Institute of Oceanology, Chinese Academy of Sciences
Article Information
- Jianing LIN(林佳宁), Tian YAN(颜天), Qingchun ZHANG(张清春), Mingjiang ZHOU(周名江)
- Impact of several harmful algal bloom (HAB) causing species, on life history characteristics of rotifer Brachionus plicatilis Müller
- Journal of Oceanology and Limnology, 34(4): 642-653
- http://dx.doi.org/10.1007/s00343-016-5065-6
Article History
- Received: Mar. 3, 2015
- Accepted: Jun. 24, 2015
2. University of Chinese Academy of Sciences, Beijing 100049, China;
3. Laboratory of Riverine Ecological Conservation and Technology, Chinese Research Academy of Environmental Sciences, Beijing 100012, China
In recent years, large-scale harmful algal blooms (HABs) have occurred frequently along the coast of China. These HABs have affected large areas (>1 000-10 000 km 2) for long periods of time (>20 d) , in the areas adjacent to the Changjiang River estuary in the East China Sea almost every spring since the 1990s (Zhou et al., 2003; Lu et al., 2005) . Marine HABs have been gradually exhibiting succession from diatom- to dinoflagellate-dominated blooms in these areas. During the 1980s and 1990s, the dominant species was the diatom Skeletonema costatum sensu lato, which reached a density of 10 5 cells/mL, accounted for 95%-99% of the total biomass of phytoplankton (Zhou et al., 2008) , and supported a high production fishery in the adjacent coastal waters. By the early 2000s, the dominant HAB species were dinoflagellates, including Prorocentrum donghaiense Lu, Karenia mikimotoi (Miyake et Kominami ex Oda) G. Hansen et Ø. Moestrup, and Alexandrium catenella (Whedon et Kofoid) Balech (Zhou, 2010) . Zhou et al. (2003) reported that maximum densities of the dinoflagellates P. donghaiense and A. catenella reached 10 5 and 10 2 cells/mL, respectively. Other toxic species such as Prorocentrum lima (Ehrenberg) Dodge, and Karlodinium veneficum (D. Ballantine) J. Larsen have also been found (Wang et al., 2011; Zhou et al., 2011) . Therefore, it is important to assess and compare the toxicities of different kinds of HABs.
Marine HAB species have been reported to adversely affect components of the marine food web, such as zooplankton, shellfish, fish, marine mammals and benthic crabs (Landsberg, 2002) . Zooplankton species, which are responsible for the exchange of materials and energy in marine ecosystems, show undesirable responses when exposed to harmful algae. Reduced survival and feeding rates, inhibition of growth and reproduction, changes in behavior, and abnormalities in embryonic and larval development have been reported (Huntley et al., 1987; Hansen, 1989; Poulet et al., 1995; Yan et al., 2009; Ianora and Miralto, 2010) .
The rotifer Brachionus plicatilis Müller, which is a polyphagous microplanktonic filter feeder characterized by rapid growth, a high reproductive rate and short generation time, has been used extensively in aquaculture and ecotoxicology (Snell et al., 1983; Snell and Janssen, 1995; Kostopoulou et al., 2012) . Previous studies demonstrate that the rotifer B . plicatilis displays inhibited swimming activity, low ingestion rates and reduced individual survival when exposed to many dinoflagellate species including: K . mikimotoi, most species of Alexandrium, Heterocapsa circularisquama, Heterosigma akashiwo, P. donghaiense and Prorocentrum micans (Kim et al., 2000; Wang et al., 2005; Xie et al., 2008; Yan et al., 2009; Zou et al., 2010; Zhang and Geng, 2012) . Nevertheless, relatively little is known about the effects of diatom and dinoflagellate blooms on rotifers.
A life table can be used to derive variables such as the duration of development, average lifespan (ML) , gross and net reproductive rates (R 0) , generation time (T) and population growth rates (Krebs, 1985) . These life history variables may be sensitive to heavy metals, pesticides and other environmental contaminants (Marcial and Hagiwara, 2007; Zha et al., 2007; Zhao et al., 2008; Huang et al., 2012) . In this study, the effects of different harmful diatom and dinoflagellate blooms on the life history characteristics of rotifers were examined.
We evaluated the effects of five different HAB species, including a diatom strain (S . costatum) and four dinoflagellate strains (P. donghaiense (non-toxin producer) , A. catenella (paralytic shellfish poison (PSP) producer) , P. lima (diarrhetic shellfish poison (DSP) producer) , and K. veneficum (karlotoxin producer) ) on the life history parameters of the rotifer, B . plicatilis . The objectives of this study were twofold: 1) to test and compare the toxicities of different diatom and dinoflagellate HABs on the life history characteristics of the rotifer B . plicatilis and 2) to discuss potential causes of adverse effects of these HAB species on B . plicatilis . The results will help to identify potential threats to the marine ecosystem posed by diatom and dinoflagellate HABs.
2 MATERIAL AND METHOD 2.1 Strains and culture conditionsThe diatom Skeletonema costatum was isolated from Jiaozhou Bay in the Yellow Sea of China. The dinoflagellates P. donghaiense, A. catenella and K . veneficum were isolated from the East China Sea and provided by two National Basic Research Priority Programs of China (CEOHAB-I, II) . Prorocentrum lima (strain CCMP1966) was obtained from the Provasoli-Guillard National Center foR marine Algae and Microbiota (NCMA, formerly the CCMP) (East Boothbay, ME, USA) . Chlorella sp. served as the control algae and was added to the mixed diet used in every treatment. Chlorella sp. was provided by the Algal Culture Center of the Institute of Oceanology, Chinese Academy of Sciences (IOCAS) . All algae, except for S . costatum, were cultured in modified f/2 medium in flasks without added silicate. All algae were cultured at 20±1°C with irradiance at 56 μE/ (m 2 ∙s) and a 12 h light:12 h dark photoperiod. The rotifer B . plicatilis was supplied by IOCAS and cultured under the same conditions as the algae throughout the year.
The natural seawater used in this study was pumped from Taipingjiao (a clean site with no known pollution history) at Qingdao and sand filtered prior to use in the laboratory. Prior to the experiments, the seawater was filtered through a 0.45 μm pore size cellulose nitrate membrane, and treated by boiling and air saturation. Salinity was adjusted to 31±1 using distilled water as determined using an ATAGO handheld refractometer. The pH of seawater was measured using a HI991000 pH instrument.
2.2 Experimental design 2.2.1 MethodThe density gradients for each algal species in the experiment were based on the bloom density measured in the field (S . costatum and P. donghaiense reached 105 cells/mL (Zhou et al., 2003, 2008) ; K. veneficum reached more than 10 3 cells/mL (Wang et al., 2011) ; and A. catenella and P. lima reached more than 10 2 cells/mL (Wang et al., 1998; Zhou et al., 2003) ) . Algal cells of the five HAB species were harvested at the exponential phase. A 1-mL subsample was taken and algal cells were counted under the microscope after fixation in Lugol’s solution. Based on this cell count, the algae were diluted to the densities shown in Table 1. Chlorella sp. (density of 2.0×10 6 cells/mL) was added into each diatom and dinoflagellate treatment to produce a mixed diet. We also measured the carbon content of the used algae in lab, except for K . veneficum (Table 1) . The densities of HAB species used in the experiment were based on the densities of algal blooms recorded in the field, therefore, the carbon levels of each species differed (P. donghaiense > P . lima > K. veneficum > S . costatum > A. catenella) .
Brachionus plicatilis neonates were hatched from gravid females for use in the experiment. Hatching took place in 96-well tissue culture plates, with one gravid rotifer in each well. Each well contained 100μL of fresh seawater and no food. Healthy and active neonates (<2 h old) were collected and placed in 24-well tissue culture plates, with one neonate per well. Each well contained 1.0 mL of test solution, which consisted of a mixed diet (one of the HAB species and Chlo rella sp. at a density of 2.0×10 6 cells/ mL) . The control solution contained only Chlorella sp. (2.0×10 6 cells/mL) . Every treatment consisted of three replicates, with thirty neonates in total. The hatching and culture of neonates was conducted at 22±1°C with irradiance at 56 μE/ (m 2 ∙s) and a 12 h light:12 h dark photoperiod.
The rotifers in the 24-well tissue culture plates were checked every 4-6 h, and the following values were recorded: time at which the first egg and neonate were produced, time at which the last egg was produced, the number of eggs and neonates produced, and the number of original test individuals that remained alive. Neonates were removed every 8 h. The original rotifers that were still alive were transferred into a freshly prepared test solution every 24h. The life table experiments were performed in darkness at 22±1°C, salinity 31±1, and pH 8.0±0.2, until each individual of every cohort died. Salinity was checked every 24 h and kept at 31±1 by adding appropriate amounts of diluted water.
2.2.2 Calculation of life history parametersUsing the data recorded in this experiment, the duration of the principal developmental periods (embryonic development (ED) , juvenile period (JP) , reproductive period (RP) , and post-reproductive period (PP) ) , mean life span (ML, ML=the sum of JP, RP and PP) , and lifetime egg production (NE) of the rotifers were calculated. Age-specific survivorship (l x, l x =proportion of survivorship per day) and fecundity (m x, m x =proportion of offspring produced per female per day) were determined for each replicate using conventional life table techniques (Poole, 1974) . Net reproductive rate (R 0, R 0 =Σ l x m x) , generation time (T, T =Σ l x m x x / R 0) , intrinsic rate of population increase (R m, R m =ln R 0 / T) , and finite rate of increase (λ, λ =anti log e R m) were calculated (Birch, 1948; Krebs, 1985; Sarma et al., 2001) .
2.3 AnalysisData were analyzed using Excel 2003, Origin 8.5 and SPSS 16.0 software packages. As the concentrations of algae used in the experiment differed among species, the data could not be analyzed using a two-way analysis of variance (ANOVA) with algal species and algal density as factors. Therefore, one-way ANOVA was applied for statistical evaluations. Prior to statistical analysis, all data were tested for normality and homogeneity (SPSS 16.0) . Mean differences were considered to be significant at the 0.05 level. If the overall ANOVA results were significant, a Fisher’s least significance difference (LSD) post-hoc test was performed to test among experimental combinations.
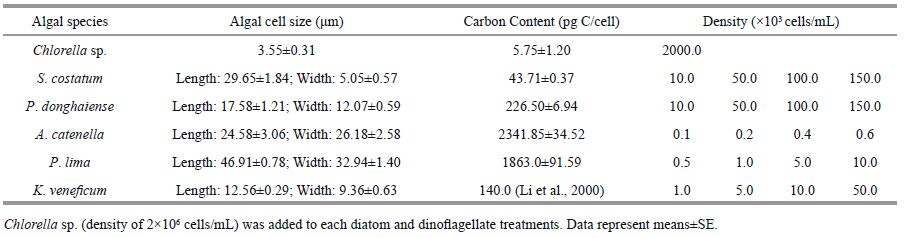
3 RESULT 3.1 Duration of developmental periods, mean life span, and lifetime egg productionThe duration of the principal developmental periods (embryonic development (ED) , juvenile period (JP) , reproductive period (RP) , and postreproductive period (PP) ) of B . plicatilis exposed to different algal densities are shown in Fig. 1. Most algal species including S . costatum, A. catenella, P . lima, and K. veneficum had no marked impact on the duration of ED. However, exposure to P. donghaiense (>1×10 5 cells/mL) significantly prolonged the duration of ED (P<0.05) , which increased with increasing algal cell density.
|
| Figure 1 Durations of embryonic development (ED) , juvenile period (JP) , reproductive period (RP) , and post-reproductive period (PP) of Brachionus plicatilis exposed to diff erent algal densities |
Rotifers did not exhibit a significantly prolonged JP (P >0.05) when exposed to S . costatum and P. donghaiense at densities ranging from 10 4 to 1.5×105 cells/mL. However when exposed to A. catenella (>400 cells/mL) , P. lima (>500 cells/mL) , and K. veneficum (>5×10 3 cells/mL) , the duration of the juvenile period was significantly prolonged (P<0.05) .
Skeletonema costatum had no significant impact on the duration of the reproductive period (RP) of rotifers (P >0.05) . Compared with the control group, an increased RP was observed in rotifers exposed to A. catenella at low densities (100, 200 and 400 cells/ mL) . Exposure to P. donghaiense (>1×10 5 cells/mL) , A. catenella (only at a density of 600 cells/mL) , P. lima, and K. veneficum significantly shortened the RP of rotifers (P<0.05) . When cultured with P. donghaiense and K. veneficum, the reproductive period of rotifers decreased as algal density increased. For every dinoflagellate species tested, harmful effects on rotifers occurred at cell densities equivalent to those found in the field. The duration of the reproductive period (Chlorella sp. (control) : 240.08 h) sharply declined to 72.20 h when rotifers were exposed to A. catenella at a density of 10 2 cells/mL and to 77.12 h when exposed to P. lima at the same concentration. Reproductive period also declined to 104.42 h when rotifers were exposed to a density of 103 cells/mL of K. veneficum, and to 154.78 h at a density of 10 5 cells/mL of P. donghaiense .
Skeletonema costatum had no significant impact on the duration of the post-reproductive period (PP) of rotifers (P >0.05) . An increased PP was observed in rotifers exposed to P. lima at densities of 10 4 cells/ mL. The duration of the post-reproductive period in rotifers showed a marked decrease when cultured with P. donghaiense (>1×10 5 cells/mL) , A. catenella, P. lima (except at a the density of 10 4 cells/mL) and K. veneficum (P<0.05) .
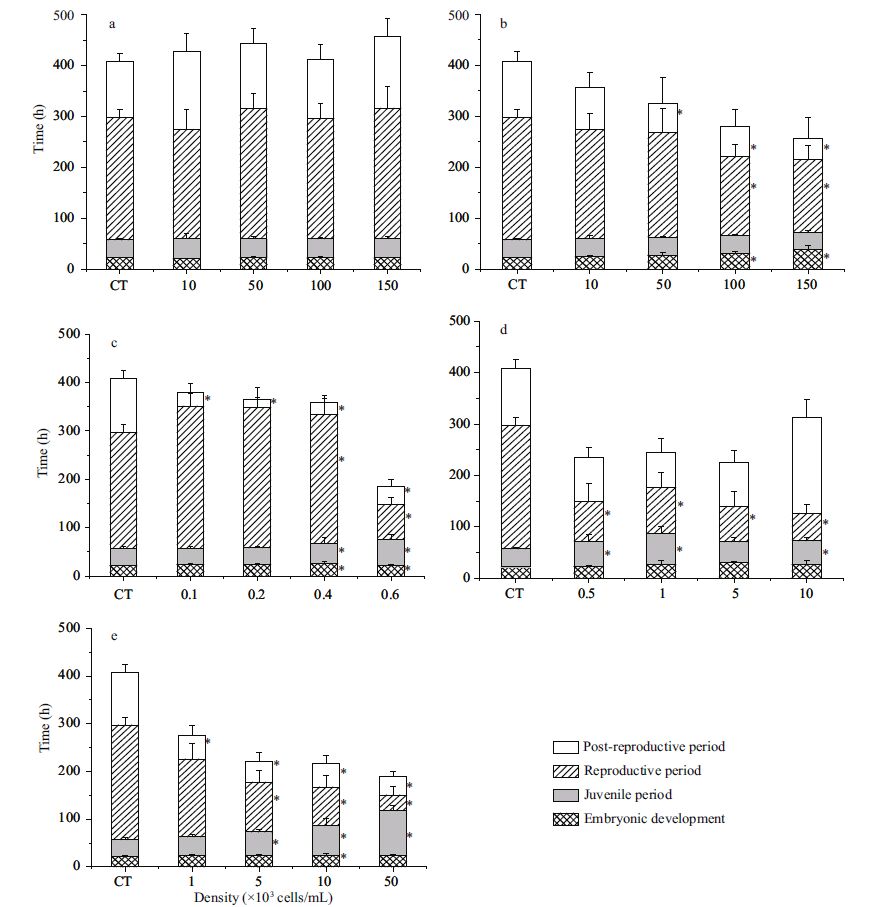
The mean life span (ML) of rotifers was calculated based on the values for JP, RP and PP (Fig. 2) . The diatom Skeletonema costatum had no significant impact on the mean life span of rotifers (P >0.05) . Exposure to P. donghaiense, A. catenella (except at a density of 100 cells/mL) , P. lima and K. veneficum significantly shortened the ML of rotifers. The mean life span of rotifers exposed to P. donghaiense and K. veneficum declined as algal densities increased (P<0.05) . However, the decline in the mean life span of rotifers exposed to A. catenella and P. lima, was not dependent on algal concentration. The duration of mean life span (Chlorella sp. (control) : 394.53 h) significantly declined to 162.90 h in rotifers exposed to a density of 10 2 cells/mL of A. catenella, and to 203.67 h for rotifers exposed to a density of 10 2 cells/ mL of P. lima . Mean life span declined to 196.00 h at a density of 10 3 cells/mL of K. veneficum and to 261.11 h at a density of 10 5 cells/mL of P. donghaiense .
|
| Figure 2 Mean life span (ML) of B. plicatilis exposed to different algal densities |
The number of eggs produced over a lifetime (NE) for B . plicatilis exposed to different algal densities is shown in Fig. 3. There was no significant impact of the diatom Skeletonema costatum on the NE of rotifers (P >0.05) . A marked decrease in NE was observed in rotifers exposed to the dinoflagellates P. donghaiense, A. catenella, P. lima and K. veneficum (P<0.05) . The NE of rotifers exposed to A. catenella, P. lima and K. veneficum declined as algal densities increased. When cultured with P. donghaiense, the decline in NE of rotifers was not dependent on the density of algae.

|
| Figure 3 Number of eggs (NE) of B. plicatilis exposed to different algal densities |
population growth of B . plicatilis Based on the age-specific survival and fertility of the rotifers exposed to several algal species that cause HABs, we calculated the net reproductive rate (R 0) , generation time (T) , intrinsic rate of population increase (R m) , and finite rate of increase (λ) for rotifers (Fig. 4) . The diatom Skeletonema costatum had no significant impact on R 0 (P >0.05) . When exposed to the dinoflagellate treatments, rotifers showed significantly shortened R 0 values. When rotifers were cultured with P. donghaiense, A. catenella or K. veneficum, R 0 decreased as algal densities increased.

|
| Figure 4 Parameters of population growth for the rotifer B . plicatilis at several algal densities |
Rotifers did not exhibit a significantly shortened T (P >0.05) when exposed to S . costatum, P. donghaiense (except at a density of 1.5×10 5 cells/mL) and A. catenella (except at a density of 600 cells/mL) . Exposure to P. lima and K. veneficum significantly shortened the T of rotifers (P<0.05) .
There were no adverse effects of S . costatum on the R m of rotifers at any of the concentrations tested (P >0.05) . The intrinsic rate of population increase (R m) significantly decreased in rotifers exposed to the dinoflagellate treatments (except for P. lima at densities of 0.5 and 1.0×10 3 cells/mL and K. veneficum at a density of 1.0×10 3 cells/mL) . The intrinsic rate of population increase (R m) gradually decreased with P. donghaiense and A. catenella .
Skeletonema costatum had no adverse effects on the λ of rotifers. The dinoflagellate treatments (except for P. lima and K. veneficum at 1.0×10 3 cells/mL) significantly decreased the λ of rotifers as algal densities increased.
4 DISCUSSION 4.1 The impacts of different HAB species on rotifersThe densities of HAB species used in the experiment were based on the densities of algal blooms recorded in the field, therefore, the carbon levels of each species differed (P. donghaiense > P. lima > K. veneficum > S . costatum > A. catenella) . Rotifers did not exhibit any adverse responses to the diatom S . costatum, even at the highest carbon content used in the experiment (6.45 μg/mL, equal to 10 4 to 1.5×10 5 cells/mL) . Similarly, S. costatum did not have any adverse effects on the copepod Calanus sinicus, during its feeding and reproduction (Jing-Jing Song, unpublished) . However, the effects of diatoms on zooplankton may be speciesspecific. Several researchers have reported adverse effects of the diatoms (e.g. Thalassiosira rotula) on copepods (e.g. Temora stylifera, Calanus helgolandicus and Pseudocalanus newmani) , including reduced hatching success, abnormal development and high larval mortality rates (Poulet et al., 1995; Carotenuto et al., 2002; Ianora et al., 2004; Halsband-Lenk et al., 2005; Ianora and Miralto, 2010) . It has been widely reported that toxic compounds (including short chain polyunsaturated aldehydes, some oxylipins) and nutritional quality may be the major cause of adverse effects of diatoms on zooplankton (Jónasdóttir, 1994; Miralto et al. 1999; Ianora et al., 2003, 2004; Fontana et al., 2007; Jónasdóttir et al., 2009; Amin, 2011; Barreiro et al., 2011) .
Rotifers in the experiment showed a decline in all of the life history parameters measured (ML, NE, R 0, R m and λ) when exposed to the dinoflagellates A. catenella, P. lima, K. veneficum and P. donghaiense, at carbon levels of <6.45 μg/mL. These dinoflagellates also have detrimental effects on copepods and some early life stages of bivalves, and may cause decreased egg production rates and hatching success, reduced ingestion rates, lower nauplii production and increased deformities (Frangópulos et al., 2000; da Costa et al., 2005; Dam and Colin, 2005; Glibert et al., 2007; Stoecker et al., 2008) . Exposure to the dinoflagellates A. catenella, P. lima and K. veneficum resulted in a reduced duration of rotifer development (JP, RP and PP) , even at a very low carbon content (i.e. A. catenella at 0.92 μg/mL carbon, equal to 400 cells/mL, P. lima at 0.93 μg/mL carbon, equal to 500 cells/mL and K. veneficum at 0.7 μg/mL carbon, equal to 5 000 cells/ mL) . As the carbon content of the dinoflagellate cells causing these effects were low, it was not possible to distinguish if any of these strains had more impact on the rotifers, compared with others. A reduction in the duration of development only occurred in rotifers exposed to a carbon content >22.65 μg/mL (or 105 cells/mL) of P. donghaiense, suggesting that P. donghaiense had the weakest effects on B . plicatilis among the dinoflagellates tested. Similarly, other Prorocentrum species, especially P . minimum, also showed weaker effects on the oyster Crassostrea ariakenisis, compared with K. veneficum (Glibert et al., 2007) .
4.2 The possible causes of adverse effects of dinoflagellates on rotifersPrevious studies have indicated that the adverse effects of several HAB species on zooplankton may be associated with algal toxicity (Ives, 1987; Hansen, 1989; Poulet et al., 1995; da Costa et al., 2005; Wu et al., 2006) , inadequate algal body size and poor nutritional quality (Dam and Colin, 2005; Zheng et al., 2011) oR mechanical damage (Wang et al., 2006) .
Rotifers prefer to consume algal cells with a diameter less than 10 μm, and seldom consume algae with a diameter of >22-30 μm (Turner et al., 1998) . For B . plicatilis, the optimal prey diameter was approximately 8 μm, and the upper limit for retention was a diameter 20-25 μm (Hansen et al., 1997) . The dinoflagellates A. catenella and P. lima are not in the optimal size range for rotifer feeding. Some algal species have poor nutritional quality. The dinoflagellate P. donghaiense has low amounts of phenylalanine, histidine and lysine (Chen et al., 2007) , and may be a poor food source for rotifers. Researchers in our lab found that B . plicatilis seldom ingest these dinoflagellate strains (Wang, 2004; unpublished data) . New-born rotifers almost died of starvation within one week when fed a diet that consisted of only a single dinoflagellate species (unpublished data) . The algae Chlorella sp. was added to every algal treatment at the same density as that used in the control. This mixed diet ruled out the possibility that large cell diameter or insufficient nutrients in the dinoflagellate HAB species led to low population growth of B . plicatilis . This suggests that algal toxicity oR mechanical damage may be the potential causes of harmful effects on the rotifers.
4.2.1 Algal toxicity of Karlodinium veneficum and Alexandrium catenellaThe strain of K. veneficum used in our experiment, produces a hemolytic toxin (Qing-Chun Zhang, unpublished) . A similar strain also isolated from the East China Sea has shown hemolytic activity, which is associated with karlotoxins (Zhou et al., 2011) . The toxin has been associated with massive fish kills and has delayed or restricted larval development of marine invertebrates (Deeds et al., 2002; Kempton et al., 2002; Brownlee et al., 2008; Zhou et al., 2011) . In this research, the life stage characteristics of rotifers were strongly reduced after exposure to the dinoflagellates. Karlodinium veneficum can produce toxic substances that are released when cells are disturbed or damaged (Deeds et al., 2002) . Therefore, direct contact between rotifers and these algal cells may facilitate the release of toxin. It is possible that sublethal effects of the toxin inhibited some facets of rotifer reproductive behavior.
The strain of A. catenella used in the experiment produces paralytic shellfish poison (PSP) (Mons et al., 1998) . The known PSP toxins are mainly stored inside the cells (Ma et al., 2009) . By measuring changes in chlorophyll a content in the gut of rotifers, it was shown that rotifers seldom ingest this strain of A. catenella (Wang, 2004) . Therefore, given that the PSP was not ingested by rotifers, the adverse effects observed may not be caused by the PSP toxin. Similarly, Wang et al. (2005) reported that the effects of Alexandrium sp. on rotifers were not related to PSP toxins. In addition to producing PSP toxin, Alexandrium sp. produces unknown non-PSP toxicants, such as polysaccharide-based compounds (Yamasaki et al., 2008) and amphipathic compounds (Ma et al., 2009, 2011) , which are harmful to marine organisms through hemolytic or other lytic activities (Simonsen et al., 1995; Bagøien et al., 1996; Lush et al., 2001; Gribble et al., 2005) . Seven strains of Alexandrium sp. studied in our lab, including A. catenella, have been shown to produce hemolytic compounds other than PSP toxins (Tan et al., 2008) . Although Chlorella sp. (carbon content of 11.4 μg/ mL) was added to each treatment in this study, rotifers exposed to A. catenella had a prolonged juvenile period (JP) . Based on the close relationship between the JP and food quality (King, 1967; Pilarska, 1977) , we hypothesized that unknown toxic substances produced by A. catenella might affect the ingestion of Chlorella sp. by B . plicatilis . Chen et al. (2007) also demonstrated that substances produced by Alexandrium sp. may inhibit the ingestion of other algal cells by the brine shrimp, Artemia salina . Further research on the ingestion of mixed diets by rotifers needs to be carried out to test this hypothesis. The unknown toxic substances may also have other effects on rotifers, such as a hemolytic or cytolytic response.
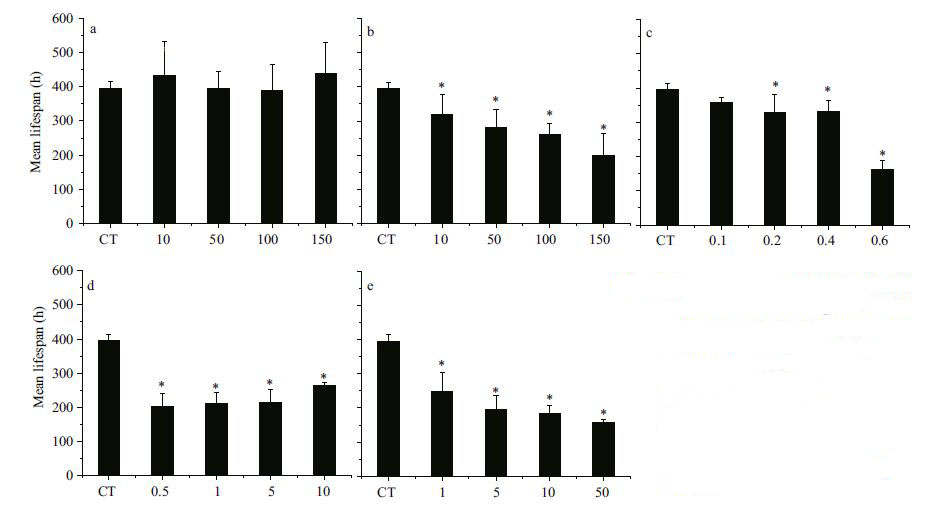
4.2.2 Mechanical damage caused by Prorocentrum lima and Prorocentrum donghaienseThe cells of P. lima appeared to be clustered and held together by mucous-like substances that seemed viscous and gummy (Fig. 5a) . Light microscopy revealed that many P. lima cells adhered to the surface of rotifers (Fig. 5b) , and inhibited them from normal swimming and movement. This phenomenon was also documented for the dinoflagellates K . mikimotoi and H . akashiwo, which exerted mechanical damage on zooplankton or juvenile sea bass by producing mucous-like substances (Wang et al., 2006; Ajuzie, 2008; Zou et al., 2010) . Interestingly, B . plicatilis tended to escape from the adhesion, expending energy to disentangle themselves from the mucus. The prolonged JP and decreased fecundity observed in the experiment may be associated with a reduced amount of energy available. This finding is supported by studies which showed that the life history characteristics of rotifers is effected by a low food intake or low levels of energy (King et al., 1967; Halbach et al., 1974 ; Pilarska et al., 1977; Schmid-Araya et al., 1991 ; Galindo et al., 1993; Xi et al., 2001) .
|
| Figure 5 Adhesion of Prorocentrum lima cells to the surface of rotifer Brachionus plicatilis |
Most planktonic rotifers (e.g. the genus Brachionus, Keratella and Anuraeopsis) , carry eggs attached to the posterior end of the body (Gilbert, 1983) , although some rotifer taxa (e.g. Epiphanes brachionus, Notholca, Trichocercidae) do not (Ruttner-Kolisko, 1974; Pontin, 1978) . When rotifers were exposed to high densities of P. donghaiense in our experiment, most eggs of B . plicatilis were easily detached. We suspect that this may be related to the presence of mucus. Wang et al. (2003) suggested that rotifers showed inhibited swimming activity due to mucus present on the cell surface of P. donghaiense . It has been reported that loose eggs have a high hatching success and could constitute up to 10% of the total production of rotifers (Sarma, 1987) . However, the dropped eggs in the P. donghaiense treatment had little chance to develop into neonates, which may result in low population reproduction rates. Determining if this is related to the mucus produced by P. donghaiense requires further investigation.
4.3 The potential threats of dinoflagellate HABs tothe marine ecosystem in China In recent years, HABs in China have been gradually exhibiting succession from diatom-dominant to dinoflagellate-dominant, and harmful dinoflagellates have become the dominant species in HABs (Zhou and Yu, 2007) . In our study the diatom S . costatum did not affect any of the life history parameters measured in the rotifer B . plicatilis, whereas the dinoflagellates P. donghaiense, A. catenella, P. lima and K. veneficum all had detrimental effects. Although rotifers are not a key species along the coast of China, they are representative of otheR micro-zooplankton of the same size and with similar characteristics. Microzooplankton, as the main food source for other organisms, play an important role in material transfer and energy flow in the marine ecosystem. If reproduction in these organisms is threatened, fisheries productivity and ecosystem balance also become endangered. Thus, the continued prevalence of dinoflagellate HABs could pose risks to the structure and function of the marine ecosystem. Additionally, zooplankton plays a key role in the occurrence of HABs and in the promotion or inhibition of HAB development. In this study, the life history characteristics of the rotifer B . plicatilis were significantly suppressed when exposed to dinoflagellates, which may promote dinoflagellate bloom formation.
5 CONCLUSIONThe diatom S . costatum had no adverse impacts on the life history parameters of the rotifer B . plicatilis . The dinoflagellates P. donghaiense, A. catenella, P. lima and K. veneficum all had adverse effects on the reproduction and growth of B . plicatilis . The continued prevalence of dinoflagellate-dominated HABs along the coast of China, could pose risks to the structure and function of the marine ecosystem.
| Ajuzie C C, 2008. Toxic Prorocentrum lima induces abnormal behaviour in juvenile sea bass. J. A ppl. P hycol., 20 (1) : 19 –27. |
| Amin R. 2011. Copepods in Skeletonema-dominated Food Webs: Toxicity and Nutritional Quality as Factors Controlling Copepod-diatom Interactions. Umeå University, Umeå. |
| Bagøien E, Miranda A, Reguera B, Franco J, 1996. Effect of the Toxic Dinoflagellate Alexandrium minutum on the Copepod Euterpina acutifrons. Intergovernmental Oceanographic Commission of UNESCO, Paris, France : 385 –388. |
| Barreiro A, Carotenuto Y, Lamari N, Esposito F, D'Ippolito G, Fontana A, Romano G, Ianora A, Miralto A, Guisande C, 2011. Diatom induction of reproductive failure in copepods: the effect of PUAs versus non volatile oxylipins. J. E xp. M ar. B iol. Ecol., 401 (1-2) : 13 –19. Doi: 10.1016/j.jembe.2011.03.007 |
| Birch L C, 1948. The intrinsic rate of natural increase of an insect population. J. Anim. Ecol., 17 (1) : 15 –26. Doi: 10.2307/1605 |
| Brownlee E F, Sellner S G, Sellner K G, Nonogaki H, Adolf J E, BachvaroffT R, Place A R, 2008. Responses of Crassostrea virginica (Gmelin) and C. ariakensis (Fujita) to bloom-forming phytoplankton including ichthyotoxic Karlodinium veneficum (Ballantine). J. Shellfish Res., 27 (3) : 581 –591. |
| Carotenuto Y, Ianora A, Buttino I, Romano G, Miralto A, 2002. Is postembryonic development in the copepod Temora stylifera negatively affected by diatom diets? J. Exp. Mar. Biol. Ecol., 276 (1-2) : 49 –66. Doi: 10.1016/S0022-0981(02)00237-X |
| Chen Y, Yan T, Zhou M J, 2007. Effects of Prorocentrum donghaiense and Alexandrium catenella on the material transfer in a simulated marine food chain. Acta Ecol. Sin., 27 (10) : 3964 –3972. Doi: 10.1016/S1872-2032(07)60086-9 |
| da CostaRm, Franco J, Cacho E, Fernández F, 2005. Toxin content and toxic effects of the dinoflagellate Gyrodinium corsicum (Paulmier) on the ingestion and survival rates of the copepods Acartia grani and Euterpina acutifrons. J. Exp. Mar. Biol. Ecol., 322 (2) : 177 –183. Doi: 10.1016/j.jembe.2005.02.017 |
| Dam H G, Colin S P, 2005. Prorocentrum minimum (clone Exuv) is nutritionally insufficient, but not toxic to the copepod Acartia tonsa. Harmful Algae, 4 (3) : 575 –584. Doi: 10.1016/j.hal.2004.08.007 |
| Deeds J R, Terlizzi D E, Adolf J E, Stoecker D K, Place A R, 2002. Toxic activity from cultures of Karlodinium micrum (= Gyrodinium galatheanum) (Dinophyceae)—a dinoflagellate associated with fish mortalities in an estuarine aquaculture facility. Harmful Algae, 1 (2) : 169 –189. Doi: 10.1016/S1568-9883(02)00027-6 |
| Fontana A, d'Ippolito G, Cutignano A, Romano G, Lamari N, Massa Gallucci A, Cimino G, Miralto A, Ianora A, 2007. LOX-induced lipid peroxidation mechanism responsible for the detrimental effect of marine diatoms on zooplankton grazers. ChemBioChem, 8 (15) : 1810 –1818. Doi: 10.1002/(ISSN)1439-7633 |
| Frangópulos M, Guisande C, Maneiro I, Riveiro I, Franco J M, 2000. Short-term and long-term effects of the toxic dinoflagellate Alexandrium minutum on the copepod Acartia clausi. Mar. Ecol. Prog. Ser., 203 : 161 –169. Doi: 10.3354/meps203161 |
| Galindo M D, Guisande C, Toja J, 1993. Reproductive investment of several rotifer species. Hydrobiol., 255 (1) : 317 –324. |
| Gilbert J J, 1983. Rotifera. In: Adiyodi K G, Adiyodi R G eds. Reproductive Biology of Invertebrates. Volume I: Oogenesis, Oviposition and Oosorption. Wiley and Sons, Chichester : 181 –209. |
| Glibert P M, Alexander J, Meritt D W, North E W, Stoecker D K. 2007. Harmful algae pose additional challenges for oyster restoration: impacts of the harmful algae |
| Karlodinium veneficum and Prorocentrum minimum on early life stages of the oysters Crassostrea virginica and Crassostrea ariakensis. J. Shellfish Res., 26 (4): 919-925. |
| Gribble K E, Keafer B A, Quilliam M A, Cembella A D, Kulis D M, Manahan A, Anderson D M, 2005. Distribution and toxicity of Alexandrium ostenfeldii (Dinophyceae) in the Gulf of Maine, USA. Deep Sea Research Part II: Topical Studies in Oceanography, 52 (19-21) : 2745 –2763. Doi: 10.1016/j.dsr2.2005.06.018 |
| Halbach U, Halbach-Keup G, 1974. Quantitative Beziehungen zwischen Phytoplankton und der Populationsdynamik des Rotators Branchionus calyciflorus Pallas. Befundeaus Laboratoriums-experimenten und Freilanduntersuchungen. Arch. Hydrobiol., 73 : 273 –309. |
| Halsband-Lenk C, Pierson J J, Leising A W, 2005. Reproduction of Pseudocalanus newmani (Copepoda: Calanoida) is deleteriously affected by diatom blooms—a field study. Prog. Oceanogr., 67 (3-4) : 332 –348. Doi: 10.1016/j.pocean.2005.09.003 |
| Hansen B, Wernberg-Møller T, Wittrup L, 1997. Particle grazing efficiency and specific growth efficiency of the rotifer Brachionus plicatilis (Muller). J. Exp. Mar. Biol. Ecol., 215 (2) : 217 –233. Doi: 10.1016/S0022-0981(97)00053-1 |
| Hansen P J, 1989. The red tide dinoflagellate Alexandrium tamarense: effects on behaviour and growth of a tintinnid ciliate. Mar. Ecol. Prog. Ser., 53 : 105 –116. Doi: 10.3354/meps053105 |
| Huang L, Xi Y L, Zha C W, Zhao L L, Wen X L, 2012. Effects of dieldrin and 17β-estradiol on life history characteristics of freshwater rotifer Brachionus calyciflorus Pallas. J. Freshwater Ecol., 27 (3) : 381 –392. Doi: 10.1080/02705060.2012.668499 |
| Huntley M E, Ciminiello P, Lopez M D G, 1987. Importance of food quality in determining development and survival of Calanus pacificus (Copepoda: Calanoida). Mar. Biol., 95 (1) : 103 –113. Doi: 10.1007/BF00447491 |
| Ianora A, Miralto A, Poulet S A, Carotenuto Y, Buttino I, Romano G, Casotti R, Pohnert G, Wichard T, Colucci-D'Amato L, Terrazzano G, Smetacek V, 2004. Aldehyde suppression of copepod recruitment in blooms of a ubiquitous planktonic diatom. Nature, 429 (3990) : 403 –407. |
| Ianora A, Miralto A, 2010. Toxigenic effects of diatoms on grazers, phytoplankton and otheRmicrobes: a review. Ecotoxicology, 19 (3) : 493 –511. Doi: 10.1007/s10646-009-0434-y |
| Ianora A, Poulet S A, Miralto A, 2003. The effects of diatoms on copepod reproduction: a review. Phycologia, 42 (4) : 351 –363. Doi: 10.2216/i0031-8884-42-4-351.1 |
| Ives J D, 1987. Possible mechanisms underlying copepod grazing responses to levels of toxicity in red tide dinoflagellates. J. E xp. M ar. B iol. Ecol., 112 (2) : 131 –144. Doi: 10.1016/0022-0981(87)90113-4 |
| Jónasdóttir S H, Visser A W, Jespersen C, 2009. Assessing the role of food quality in the production and hatching of Temora longicornis eggs. Mar. Ecol. Prog. Ser., 382 : 139 –150. Doi: 10.3354/meps07985 |
| Jónasdóttir S H, 1994. Effects of food quality on the reproductive success of Acartia tonsa and Acartia hudsonica: laboratory observations. Mar. Biol., 121 (1) : 67 –81. Doi: 10.1007/BF00349475 |
| Kempton J W, Lewitus A J, Deeds J R, Law J M, Place A R, 2002. Toxicity of Karlodinium micrum (Dinophyceae) associated with a fish kill in a South Carolina brackish retention pond. Harmful Algae, 1 (2) : 233 –241. Doi: 10.1016/S1568-9883(02)00015-X |
| Kim D, Sato Y, Oda T, Muramatsu T, Matsuyama Y, Honjo T, 2000. Specific toxic effect of dinoflagellate Heterocapsa circularisquama on the rotifer Brachionus plicatilis. Biosci. Biotechnol. Biochem., 64 (12) : 2719 –2722. Doi: 10.1271/bbb.64.2719 |
| King C E, 1967. Food, age, and the dynamics of a laboratory population of rotifers. Ecology, 48 (1) : 111 –128. Doi: 10.2307/1933423 |
| Kostopoulou V, Carmona M J, Divanach P, 2012. The rotifer Brachionus plicatilis: an emerging bio-tool for numerous applications. Journal of Biological Research, 17 : 97 –112. |
| Krebs C J. 1985. Ecology: The Experimental Analysis of Distribution and Abundance. Harper and Row, New York. |
| Landsberg J H, 2002. The effects of harmful algal blooms on aquatic organisms. Rev. Fish. Sci., 10 (2) : 113 –390. Doi: 10.1080/20026491051695 |
| Li A S, Stoecker D K, Coats D W, 2000. Mixotrophy in Gyrodinium galatheanum (DINOPHYCEAE): grazing responses to light intensity and inorganic nutrients. J. Phycol., 36 (1) : 33 –45. Doi: 10.1046/j.1529-8817.2000.98076.x |
| Lu D D, Goebel J, Qi Y Z, Zou J Z, Han X T, Gao Y H, Li Y G, 2005. Morphological and genetic study of Prorocentrum donghaiense Lu from the East China Sea, and comparison with some related Prorocentrum species. Harmful Algae, 4 (3) : 493 –505. Doi: 10.1016/j.hal.2004.08.015 |
| Lush G J, Negri A, HallegraeffG M. 2001. Exotoxins produced by the toxic dinoflagellate Alexandrium minutum: characterisation by radioreceptor and neuroblastoma assays during the growth cycle. In: HallegraeffG M, Blackburn S I, Bolch C J S, Lewis R eds. 9th International Conference-Harmful Algal Blooms 2000. IOC UNESCO. p.268-271. |
| Ma H Y, Krock B, Tillmann U, Cembella A, 2009. Preliminary characterization of extracellular allelochemicals of the toxic marine dinoflagellate Alexandrium tamarense using a Rhodomonas salina bioassay. Marine Drugs, 7 (4) : 497 –522. Doi: 10.3390/md7040497 |
| Ma H Y, Krock B, Tillmann U, Muck A, Wielsch N, Svatoš A, Cembella A, 2011. Isolation of activity and partial characterization of large non-proteinaceous lytic allelochemicals produced by the marine dinoflagellate Alexandrium tamarense. Harmful Algae, 11 : 65 –72. Doi: 10.1016/j.hal.2011.07.004 |
| Marcial H S, Hagiwara A, 2007. Effect of diazinon on life stages and resting egg hatchability of rotifer Brachionus plicatilis. Hydrobiologia, 593 (1) : 219 –225. Doi: 10.1007/s10750-007-9070-9 |
| Miralto A, Barone G, Romano G, Poulet S A, Ianora A, Russo G L, Buttino I, Mazzarella G, Laabir M, Cabrini M, Giacobbe M G, 1999. The insidious effect of diatoms on copepod reproduction. Nature, 402 (6758) : 173 –176. Doi: 10.1038/46023 |
| Mons M P, van Egmond H P, Speijers G J A. 1998. Paralytic Shellfish Poisoning; A Review. National Institute of Public Health and the Environment, the Netherlands. |
| Pilarska J, 1977. Eco-physiological studies on Brachionus rubens Ehrbg (Rotatoria). II. Production and respiration. Pol. Arch. Hydrobiol., 24 : 329 –341. |
| Pontin Rm. 1978. A Key to the Freshwater Planktonic and Semi-Planktonic Rotifera of the British Isles. Freshwater Biological Association Ambleside, Scientific Publications. 38 p. |
| Poole R W. 1974. Introduction to Quantitative Ecology. McGraw-Hill, New York. |
| Poulet S A, Laabir M, Ianora A, Miralto A, 1995. Reproductive response of Calanus helgolandicus. I. Abnormal embryonic and naupliar development. Mar. Ecol. Prog. Ser., 129 : 85 –95. |
| Ruttner-Kolisko A, 1974. Plankton rotifers: biology and taxonomy. In: Die Binnengewasser. E. Schweizerbartsche Verlagsbuchhandlung, Stuttgart, 26 : 1 –146. |
| Sarma S S S, Nandini S, Flores J L G, 2001. Effect of methyl parathion on the population growth of the rotifer Brachionus patulus (O. F. Müller) under different algal food ( Chlorella vulgaris) densities. Ecotoxicol. Environ. Safety., 48 (2) : 190 –195. |
| Schmid-Araya J, 1991. The effect of food concentration on the life histories of Brachionus plicatilis (OFM) and Encentrum linnhei Scott. Arch. Hydrobiol., 111 : 87 –102. |
| Simonsen S, Møller B, Larsen J, Ravn H, 1995. Haemolytic activity of Alexandrium tamarense cells. In: Lassus P, Arzul G, Erard-Le-Denn E, Gentien P eds. Harmful Marine Algal Blooms. Lavoisier, Paris : 513 –518. |
| Snell T W, Bieberich C J, Fuerst R, 1983. The effects of green and blue-green algal diets on the reproductive rate of the rotifer Brachionus plicatilis. Aquaculture, 31 (1) : 21 –30. Doi: 10.1016/0044-8486(83)90254-5 |
| Snell T W, Janssen C R, 1995. Rotifers in ecotoxicology: a review. Hydrobiologia, 313-314 (1) : 231 –247. Doi: 10.1007/BF00025956 |
| Stoecker D K, Adolf J E, Place A R, Glibert P M, Meritt D W, 2008. Effects of the dinoflagellates Karlodinium veneficum and Prorocentrum minimum on early life history stages of the Eastern Oyster ( Crassostrea virginica). Mar. Biol., 154 (1) : 81 –90. Doi: 10.1007/s00227-007-0901-z |
| Tan Z J, Yan T, Yu R C, Zhou M J, 2008. The preliminary research on the hemolytic activity of the dinoflagellate Alexandrium spp. Marine Sciences, 32 (12) : 75 –81. |
| Turner J T, Tester P A, Hansen P J, 1998. Interactions between toxic marine phytoplankton and metazoan and protistan grazers. In: Anderson D M, Cembella A D, HallegraeffG M eds. Physiological Ecology of Harmful Algal Blooms, NATO-Advanced Study Institute Series, V 41. Springer-Verlag, Heidelberg : 453 –473. |
| Wang H X, Lu D D, Huang H Y, Göbel J, Dai X F, Xia P, 2011. First observation of Karlodinium veneficum from the East China Sea and the coastal waters of Germany. Acta Ecol. Sin., 30 (6) : 112 –121. |
| Wang L P, Yan T, Tan Z J, Zhou M J, 2003. Effects of Alexandrium tamarense and Prorocentrum donghaiense on rotifer Brachionus plicatilis population. Chin. J. Appl. Ecol., 14 (7) : 1151 –1155. |
| Wang L P, Yan T, Yu R C, Zhou M J, 2005. Experimental study on the impact of dinoflagellate Alexandrium species on populations of the rotifer Brachionus plicatilis. Harmful Algae, 4 (2) : 371 –382. Doi: 10.1016/j.hal.2004.06.014 |
| Wang L P, Yan T, Zhou M J, 2006. Impacts of HAB species Heterosigma akashiwo on early development of the scallop Argopecten irradians Lamarck. Aquaculture, 255 (1-4) : 374 –383. Doi: 10.1016/j.aquaculture.2005.11.057 |
| Wang L P, 2004. Impacts of HAB Species on Early Development of the Scallop Argopecten irradians Lamark and Populations of the Rotifer Brachionus plicatilis. Qingdao: University of Chinese Academy of Sciences. |
| Wang Z H, Lv S H, Chen J F, Xu N, Qi Y Z, 1998. Taxonomic studies on red tide causative algae on the Guangdong coast, south China sea. Journal of Wuhan Botanical Research, 16 (4) : 310 –314. |
| Wu Z X, Zou Y L, Zhu M Y, Wang Z L, Wang D, 2006. Effects of toxic Alexandrium species on the survival and feeding rates of brine shrimp, Artemia salina. Acta Ecol. Sin., 26 (12) : 3942 –3947. Doi: 10.1016/S1872-2032(07)60004-3 |
| Xi Y L, Huang X F, Jin H J, Liu J K, 2001. The effect of food concentration on the life history of three types of Brachionus calyciflorus females. Internat. Rev. Hydrobiol., 86 : 211 –217. Doi: 10.1002/(ISSN)1522-2632 |
| Xie Z H, Xiao H, Tang X X, Lu K H, Cai H J, 2008. Interactions between red tide microalgae and herbivorous zooplankton: effects of two bloom-forming species on the rotifer Brachionus plicatilis (O. F. Muller). Hydrobiologia, 600 (1) : 237 –245. Doi: 10.1007/s10750-007-9237-4 |
| Yamasaki Y, Katsuo D, Nakayasu S, Salati C, Duan J J, Zou Y N, Matsuyama Y, Yamaguchi K, Oda T, 2008. Purification and characterization of a novel high molecular weight exotoxin produced by red tide phytoplankton, Alexandrium tamarense. J. B iochem. M ol. T oxicol., 22 (6) : 405 –415. |
| Yan T, Wang Y F, Wang L P, Chen Y, Han G, Zhou M J, 2009. Application of rotifer Brachionus plicatilis in detecting the toxicity of harmful algae. Chin. J. Oceanol. Limnol., 27 (2) : 376 –382. Doi: 10.1007/s00343-009-9104-4 |
| Zha C W, Xi Y L, Huang L, Zhao L L, 2007. Effect of sublethal exposure to chlordecone on life history characteristics of freshwater rotifer Brachionus calyciflorus Pallas. Bull. Environ. Contam. Toxicol., 78 (1) : 79 –83. Doi: 10.1007/s00128-007-9003-3 |
| Zhang X, Geng H, 2012. Effect of Microcystis aeruginosa on the rotifer Brachionus calyciflorus at different temperatures. Bull. Environ. Contam. Toxicol., 88 (1) : 20 –24. Doi: 10.1007/s00128-011-0450-5 |
| Zhao L L, Xi Y L, Huang L, Zha C W, 2008. Effects of three phthalate esters on the life-table demography of freshwater rotifer Brachionus calyciflorus Pallas. Aquat. Ecol., 43 (2) : 395 –402. |
| Zheng Y, Dam H G, Avery D E, 2011. Differential responses of populations of the copepod Acartia hudsonica to toxic and nutritionally insufficient food algae. Harmful Algae, 10 (6) : 723 –731. Doi: 10.1016/j.hal.2011.06.003 |
| Zhou C X, Fernández N, Chen H M, You Y R, Yan X J, 2011. Toxicological studies of Karlodinium micrum (Dinophyceae) isolated from East China Sea. Toxicon, 57 (1) : 9 –18. Doi: 10.1016/j.toxicon.2010.08.014 |
| Zhou M J, Shen Z L, Yu R C, 2008. Responses of a coastal phytoplankton community to increased nutrient input from the Changjiang (Yangtze) River. Cont. Shelf Res., 28 (12) : 1483 –1489. Doi: 10.1016/j.csr.2007.02.009 |
| Zhou M J, Yan T, Zhou J Z, 2003. Preliminary analysis of the characteristics of red tide areas in Changjiang River estuary and its adjacent sea. Chin. J. Appl. Ecol., 14 (7) : 1031 –1038. |
| Zhou M J, Yu R C, 2007. Mechanisms and impacts of harmful algal blooms and the countmeasures. Chinese Journal of Nature, 29 (2) : 72 –77. |
| Zhou M J, 2010. Environmental settings and harmful algal blooms in the sea area adjacent to the Changjiang River estuary. TERRAPUB and Nagasaki University, Nagasaki, Japan : 133 –149. |
| Zou Y, Yamasaki Y, Matsuyama Y, Yamaguchi K, Honjo T, Oda T, 2010. Possible involvement of hemolytic activity in the contact-dependent lethal effects of the dinoflagellate Karenia mikimotoi on the rotifer Brachionus plicatilis. Harmful Algae, 9 (4) : 367 –373. Doi: 10.1016/j.hal.2010.01.005 |
 2016, 34
2016, 34