Institute of Oceanology, Chinese Academy of Sciences
Article Information
- Yulan JIN(金玉兰), Yurong WANG(王玉荣), Lin XIAO(肖琳), Xiukun LIN(林秀坤)
- Characterization of proteases from Planomicrobium sp. L-2 isolated from the gastrointestinal tract of Octopus variabilis (Sasaki)
- Journal of Oceanology and Limnology, 34(3): 559-566
- http://dx.doi.org/10.1007/s00343-016-5040-2
Article History
- Received: Feb. 3, 2015
- Accepted: Mar. 27, 2015
2. Department of Pharmacology, Capital Medical University, Beijing 100069, China
Proteases are important industrial enzymes,accounting for approximately 40% of the total industrial enzyme market. They are used in the likes of detergents,food,pharmaceuticals,leather,diagnostics,waste management and silver recovery (Lata et al.,2014) . However,until recently,the largest share of the enzyme market has been held by detergent alkaline proteases,which are only active and stable in alkaline pH ranges (Gupta et al.,2002) . Proteases are derived mainly from animal,plant and microbial sources. Bacteria are the most important producers of large amounts of proteases with significant proteolytic activity and stability at high pH and temperature (Venugopal and Saramma,2007) . Alkaline proteases from several species of bacteria (e.g. Bacillus sp. HR-08,B. circulans BM15,B. sp. YaB,B. sp. EMB9,B. licheniformis RP1,B. sp. NG312,B. cereus,B. pumilus CBS and Nocardiopsis sp. (Shimogaki et al.,1991; Jasvir et al.,1999; Moreira et al.,2002;Banik and Prakash,2004; Venugopal and Saramma,2007; Jaouadi et al.,2008; Sellami-Kamoun et al.,2008; Moradian et al.,2009; Sinha et al.,2014) ) have been isolated and thoroughly characterized based on physiochemical and enzymatic properties. However,the appropriateness of the majority of these proteases as additives in detergent is limited by their stability and activity in oxidants and surfactants,both common detergent ingredients. Therefore,it is necessary to identify proteases from new sources that have high thermal stability,alkaline activity and are more compatible with washing systems.
Octopus variabilis (Sasaki,1929) is an active predator that feeds primarily on gastropod and bivalve mollusks,and crustaceans like crabs and crayfish. This species is found throughout tropical and semitropical waters,from coastal shallows to depths of 200 m. It is an abundant species along the northern and southern coasts of China,especially in the Bohai and Huanghai seascank (Zhong et al.,2009) . In our previous study,a protease,80.5 kDa in molecular weight,was purified from the digestive tract of this octopus species; it manifested relatively high activity in the pH range of 7.0-9.0 and temperatures of 55-60°C (Ren et al.,2012) . In this current study,Planomicrobium sp. L-2 was isolated from the gastrointestinal tract of this same octopus species using a casein medium. The crude proteinases from Planomicrobium sp. L-2 manifest relatively thermostable and solvent-stable characteristics,which both show promise for industrial detergent formulation applications.
2 MATERIAL AND METHOD 2.1 Bacterial strains,media and culture conditionsPlanomicrobium sp. L-2 (GenBank accession number KF358213) was isolated from intestines of O. variabilis which collected from Jiaozhou gulf wildly,purchased from the aquatic product market of Qingdao,People’s Republic of China. Planomicrobium sp. L-2 was cultured in a fermentation medium (100 mL seawater,0.5 g peptone,0.5 g starch soluble,0.1g yeast extract,and 0.01 g FePO4·4H2O) at 25°C in a shaker (180 r/min) .
2.2 Preparation of crude extracellular proteasePlanomicrobium sp. L-2 was cultured in a fermentation medium at 25°C for 3 d in a shaker (180 r/min) . The bacterial culture was centrifuged at 8 000×g for 15 min at 4°C. The supernatant was fractional precipitated by ammonium sulfate with a final saturation of 80%,and the mixture was centrifuged at 6 000×g for 15 min at 4°C. The precipitation was dialyzed against 0.01 mol/L Tris-HCl (pH 7.6) with four changes in 24 h (Moreira et al.,2002) . The dialyzed sample was used as the crude protease for characterization and its application in detergent formulation experiments.
2.3 Protease activity assayProteolytic activities in the crude extract were measured using 1% azocasein as substrate with a slight modification from that method described by Temiz et al. (2013) . Enzyme extract (50 μL) was mixed with 1% azocasein (50 μL) in 0.02 mol/L pH8.0 phosphate buffer saline (PBS) ,and incubated for 60 min at 40°C. The reaction was stopped by adding of 10% trichloroacetic acid (300 μL) . The mixture was then centrifuged at 12 000×g for 5 min. The supernatant (100 μL) was mixed with 1 mol/L NaOH (100 μL) and the mixture absorbance was measured at 450 nm. One unit (U) of protease activity was defined as an increase of 0.001 in absorbance at 450 nm per minute. Protein concentration was determined by referring to bovine serum albumin (BSA) standard in accordance with Bradford (1976) . Specific activity was expressed as the amount of unit activity per milligram of protein.
2.4 Polyacrylamide gel electrophoresis and zymogramSodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) was performed as described by Ktari et al. (2014) using 5% (w/v) stacking and 10% (w/v) separating gels (2% casein) . Samples were mixed at 4:1 (v/v) ratio with the SDS-PAGE sample buffer (1 mol/L Tris-HCl,pH 6.8; 10% SDS; 25% glycerol; 5% β-mercaptoethanol; 0.1% bromophenol blue) . Samples were not heated before electrophoresis. After electrophoresis,the gel was submerged in 2.5% Triton X-100,and shaken for 60 min to remove SDS and allow enzyme renaturation. The gel was then immersed in buffer A (0.1 mol/L Tris-HCl buffer (pH 8.0) ) for 120 min at 40°C. Finally,the gel was stained with 0.1% Coomassie brilliant blue G-250 in 45% ethanol and 10% acetic acid,and destained with 10% methanol and 10% acetic acid. The development of clear bands on the blue background of the gel indicated the presence of protease activity.
2.5 Effects of pH and temperature on protease activityProtease activity was measured at different pH values under standard assay conditions with azocasein as a substrate. The activities of proteases at pH 4.5- 11.0 were assayed with 0.1 mol/L acetate buffer (pH 4.5-7.0) ,0.1 mol/L Tris-HCl buffer (pH 7.0-9.0) and 0.1 mol/L glycine-NaOH buffer (pH 9.0-11.0) for 60 min at 40°C. At various temperatures (10-80°C) ,at pH 7.8,the protease activity was also assayed to examine the optimum temperature for its activity.
2.6 Effects of pH and temperature on protease stabilityThe enzyme was incubated in buffers of different pH values (pH 4.5-11.0,0.1 mol/L buffers as above) at 4°C for 30 min. Protease activity was measured at 40°C,using 0.1 mol/L Tris-HCl buffer,pH 7.8. Thermal stability was examined by incubating the enzyme preparation for 100 min at different temperatures from 40 to 60°C. Aliquots were withdrawn at desired time intervals to test the remaining activity at pH 7.8 towards azocasein 1% (w/v) as substrate at 40°C. The unheated crude enzyme was taken as 100%.
2.7 Effects of chemical reagents on the crude proteaseThe effects of enzyme inhibitors on protease activity were studied using phenyl methyl sulfonyl fluoride (PMSF) ,soybean trypsin inhibitor (SBTI) ,β-mercaptoethanol,pepstatin A,ethylene-diamine tetraacetic acid (EDTA) and various metal ions. The crude protease was preincubated with each chemical reagent for 30 min at 25°C,and then the remaining protease activity was tested using casein as a substrate. The activity of the protease assayed in the absence of inhibitors was taken as control.
2.8 Effects of oxidizing agent and surfactants on proteaseThe suitability of the crude protease as a detergent additive was determined by testing its stability towards some surfactants (Triton X-100,Tween-80 and SDS) and oxidizing agents (H2O2) . The enzyme preparation (200 μL) was incubated with 200 μL different concentrations of surfactants (0.05%,0.1%,0.5%,1% and 5%) for 30 min,and 60 min at 40°C,respectively,and then the residual proteolytic activities were measured under standard assay conditions. The hydrogen peroxide stability of the crude protease was assayed by incubating samples (300 μL) with H2O2 (300 μL) at different concentrations (1%,5%,10% and 15%) at 40°C. Aliquots were withdrawn at desired time intervals to test the activity at pH 7.8 and using azocasein 1% (w/v) as substrate at 40°C. The activity of the crude protease,incubated under similar conditions without any additives,was taken as 100%.
2.9 Commercial detergent compatibilityThe compatibility of the crude protease extract with liquid laundry detergents was studied using commercially available detergents: Dixan (Henkel-Spain) ,Nadhif (Henkel-Alki-Tunisia) ,Lav+ (STID-Tunisia) ,Carrefour (U.E/Geproduceerd-France) and Tex’til (U.E/Vervaardigd-Belgium) . Commercial detergents were diluted with tap water to a final concentration of 7 mg/mL to simulate washing conditions (Espósito et al.,2009) . The enzymes contained in detergents were inactivated by heating the diluted detergents for 1 h at 65°C prior to the addition of the crude protease. The crude protease at a concentration of 897 U/mL was incubated with different detergents for 1 h at 40°C,and then the remaining activities were determined under the standard assay conditions. The enzyme activity of a control,without detergent,incubated under the similar conditions,was taken as 100%.
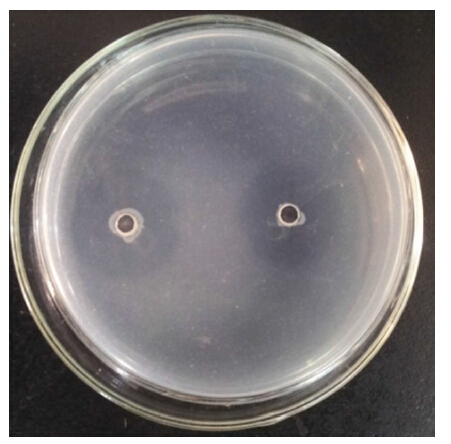
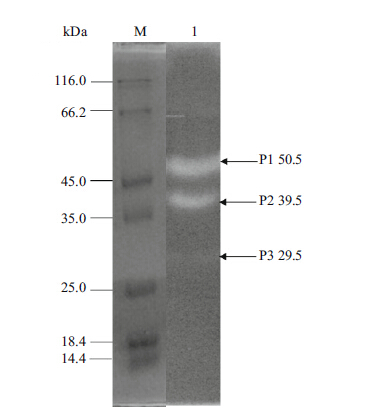
3 RESULT 3.1 Zymography of crude protease extractPlanomicrobium sp. L-2 demonstrated a prominent hydrolyzed clearing zone of 2.58-cm diameter on 1% casein agar plates (Fig. 1) . To estimate the number of proteases in the crude enzyme extract,the sample was separated by SDS-PAGE and then proteolytic activity was evaluated by casein zymogram activity staining. As illustrated in Fig. 2,the crude enzyme extract showed at least three major clear bands of protease activity with molecular weights of 50.1,39.5 and 29.5 kDa.
|
| Figure 1 Hydrolyzed clearing zone of Planomicrobium sp.L-2 |
|
| Figure 2 Zymogram of the crude protease produced from Planomicrobium sp. L-2 |
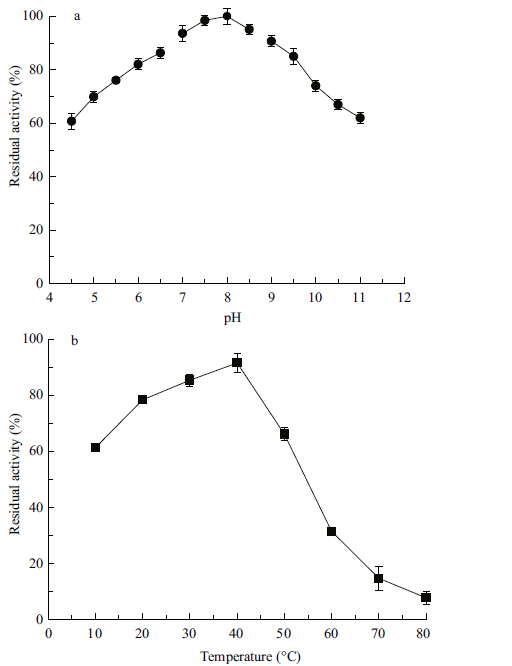
The relative activity values at certain pH values between 4.5 and 11.0 are presented in Fig. 3a. The enzyme preparation was highly active between pH 7.0 and 9.0 with an optimum at pH 8.0 when incubated for 60 min at 40°C. The relative activities at pH 9.0 and 10.0 were about 90.9% and 75.0%,respectively,suggesting these enzymes are highly active at high pH conditions.
|
| Figure 3 pH (a) and temperature (b) profiles of crude proteases produced from Planomicrobium sp. L-2 |
The effect of temperature on protease activity was determined by checking enzyme activity at different temperatures (Fig. 3b) . The crude protease produced from Planomicrobium sp. L-2 was incubated with the substrate at temperatures ranging 10-80°C. Results revealed the optimum temperature of the crude enzyme was between 30°C and 40°C. The relative activities of the crude enzyme were about 61% and 78% at 10°C and 20°C respectively,though an appreciable decrease in enzyme activity occurred above 40°C,a decrease we attribute to thermal denaturation. At 80°C,the relative activity was only 8% of that of 40°C.
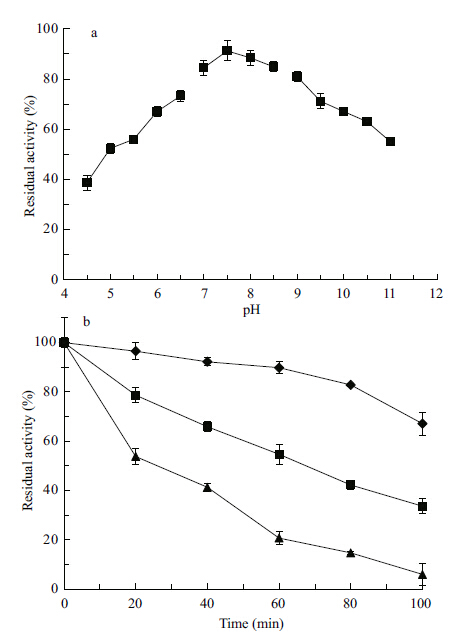
3.3 Effects of pH and temperature on protease stabilityThe thermal and pH stabilities of crude protease from Planomicrobium sp. L-2 are shown in Fig. 4a and b respectively. The enzymes are highly stable between pH 7.0 and 9.0,retaining more than 80% of their original activity after incubation for 30 min at 4°C. Residual activities at pH 10.0 and 11.0 were about 67% and 55% respectively. The crude protease from Planomicrobium sp. L-2 remained thermally stable,retaining about 66% and 55% of its activity after being incubated at 50°C for 40 min and 60 min,respectively. About 92% of the original activity was retained after incubation at 40°C for 40 min,and 90% after 60 min.
|
| Figure 4 pH (a) and thermal (b) stability of crude protease produced from Planomicrobium sp. L-2 |
The effects of various enzyme inhibitors,such as chelating agents,group-specific reagents and various metal ions,on enzyme activity are presented in Table 1. The proteases were active without NaCl; however,their activities were not influenced by 500 mmol/L NaCl. PMSF (2 mmol/L) ,a serine enzyme inhibitor,strongly influenced crude protease proteolytic activity,resulting in 79% of original activity being lost,indicating the existence of serine protease,in particular trypsin and chymotrypsin (Ktari et al.,2014) . Antinutritional factors (SBTI) also decreased proteolytic activity by considerably,by 64%,indicating these proteases were not suitable for hydrolysis of soybean protein. In contrast,β-mercaptoethanol (5 mmol/L) significantly enhanced enzyme activity by 48%,suggesting the presence of thiol-dependent serine proteases. EDTA (2 mmol/L) and pepstatin A had no effect on protease activity. The enzyme preparations proved sensitive to ions,mainly Cu2+,Zn2+,Fe3+ and Fe2+.
A good protease when applied in detergent should be stable in the presence of surfactants and oxidizing agents. To determine the effect of detergent on protease activity,we checked for protease activity in the presence of different kinds of detergents. As shown in Fig. 5,protease from Planomicrobium sp.L-2 proved to be highly stable in or even stimulated by the presence of surfactants,including Tween 80,Triton-X-100 and SDS; retaining 108% of its initial activity in the presence of 5% Tween 80 and the activity increased more than 50% when treated with 5% Triton-X-100. Additionally,the crude protease was relatively stable in the presence of SDS,retaining 89% and 77% of its initial activity in the presence of 0.05% and 0.1% SDS,respectively,after 30 min of incubation. SDS usually denatures the protein native structure (Ghosh,2008) . We also investigated the effects of oxidizing agents (H2O2) on the enzymatic activity of the crude enzyme extract. The activity of proteolytic enzymes was little influenced by H2O2,retaining about 90% and 70% of its activity in the presence of 1% and 5% H2O2,respectively,when incubated for 1 h at 40°C (Fig. 6) . Our results confirm the possibility that the protease from Planomicrobium sp. L-2 would be valuable as additive components in detergents.
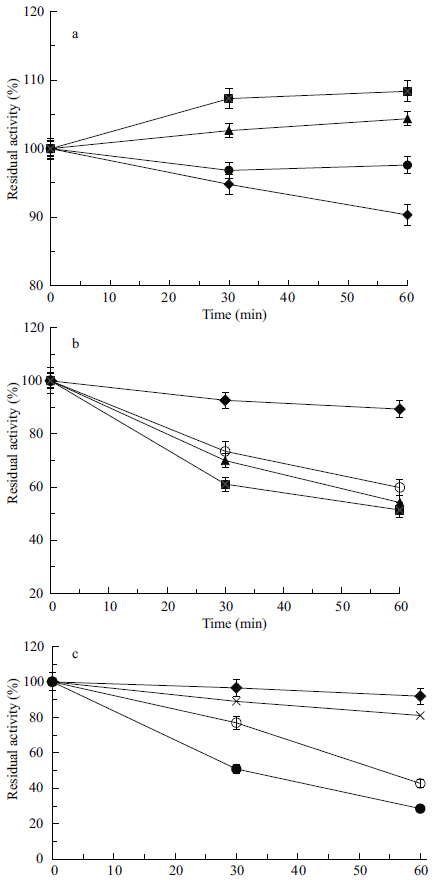
|
| Figure 5 Effect of various concentrations [control, 0% (♦) ,0.05 % (×) , 0.1 % (○) , 0.5% (●) , 1.0% (▲) , and 5.0% (■) ] of Tween-80 (a) , Triton X-100 (b) and SDS (c) on protease activity of Planomicrobium sp. L-2 at 40°C |
|
| Figure 6 Peroxide inactivation curve of proteases from Planomicrobium sp. L-2 in presence of varying concentrations [control,0% (♦) ,0.5% (●) ,1.0% (▲) ,and 5.0% (■) ] of H2O2 at 40°C |
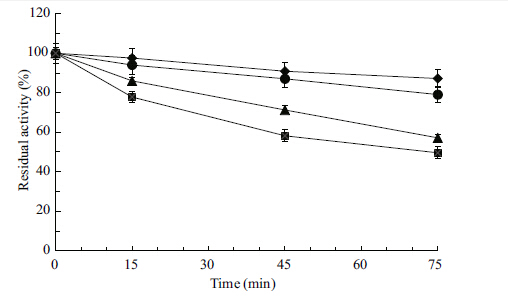
An ideal detergent enzyme should remain stable and active in the detergent solution for a long period of time. Currently Tide®,Eagle Brand® and Liby® are the most popular brands of powdered detergent in China. To appraise the compatibility of the protease with commercial laundry detergents,the crude enzyme was pre-incubated in the presence of certain commercial laundry detergents for 1 h at 40°C. The data presented in Fig. 7 demonstrate the alkaline protease to be relatively stable in the presence of select detergents,retaining more than 50% of its activity in the presence of Tide® and Eagle Brand®,and more than 45% of its activity with Liby®. The greatest stability was experienced in the Tide® treatment,with the enzyme retaining 53% of its activity after incubation with detergent at 40°C for 1 h. These results further support our contention that these kinds of proteases might be highly effective as detergent additives.

|
| Figure 7 Stability of crude protease in various commercial detergents [control (♦) , Tide® (●) , Eagle Brand® (▲) ,and Liby® (■) ] |
In the present study,proteases from Planomicrobium sp. L-2 are recognized to be highly tolerant of a range of temperature and high pH conditions,in addition to greater stability in the presence of some detergents. In our previous study,a protease of molecular weight 80.5 kDa was isolated from the intestine of O. vulgaris (Ren et al.,2012) ,but what we didn’t know then was whether this enzyme was secreted by O. variabilis or whether it is produced by some bacterium. Herein we report three kinds of protease with molecular weights of 50.1,39.5 and 29.5 kDa from the gastrointestinal tract of O. variabilis,suggesting bacterial strains parasitizing this tract may be rich sources of novel and valuable proteases. Additionally,the diversity of proteases suggests that enzymes in Planomicrobium sp. L-2 may play important roles in protein degradation in the gastrointestinal tract of O. variabilis.
We demonstrate crude proteases from the gastrointestinal tract of O. vulgaris to be highly tolerant of temperature,with an optimum activity at 40°C and pH 8.0,though temperature tolerance is somewhat lower than that earlier reported from the O. vulgaris intestine (Ren et al.,2012) . Many other temperature-tolerant proteases have been isolated from other species,including marine yeast Metschnikowia reukaufii W6b (Li et al.,2010) . Another interesting characteristic of the enzymes from Planomicrobium sp. L-2 is that they demonstrate high stability at high pH,an alkaline tolerance that renders them of potential use for industrial applications. However,because our results have been obtained from crude enzymes only,additional research is required to identify differences in the enzymatic activity of these proteases,as well as the diversity of their amino acid sequences.
We also demonstrate the proteolytic enzymes found in the Planomicrobium sp. L-2 to be extremely stable in the presence of two surfactants (5% Tween 80,1% Triton X-100 and 0.05% SDS) and an oxidizing agent (1% hydrogen peroxide) . In this regard our results are similar to those of proteases from Zebra blenny (Salaria basilisca) viscera,already demonstrated to be highly stable in the presence of 5% Tween-80 and relatively effective in the deproteinization of shrimp waste powder (Ktari et al.,2014) . The performance of Planomicrobium sp. L-2 protease with 0.5% SDS was consistent with the protease from pyloric caeca and intestines of black pacu,Colossoma macropomum (Espósito et al.,2009) . That the activity of the crude protease increases significantly in the presence of Tween-80 is unusual,given the activities of most micro-organism-sourced proteases are inhibited by it; treatment of the protease from the bacterium Nocardiopsis sp. with 1% Tween-80 results in significant loss of the enzymatic activity (Moreira et al.,2002) . The mechanism underlying the increased activity in the presence of the detergent is unknown. It is possible that some detergents can keep the 3D structure of proteases more stable.
5 CONCLUSIONWe extract and characterize proteases from Planomicrobium sp. L-2 isolated from the gastrointestinal tract of an octopus,O. variabilis. We also evaluate their appropriateness as potential detergent additives. Proteases were most active at pH 8.0 and 40°C. They are identified based on molecular weight by zymography of crude protease extract. Crude proteases were active throughout a pH range of 7.0-9.0,and were highly stable in the presence of an oxidizing agent and various laundry liquid detergents. Three separate proteases with different molecular-weight were isolated from Planomicrobium sp. L-2.
Our results suggest proteases from Planomicrobium sp. L-2 could be used as commercial detergent additives,however,additional modification and reconstruction are required before they can be used as detergent additives. Additional research is also necessary to isolate each of the proteases and to identify their properties as potential biotechnological tools in detergent-processing industries. Moreover,fermentation conditions for these strains need to be studied to enable production of appropriate quantities of enzyme to meet industrial demands.
6 ACKNOWLEDGEMENTThe authors are grateful to all members of the laboratory for their continuous technical advice and helpful discussion.
| Banik R M, Prakash M, 2004. Laundry detergent compatibility of the alkaline protease from Bacillus cereus. Microbiol. Res., 159 (2) : 135 –140. Doi: 10.1016/j.micres.2004.01.002 |
| Bradford M M, 1976. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem., 72 (1-2) : 248 –254. Doi: 10.1016/0003-2697(76)90527-3 |
| Espósito T S, Amaral I P G, Buarque D S, Oliveira G B, Carvalho L B Jr, Bezerra R S, 2009. Fish processing waste as a source of alkaline proteases for laundry detergent. Food Chem., 112 (1) : 125 –130. Doi: 10.1016/j.foodchem.2008.05.049 |
| Ghosh S, 2008. Interaction of trypsin with sodium dodecyl sulfate in aqueous medium:a conformational view. Colloids Surf., 66 (2) : 178 –186. Doi: 10.1016/j.colsurfb.2008.06.011 |
| Gupta R, Beg Q, Lorenz P, 2002. Bacterial alkaline proteases:molecular approaches and industrial applications. Appl. Microbiol. Biotechnol., 59 (1) : 15 –32. Doi: 10.1007/s00253-002-0975-y |
| Jaouadi B, Ellouz-Chaabouni S, Rhimi M, Bejar S, 2008. Biochemical and molecular characterization of a detergent-stable serine alkaline protease from Bacillus pumilus CBS with high catalytic efficiency. Biochimie., 90 (9) : 1291 –1305. Doi: 10.1016/j.biochi.2008.03.004 |
| Jasvir S, Gill N, Devasahayam G, Sahoo D K, 1999. Studies on alkaline protease produced by Bacillus sp. NG312. App. Biochem. Biotechnol., 76 (1) : 57 –63. Doi: 10.1385/ABAB:76:1 |
| Ktari N, Khaled H B, Younes I, Bkhairia I, Mhamdi S, Hamza I, Nasri M, 2014. Zebra blenny(Salaria basilisca)viscera as a source of solvent-stable proteases:characteristics, potential application in the deproteinization of shrimp wastes and evaluation in liquid laundry commercial detergents. J. Food Sci. Technol., 51 (11) : 3094 –3103. Doi: 10.1007/s13197-012-0817-6 |
| Lata C, Negi D S, Mehrotra V, 2014. Potential of microbial proteases. Indian Streams Res. J., 4 (2) . |
| Li J, Peng Y, Wang X H, Chi Z M, 2010. Optimum production and characterization of an acid protease from marine yeast Metschnikowia reukaufii W6b. J. Ocean Uni v. China., 9 (4) : 359 –364. Doi: 10.1007/s11802-010-1765-2 |
| Moradian F, Khajeh K, Naderi-Manesh H, Sadeghizadeh M, 2009. Isolation, purification and characterization of a surfactants-, laundry detergents- and organic solventsresistant alkaline protease from Bacillus sp. HR-08. Appl. Biochem. Biotechnol., 159 (1) : 33 –45. Doi: 10.1007/s12010-008-8402-1 |
| Moreira K A, Albuquerque B F, Teixeira M F S, Porto A L F, Lima F J L, 2002. Application of protease from Nocardiopsis sp. as a laundry detergent additive. World J. Microbiol. Biotechnol., 18 (4) : 307 –312. |
| Ren P, Wang Y, Jin Y L, Piao M Z, 2012. Extraction, purification and characterization of protease from digestive tract of Octopus vulgaris. Food Sci., 33 (7) : 168 –171. |
| Sellami-Kamoun A, Haddar A, Ali N E-H, Ghorbel-Frikha B, Kanoun S, Nasri M, 2008. Stability of thermostable alkaline protease from Bacillus licheniformis RP1 in commercial solid laundry detergent formulations. Microbiol. Res., 163 (3) : 299 –306. Doi: 10.1016/j.micres.2006.06.001 |
| Shimogaki H, Takeuchi K, Nishino T, Odhera M, Kudo T, Ohba K, Iwama M, Irie M, 1991. Purification and properties of a novel surface-active agent- and alkalineresistant protease from Bacillus sp. Y. Agric. Biol. Chem., 55 (9) : 2251 –2258. |
| Sinha R, Srivastava A K, Khare S K, 2014. Efficient proteolysis and application of an alkaline protease from halophilic Bacillus sp. EMB9. Prep. Biochem. Biotechnol., 44 (7) : 680 –696. Doi: 10.1080/10826068.2013.844711 |
| Temiz H, Ustun N S, Turhan S, Aykut U, 2013. Partial purification and characterization of alkaline proteases from the Black Sea anchovy(Engraulis encrasicholus)digestive tract. Afr. J. Biotechnol., 12 (1) : 56 –63. Doi: 10.5897/AJB |
| Venugopal M, Saramma A V, 2007. An alkaline protease from Bacillus circulans BM15, newly isolated from a mangrove station:characterization and application in laundry detergent formulations. Indian J. Microbiol., 47 (4) : 298 –303. Doi: 10.1007/s12088-007-0055-1 |
 2016, 34
2016, 34



