On the determination of the Manchu minnow Gnathopogon mantschuricus(Cypriniformes:Cyprinidae)in the Kenon Lake. Journal on Ichthyology, 52 (5) : 604 –606. |
| Kachaeva M I, 1976. Algae of bottom epibioses in the Ingoda River. Gidrobiol. Zh., 12 (5) : 68 –72. |
| Karasev G L, 1968. Linear, weight growth and fatness of fish of Lake Kenon. Bulletin of Chita State Pedagogical Institute, 19 : 96 –195. |
| Karasev G L. 1970. Ichthyofauna of Kenon Lake. In:Materials of the XXI and XXII scientific. conf. ChPGI. Chita. p.63-65.(in Russian) |
| Katanskaya V M. 1981. High Plants of Continental Reservoirs of USSR:Study Methods. Publishing House of AS of the USSR, Leningrad. 187p.(in Russian) |
| Korneeva G A, 1993. Evaluation of the functional state of the Black Sea waters in terms of hydrological enzymatic activities. Proceedings of the Russian Academy of Sciences. Biology Series, 6 : 909 –913. |
| Kuklin A P, 2014. Filamentous algae of Kenon Lake:variety, indication of water quality. Water:Chemistry and Ecology, 74 (8) : 49 –54. |
| Kuklin A P, Tsybekmitova G Ts, Gorlacheva E P, 2013. State of lake ecosystems in Onon-Torei plain in 1983-2011(Eastern Transbaikalia). Arid Ecosystems, 3 (3) : 122 –130. Doi: 10.1134/S2079096113030062 |
| Küpper H, Küpper F, Spiller M, 1996. Environmental relevance of heavy metal-substituted chlorophylls using the example of water plants. J. Exp. Bot, 47 : 259 –266. Doi: 10.1093/jxb/47.2.259 |
| Lamai C, Kruatrachue M, Pokethitiyook P, Upathamb E S, Soonthornsarathool V, 2005. Toxicity and accumulation of lead and cadmium in the filamentous green alga Cladophora fracta(O. F. Muller ex Vahl)kutzing:a laboratory study. Science Asia, 31 (2) : 121 –127. |
| Nikolsky G V. 1956. Fishes of Amur Basin. Publishing House of the AS of USSR, Moscow. 551p.(in Russian) |
| Obyazov V A, 2013. Long-term changes of river runoff in the Transbaikal region during wet and dry periods. Doklady Earth Sciences, 450 (2) : 676 –678. Doi: 10.1134/S1028334X13060196 |
| Ogly Z P, Anisimova E G. 2005. Water bloom in Lake Kenon(Transbaikalia). In:Proceedings of the 5th Russian scientificpractical conference. Chita, CSU. р.19-23.(in Russian) |
| Ogly Z P, 2008. On the problem of eutrophication of Lake Kenon. Water Management of Russia:Problems, Technology, Management, 2 : 93 –100. |
| Pravdin I F. 1966. A Guide to Fish Study. Food Industry, Moscow. 376p.(in Russian) |
| Semyonov A D. 1977. A Guide on Chemical Analysis of Surface Waters. Hidrometeoizdat, Leningrad. 541p.(in Russian) |
| Shishkin B A, Lokot' L I. 1973. Mode of nutrients and phytoplankton production of Lake Kenon. In:Limnological research in Trans-Baikal region. Scholarly notes of Trans-Baikal branch of the Geographical Society of the USSR. Chita. p. 28-48.(in Russian) |
| Taranets A Ya, 1937. Ichthyofauna of upper Amur and frontier areas of ingoda, selenga and vitima river basins. Vestn. Dal'nevost. Otd. Akad. Nauk USSR, 27 : 101 –123. |
| Tashlykova N A. 2012. Species composition and ecological and geographical characteristics of summer phytoplankton of the lake Kenon(Trans-Baikal region). In:Bioraznoobraziei Ustoichivoe Razvitie:Tezisy Dokladov II Mezhdunarodnoi Nauchno-Prakticheskoi Konferentsii. Krymskii Nauchnyi Tsentr NAN Ukrainy i MON Ukrainy, Simferopol. p.123-126.(in Russian) |
| Tashlykova N A, Afonina E Yu, Itigilova M Ts, 2013. Assessment of water quality of Lake Kenon by plankton state(Trans-Baikal region). Vestnik of Astrakhan State Technical University. Series Fishery, 1 : 100 –105. |
| Thermal Regime and Biology Lake Kenon. 1972. Notes of the Trans-Baikal branch of the Geographical Society SSSR, Chita. p.83.(in Russian) |
| Tsybekmitova G Ts. 1998. Biochemical parameters of some fish species. In:Ecology of the City Basin. Publishing House of the SB RAS, Novosibirsk. p.189-197.(in Russian) |
| Tsybekmitova G Ts, 2014. Content of biogenic elements(nitrogen and phosphorus)in the Lake Kenon-the cooling pond of thermal power station-1. International Journal of Applied and Fundamental Research, 7 : 39 –43. |
| Usmanova L I. 2012. Contemporary chemical and ecological status of Lake Kenon-the heat-sink of Chita CHP1. In:Geological Evolution of Water and Rocks Interaction. Publishing House "Scientific and Technical Literature", Tomsk. p.179-181.(in Russian) |
| Vladimirova Z F. 1979. Aquatic vegetation and its regulation in the water basin-cooler of Chita CHP(Lake Kenon). In:Nature Protection and Natural Resources Reproduction. Publishing House of the State Pedagogical Institute, Chita. p.113-114.(in Russian) |
| Zolotareva L N. 1998. Aquatic Vegetation and Its Growth Dynamics in the Kenon Lake(Eastern Transbaikalia, Extended Abstract of Cand. Sci. Biol.). Dissertation. BSC, SB RAS, Ulan-Ude. 19p.(in Russian) |
 2016, 34
2016, 34