Institute of Oceanology, Chinese Academy of Sciences
Article Information
- Majid SHAYEGAN(SHAYEGANMajid), FEREIDOUNI Abolghasem ESMAEILI(ESMAEILIFEREIDOUNIAbolghasem), Naser AGH(AGHNaser), KHALILI Khosrow JANI(JANIKHALILIKhosrow)
- Effects of salinity on egg and fecal pellet production, development and survival, adult sex ratio and total life span in the calanoid copepod, Acartia tonsa: a laboratory study
- Journal of Oceanology and Limnology, 34(4): 709-718
- http://dx.doi.org/10.1007/s00343-016-5030-4
Article History
- Received: Jan. 25, 2015
- Accepted: Jun. 28, 2015
2. Fisheries Department, Faculty of Animal Sciences and Fisheries, Sari Agricultural Sciences and Natural Resources University (SANRU), Sari, Iran;
3. Artemia Reference Center, Urmia University, Urmia, Iran
Copepods are very importantin marine food chains and are considered as good, naturalfood for marine fish larvae because of such advantages as high levels of highly unsaturated fatty acids (HUFA) , antioxidants, astaxanthins, vitaminsE and D, digestive enzymes, and they represent suitablesizes in different species and at various life stages (Stttrup and McEvoy, 2003; McKinnon et al., 2003;Drillet et al., 2011) .
Among the environmental parameters, temperature and salinityhave been considered as principal factors affecting seasonaland spatial distribution patterns of copepods in nature. Previousresearch on calanoid copepod productivity have shown clear effects of differencesin salinity on egg production rates (Castro-Longoria, 2003; Chen et al., 2006;Holste and Peck, 2006; Camus and Zeng, 2009) , development and survival (Trujillo-Ortiz, 1990; Chinnery and Williams, 2004) , as well as sex ratioand total life span (Milione and Zeng, 2008) .
The genus Acartia is a member of the subtropical, coastal pelagiczooplankton, and is dominant in many estuaries and semi-enclosed systems.Different species of genus Acartia such as A. tonsa, A. clausi, A. bifilosa and A. sinjiensis are frequently used in marine fish larviculture (Stttrup and McEvoy, 2003; Drillet et al., 2011) . Among various copepod candidates for mass culture, those with more fecundity, shorter generation times and more resistance against water temperature and salinity fluctuations have received the most attention. A. tonsa is more abundantoutside estuaries, occurringduring spring-autumnand shows maximumdensities in August in the CaspianSea (Bagheri et al., 2013) .This species is completely adapted to the Caspian Sea brackish water (13) ; however, it seems that this species is euryhaline and can probably toleratesalinity fluctuations and even reproduce in different salinities. In spite of the marked influences of salinity on egg production, feeding, respiration and development of copepods (Rokneddine, 2005; Peck and Holste, 2006; Milione and Zeng, 2008;Devreker et al., 2009; Ohs et al., 2010) , there are very few published quantitative data on the salinity effects upon the reproductive and feeding performances, development, and sex ratio of the Caspian Sea calanoid copepod, A. tonsa.Therefore, the main goal of the present investigation has been to evaluate, under laboratory conditions, the reproductiveresponses (daily egg production and egg hatching success) , fecal pellet production, development, survival, adult sex ratio and longevity to salinitychanges.
2 MATERIAL AND METHOD 2.1 Microalgal culturesPure stocksof the microalga Isochrysis galbana and Chaetoceros calcitrans were supplied by Fisheries Research Center in Hormozganprovince (Bandar-e-Lengeh) , Iran. Batch culturesof the algae were maintained in f/2 media (Guillard, 1975) at 26℃ and four salinities (13, 20, 35, and 45) under constant illumination (3 000 lx) and 12 h L:12 h D (light:dark) photoperiod. Concentrations of algal cultureswere assessed using a hemocytometer. Salinity was measured using a refractometer.
2.2 A. tonsa stock culturesA. tonsa was collected from zooplankton samples captured in the Iraniansouthern parts of the Caspian Sea, Mahmood Abad coasts (salinity 13) . Samples were immediately transferred to the Sari Agricultural Sciences and Natural Resources University laboratory. A total of 200 pairs of adults copepodwere isolated under a stereo dissecting microscope and equally divided into four 5-L jars with 1μm filtered brackish water. Water temperature was maintained at 26±1℃ and photoperiod set to 12 h light:12h dark. All copepods were fed daily with a mixture of I. galbana and C. calcitrans (at a ratio 1:1) to obtain a feeding density of 3×104 cells/mL. Gentle aerationwas provided in jars, and 50% of the water was replaced every three days. The copepods were gradually acclimated from 13 to 20, 35, and 45, respectively, within 7 days (Castro-Longoria, 2003; Chen et al., 2006) . Then, the copepodswere reared for two successive generations during four weeksunder controlled conditions in eight carboys (20 L) at each salinity level.A salinity of 13 corresponds toambient conditions experienced by A. tonsa populations in the Caspian Sea. Salinities ranging from 20 to 35 represent the natural salinityrange of A. tonsa reported in literatures, and a salinityof 45 was chosen to test the tolerance of this speciesto salinity beyond those values. At 45, most of the adult copepods died due to stressedshocks and the survivors were apparently severelyaffected by slower than normal movements and/or abnormal swimming.
2.3 Egg and fecal pellet production rates in short-term study (7 consecutive days) and long-term study (total females’ reproductive periods)At the beginningof the experiment, four 2-L beakers were incubated at 26±1℃ under four salinity regimes (13, 20, 35, and 45) . In each beaker, copepods were fed daily with a mixture of I. galbana and C. calcitrans (at a ratio 1:1) to obtain a feeding density between 3×104 cells/mL. Then, one male-female pair (n=15 pairs) of A. tonsa was individually placed in Plexiglas cylinderswith mesh false bottoms (100 μm) to separatethe adults from the eggs and nauplii at each salinitylevel (15 replicates for each treatment) . The cylinderswere suspended in 250-mL beakersto monitor their egg production rate (EPR) and fecal pellet production rate (FPR) during 24 h. Egg cannibalism was discarded, and the copepodswere continuouslymonitored. During the experiments, no escape was registered for females throughthe mesh for separating eggs. Every 24 h, each pair was transferred to a new beaker to facilitate observation of the subsequent reproduction, and then eggs and fecal pellets were collected usinga 40-μm sieve (Trujillo-Ortiz, 1990; Carlotti et al., 1997; Devreker et al., 2012) . The experiment for each individual started from the last molting (CopepodidV) and continued in two separateperiods. In experiment 1 (short-term study) , the individual experiments were conducted for 7 consecutive days in four salinities (13, 20, 35, and 45) . Then in experiment 2 (long-term study) , the individual experiments were continuously maintained in threesalinities (13, 20, and 35) until all females in a treatment died. In the above two experiments, the males were replaced following death. Eggs and fecal pellets producedwere counted under a stereomicroscope (Model Nikon, SMZ-1500, Tokyo, Japan) after sieving (40 μm) the remaining water of beakers.
2.4 Egg hatching successThe numberof un-hatched eggs was counted periodically until no furtherhatching was noticed over a two-day period.Un-hatched eggs in each replicate were counted under a stereomicroscope. Egg hatchingsuccess (HS) (%) was calculated as: [ (No. of eggs produced initially-No. of un-hatched eggs) ×100]÷No. of eggs produced initially.
2.5 Development time, survival, adults' length, sex ratio, and longevitySimilar numbersof nauplii (n=150) were counted and randomlydistributed in 500-mLplastic flasks filled with filtered Caspian Sea water (1 μm) . All treatments were run in three replicates. Water temperature and food supplywere the same as those previously described across all treatments. Before feeding, between40% and 50% of water was siphoned every otherday through a 20-μm mesh. Every week, flasks were drained througha 150-μm sieve to remove detritus. The development time of A. tonsa was followed from hatchingto the adult stage (Copepodid V) in three salinityregimes (13, 20, and 35) . These conditions were maintained until fifty percent of population matured, when morphological differentiation began (Carlotti et al., 1997; Devreker et al., 2009) . At the end of trials, the development time and survival rate of A. tonsa were determined in each treatment. Adults were also sexed to obtain their sex ratio. The prosomelength of males and females were measuredusing a micrometer (Camus and Zeng, 2008) .
2.6 Data analysesThe results of reproductive traits, mean hatching success, fecal pellet production, development and survival, adult sex ratio, and longevity were analyzed by standard one-wayanalysis of variance (ANOVA) using SPSS, 17.0 (Statistical Program for Social Sciences 17.0) . Prior to testing for statistical differences, the Kolmogorov-Smirnov and Bartlett’s tests were applied to check the normality and homogeneity of variances (Sokal and Rohlf, 1981) . When significant differences were found among the treatments (P<0.05) , the mean valueswere compared with Duncan’s multiple range test. Only the pairs having reproduced more than threetimes in theirtotal life span were selected for analysis (Holste and Peck, 2006) . Data are presentedas mean ± standard error (SE) .
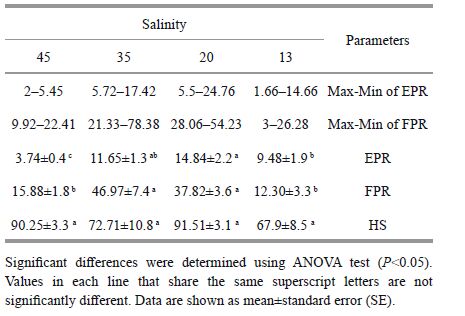
3 RESULT 3.1 Egg and fecal pellet production rates, and egg hatching success over 7 consecutive daysThe mean daily trend of EPR and FPR in A. tonsa over 7 consecutive days is shown in Fig. 1.In the newly molted females, salinityhad a significant effect on EPR. Mean egg production per female per day in the two salinities of 20 and 35 was significantly (P=0.030) higher than that in salinities of 13 and 45. Also, the mean fecal pelletproduction (FPR) in 20 and 35 was significantly (P=0.015) higher than that in 13 and 45. The highest (46.97±7.4) and lowest (12.30±3.3) of FPR values per female per day were recorded in salinities of 35 and 13, respectively (Table 1) .
|
There were no significant differences (P=0.621) in hatching successof A. tonsa eggs among treatments; however, an increasing trend was observedfrom 13 (67.9%) to 20 (91.51%) and 45 (90.25%) , respectively (Table 1) .
3.2 Egg and fecal pellet productionrates, and egg hatching success over females’reproductive periodMean egg production rate of A. tonsa over the females’ reproductive period was significantly (P=0.013) affectedby increased salinities (Fig. 1) . The mean number of egg produced per female per day gradually (P=0.026) increased when the salinity increased from 13 to 35. However, therewere no significant differences (P=0.136) between the two salinities of 20 and 35. The overall pattern of egg production at different salinities was as 35>20>13 (Table 2) . In addition, mean fecal pellet production per femaleper day (22.7±6.3) was higher (P=0.028) at 35. The overall pattern of fecal pellet production at different salinities was 35>13>20. Femaleswhich had stopped spawninglong before their death continued to produce fecal pellets in all treatments. The average daily egg and fecalpellet production rates at various salinities were a functionof the females’ ages (Fig. 2) . There was a generalincrease in egg hatching success with increasing salinity (P=0.074) , although A. tonsa showed relatively lower hatching rates at the lowest salinity level tested. Maximum and minimum means of hatching success were recordedin 35 (80.09%) and 13 (71.32%) , respectively (Table 2) .

|
| Figure 1 Egg and fecal pellet production rates of A. tonsa in foursalinities of 13, 20, 35, and 45 within 7 consecutive days |

|
| Figure 2 Egg and fecal pellet production rates of A.tonsain three salinitiesof 13, 20, and 35 over total females’ reproductive experiments |
Mean development times were relatively similar (7.5-8.1 days) at different salinity treatments (P=0.183) . The salinity levels significantly (P=0.019) affected the survival rate prior to adult stage. Even though the reproductive duration of females was not statistically different (P=0.153) among treatments, a shorter reproductive period (20.08 days) was obtained at 35 than at salinities of 20 (21.13 days) and 13 (23.07 days) . There were no significant differences (P=0.324) in terms of the longevity of A. tonsa among the three salinitylevels examined (Table 2) .

|
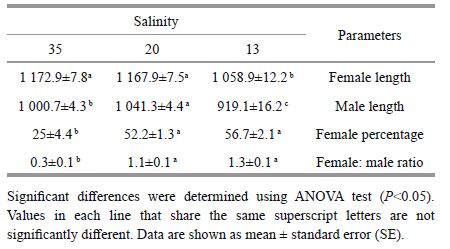
With the exception of prosome lengthin males at 35, salinity was positively correlated with themean length of A. tonsa for both femalesand males. Relatively similar increasing trends in prosome length of both females and males were observed with the increasing of salinity. There were no significant differences (P=0.251) in prosome length of females between salinitiesof 20 and 35. Female percentage clearly decreasedwith increasing salinity. Higher female percentages (56.7% and 52.2%, respectively) were significantly (P=0.032) observed across two salinities of 13 and 20 comparedto that in 35 (25%) . The sex ratios variedsignificantly (P=0.045) from 0.3 (35) to 1.3 (13) between treatments (Table 3) .
|
Many calanoidspecies inhabit estuarine waters and have a wide salinitytolerance. Their productivity may vary greatly, however, at different salinities (Castro-Longoria, 2003) . In the field, A. tonsa can be found at salinities from <5 up to ca. 30 (Cervetto et al., 1999) , and tolerate a wide salinityrange from nearly 0.0 to 52 (Rey et al., 1991) .
Most of the previous studieson the copepod egg production rate normally examinedegg output within 24 h or 48 h (Hirst and McKinnon, 2001) , or daily egg production for six (Castro-Longoria, 2003) and eight (Camus and Zeng, 2008) consecutive days. However, in our investigation, effects of salinity were examined throughout two separate periods.Our findings reveal that salinitiesof 20 and 35 resultedin the best conditions for the total egg production of A. tonsa, followed by 13 and 45, respectively, in trials lasting for seven consecutive days. The individual-based egg productionrate obtained in our study is favorably comparable to the rates obtained in different conditions reported by several authors (Matias-Peralta et al., 2005; Holste and Peck, 2006; Peck and Holste, 2006; Milione and Zeng, 2008) . For example, Holste and Peck (2006) reported the lowestand highest meanegg productionsat 30 (17 eggs/ (femalefld) ) and 14 (40 eggs/ (femalefld) ) in Baltic A. tonsa during five days with a 48-h egg hatching successof >75% for all salinities >13. Peck and Holste (2006) reported a direct effect of salinity regimes on the total egg production of A. tonsa; however contrary to our results, salinities of 14 and 20 gave more egg production than the three salinities of 6, 10 or 30. Additionally, mean final population growth of A. sinjiensis under salinities ranging (from 10 to 50) was significantly higher in 25, 30, and 35 than in other salinities after eight days culture (Milione and Zeng, 2008) . According to their results, there were clear trends of decline in mean final population as salinity changed below or above 30, with a much sharper decline at levels below 30. Also, the egg hatching rates of A. sinjiensis were relatively high (>80%) with no significant differences acrossthe wide range of salinities tested by Milione and Zeng (2008) . In A. clausi collected from the Solent-Southampton Water of United Kingdom, the egg production rates were noticeably lower in 15 and 20 after six days, with maximum daily egg production at 35 (22.4 eggs/ (femalefld) ) showing a clear decreasing pattern with decreasing salinity (Castro-Longoria, 2003) . They further found the highestpercentage of hatchedeggs at 35 (88.6%) followed by 30 (70%) , 25 (54.6%) , and 20 (25.3%) . In A. tonsa, daily egg production showed a generalincrease with time at four high-salinity incubations with low numbers of eggs produced at 15; a mean egg production of 16.3 eggs/ (femaleflday) was found at 25. Moreover, except for 15, the other sal inities gave high percentages of hatching success namely 82.6% (35) , 90.6% (30) , 94% (25) , and 62.6% (20) (Castro-Longoria, 2003) . A different type of response to salinity variations was shown by Chinnery and Williams (2004) in A. tonsa, in whichthe egg hatching successvaried from 55.9% (15.5) , 55.6% (20) to 85.4% (33.5) .
Expressed in terms of egg production rate per female per day over the entirelife span of a female A. tonsa, this gives fewer eggs produced at various salinities compared to other field and/or laboratory experiments. Examplesinclude a mean production of 19 eggs/ (femaleflday) in A. tonsa cultured in circular outdoor tanks for six months with natural phytoplankton diets (Ogle 1979) , a mean production of 23-27 eggs/ (femaleflday) in A. tonsa cultured in 200-L tanks (Stottrup et al., 1986) , and the egg numbers of 16 and 25/ (femaleflday) in A. clausi in multiple generation culturesfed two mixed diets of I.galbana and Pavlova lutheriat two concentrations of 1×106 and 1.5×106 cells/mL, respectively (Iwasaki et al., 1977) .However, Johnson (1974) found an average of 30.5 eggs/ (femaleflday) for A. tonsa in Yaquina Bay, Oregon (21℃ and 25) .
According to Srensen et al. (2007) and Rodriguez-Graoa et al. (2010) , mean egg production of individual females continuesfor about 3-4 weeks, and each female calanoidcopepod can produce20-30 eggs per day. On the other hand, Mauchline (1998) stated that the free-spawning calanoids such as various Acartia species may producebetween 11-50 eggs/ (femalefld) . Contrary to our results, Ohs et al. (2010) indicated that the fecundity of Pseudodiaptomus pelagicus wasnot significantly different among salinities except that fewer eggs were producedin egg sacs at the lowest and highestsalinities examined. Devrekeret al. (2009) did not observe any differencesin fecundities of the calanoid copepod Eurytemora affnis cultured at various salinities. Some of the differences in egg production, fecundity, and hatchability of the copepod in different studiesand also opposingdata from this study with those other studies can be attributed to inter-specific differences, geographic differences among different populations as well as different experimental conditions (food quality and quantity, water temperature, salinity and etc.) .
Studies on animalsacclimatized to salinity fluctuations show that wider salinity tolerance in A. tonsa is linked to a more stable energetic balanceat low salinities (i.e., gross growth effciency, cost of growth) , and that such animals perform better in terms of feeding, egg production rate, egg hatching success, and naupliar survivalrate over a wider salinity range (Castro-Longoria, 2003; Chinnery and Williams, 2004; Calliari et al., 2006) . Lance (1965) demonstrated that the respiratory rates of A. tonsa increased by a factor of 78% when the originalsalinity (36.4) was lowered to 14.7. The increasingtrend in the needof supplementary energy for osmoregulation in copepodsmay be one of the most important parameters in the shifting of higher energy sourcesfor osmoregulation at the expenseof other substantial processes such as egg and fecal pellet production (Péqueux, 1995) .
The highest fecal pellet productionrates of A. tonsa in the current study were obtained at 35 indicatingthe reduction of feeding (estimated as fecal pellet production) in A. tonsa in two lowersalinities 13 and 20. Previousfindings of reductions in feeding and fecal pellet production beyond a thresholdof 50% seawater dilutionwere reported by Mauchline (1998) in Acartia discaudata and A. bifilosa. In the present study, the production of fecal pelletsoccurred during the whole life, being more regular than egg production with a maximum daily rate in the first part of life, and showed a decrease until death.
4.2 Developmenttime, naupliar survival, prosome length, adult sex ratio, and longevitySeveral environmental factors such as salinity, temperature and food supply can affect the development time in copepods (Mauchline, 1998; Matias-Peralta et al., 2005) . In the currentexperiment, a n accelerated mean development time was found from naupliar to adult when the salinityincreased. A. tonsa incubated at high salinity levels showed higher growth rates and reached maturityin a shortertime. Also, the time taken for nauplii to reach maturitywas affected by salinity; lower salinities produced slower development times, but this difference was not often significant between the three salinity regimes tested. The development time of A. tonsa in the current investigation is in agreement with those reported by Hagiwara et al. (1995) and Chinnery and Williams (2004) . The fastest development was attainedby harpacticoid copepods (Nitocra affnis f. californica) reared under salinities of 30-35 comparedto other treatments (5-25) (Matias-Peralta et al., 2005) . The time of development from the first naupliarstage to adult in Tigriopus japonicusbecame shorter with increasing salinity (Hagiwara et al., 1995) . Contraryto our results, the development times in the calanoid copepod, Arctodiaptomus salinus decreased with an increase in salinity at 10℃ (Rokneddine, 2005) .
In general, Klein Breteler (1982) estimatedsurvival rates of 46%-75%during one generation for three calanoid speciesincluding A. clausi, Temora longicornis, and Centropageshamatus (in comparison with the survival rates of 47%-63% in the present study) . Similarlyto our results, Chinnery and Williams (2004) reportedthat the greatestnaupliar survival of A. tonsa was recorded at 33.3 (72.9%) , which generally decreasedwith reduced salinity (20.9% at 15.5) .
Mean prosome length in females of A. tonsa was greater at higher (20 and 35) than at low salinity (13) ; in the present investigation however, such a trend was not observedin the prosome length of males during one generation. Nonetheless, the female prosome length of Eurytemora affnis at temperatures from 10 to 20℃ was not considerably affected by different salinities from 8 to 35. At 14℃, female E. herdmani showed a linear and significant size increase with decreasing salinity from 33 (0.96 mm) to 20 (1.127 mm) and 15 (1.227 mm) but the differencewas less pronounced in males (Katona, 1970) . Gaudy and Verriopoulos (2004) found that salinity can be a decisive factor in the determination of copepod length in environments characterized by salinity variations. It appearsthat several environmental factors such as temperature, food supply, size-selective predation, and salinityhave been recognized to influence the final body size in copepods (Mauchline, 1998; Gaudy and Verriopoulos, 2004) .Furthermore, correlations of prosome length with salinitywere statistically positive for almost all small copepods. At low temperatures, an increasein salinity was shown to evoke an increase in size of the various developmental stages of Arctodiaptomus salinus, whereas the reverse was observed at higher temperatures (Rokneddine, 2005) . In the copepod Sinocalanus tenellus, Kimoto et al. (1986) recordedsuccessful post-embryonic development in salinities of 5-30, but both the rate of growth and overall body size decreasedat both the lowest and the highest salinities.
Sex ratio in copepods has been found to be influenced by temperature (Miliou, 1993, Mauchline, 1998) , salinity (Katona, 1970; Miliou, 1993) , geographicaldistribution (Gusmo and McKinnon, 2009) , degree of inbreeding and delay of mating (Mauchline, 1998) , food concentration (Irigoien et al., 2000) , and inter-sexes of the populations (Gusmo and McKinnon, 2009) . The proportions of males and females in adult A. tonsa observed in the present experiment were significantly affected by salinity, in which, a relatively clear trend of accelerated female ratio was obtained with decreasing salinitylevels (20 and 13) . These results correspond well to those obtained by Katona (1970) and Miliou (1993) .More laboratory and field studies are requiredto provide data on sex ratio in order to understandthese hypotheses better in future.
It appears that, depending on the speciesand the environmental factors, particularly temperature and foodsupply, calanoids may mature (from eggs or nauplii to adults) withina few days to a few weeks and the total life span of the animalis about several months (Mauchline, 1998; Stttrup and McEvoy, 2003) . The mean life span of A. tonsa varied between 29-32 days in our treatments with no significant effect of salinity in laboratorycultures. These results are consistent with Matias-Peralta et al. (2005) in the harpacticoid Nitocra affnis f. californica and Ohs et al. (2010) in Pseudodiaptomus pelagicus. Matias-Peralta et al. (2005) further reportedthat the longevity of Nitocra affnis f. californica was significantly shortened in lower (10-25) compared to higher salinities (30-35) .
5 CONCLUSIONThe adaptability of A. tonsa as a live feed for marine larviculture, to low and high salinities is a useful attribute for aquaculture purposes.Therefore, maximizing copepod productivity is the major goal, which is probably affected by salinity conditions. Our results clearly demonstrate that, although the A. tonsa can tolerate a wide range of salinitiesfrom 13 to 45, prolonged exposuresto subnormal salinities (such as 45) affect its reproduction. Thereby, becauseof relativelyhigher egg production rates and feeding performance, better egg hatching success, and faster growth to maturity in A. tonsa specimens, this copepod could be maintained and cultured in a salinity of 35 in order to maximizeits productivity.
6 ACKNOWLEDGEMENTWe are most gratefulto Dr. Mohammad Kazem Khalesi for the technicalassistance with laboratory equipments and English revision.
| Bagheri S, Sabkara J, Mirzajani A, Khodaparast S H, Yosefzad E, Yeok F S, 2013. List of zooplankton taxa in the Caspian Sea waters of Iran. J. Mar. Biol., 2013 : 134263 . |
| Calliari D, Andersen C M, Thor P, Gorokhova E, Tiselius P, 2006. Salinity modulates the energy balance and reproductive success of co-occurring copepods Acartia tonsa and A. clausi in different ways. Mar. Ecol. Prog. Ser., 312 : 177 –188. Doi: 10.3354/meps312177 |
| Camus T, Zeng C S, 2008. Effects of photoperiod on egg production and hatching success, naupliar and copepodite development, adult sex ratio and life expectancy of the tropical calanoid copepod Acartia sinjiensis. Aquaculture, 280 (1-4) : 220 –226. Doi: 10.1016/j.aquaculture.2008.05.008 |
| Camus T, Zeng C S, 2009. The effects of stocking density on egg production and hatching success, cannibalism rate, sex ratio and population growth of the tropical calanoid copepod Acartia sinjiensis. Aquaculture, 287 (1-2) : 145 –151. Doi: 10.1016/j.aquaculture.2008.10.005 |
| Carlotti F, Rey C, Javanshir A, Nival S, 1997. Laboratory studies on egg and faecal pellet production of Centropages typicus: effect of age, effect of temperature, individual variability. J. Plankton Res., 19 (8) : 1143 –1165. Doi: 10.1093/plankt/19.8.1143 |
| Castro-Longoria E, 2003. Egg production and hatching success of four Acartia species under different temperature and salinity regimes. J. Crustacean Biol., 23 (2) : 289 –299. Doi: 10.1163/20021975-99990339 |
| Cervetto G, Gaudy R, Pagano M, 1999. Influence of salinity on the distribution of Acartia tonsa (Copepoda, Calanoida). J. Exp. Mar. Biol. Ecol., 239 (1) : 33 –45. Doi: 10.1016/S0022-0981(99)00023-4 |
| Chen Q X, Sheng J Q, Lin Q, Gao Y L, Lv J Y, 2006. Effect of salinity on reproduction and survival of the copepod Pseudodiaptomus annandalei Sewell, 1919. Aquaculture, 258 (1-4) : 575 –582. Doi: 10.1016/j.aquaculture.2006.04.032 |
| Chinnery F E, Williams J A, 2004. The influence of temperature and salinity on Acartia (Copepoda: Calanoida) nauplii survival. Mar. Biol., 145 (4) : 733 –738. |
| Devreker D, Pierson J J, Souissi S, Kimmel D G, Roman M R, 2012. An experimental approach to estimate egg production and development rate of the calanoid copepod Eurytemora affinis in Chesapeake Bay, USA. J. Exp. Mar. Biol. Ecol., 416-417 : 72 –83. Doi: 10.1016/j.jembe.2012.02.010 |
| Devreker D, Souissi S, Winkler G, Forget-Leray J, Leboulenger F, 2009. Effects of salinity, temperature and individual variability on the reproduction of Eurytemora affinis (Copepoda; Calanoida) from the Seine estuary: a laboratory study. J. Exp. Mar. Biol. Ecol., 368 (2) : 113 –123. Doi: 10.1016/j.jembe.2008.10.015 |
| Drillet G, Frouël S, Sichlau M H, Jepsen P M, Højgaard J K, Joarder A K, Hansen B W, 2011. Status and recommendations on marine copepod cultivation for use as live feed. Aquaculture, 315 (3-4) : 155 –166. Doi: 10.1016/j.aquaculture.2011.02.027 |
| Gaudy R, Verriopoulos G, 2004. Spatial and seasonal variations in size, body volume and body proportion (prosome: urosome ratio) of the copepod Acartia tonsa in the semiclosed ecosystem (Berre lagoon, Western Mediterranean). Hydrobiologia, 513 (1) : 219 –231. Doi: 10.1023/B:hydr.0000018190.34856.d2 |
| Guillard R R L. 1975. Culture of phytoplankton for feeding marine invertebrates. In: Smith W L, Chanley M H eds. Culture of Marine Invertebrate Animals. Plenum Press, New York, USA. p.26-60. |
| Gusãmo L F M, McKinnon A D, 2009. Sex ratios, intersexuality and sex change in copepods. J. Plankton Res., 31 (9) : 1101 –1117. Doi: 10.1093/plankt/fbp059 |
| Hagiwara A, Lee C S, Shiraishi D J, 1995. Some reproductive characteristics of the broods of the harpacticoid copepod Tigriopus japonicus cultured in different salinities. Fish. Sci., 61 (4) : 618 –622. |
| Hirst A G, McKinnon A D, 2001. Does egg production represent adult female copepod growth? A call to account for body weight changes. Mar. Ecol. Prog. Ser., 223 : 179 –199. Doi: 10.3354/meps223179 |
| Holste L, Peck M A, 2006. The effects of temperature and salinity on egg production and hatching success of Baltic Acartia tonsa (Copepoda: Calanoida): a laboratory investigation. Mar. Biol., 148 (5) : 1061 –1070. Doi: 10.1007/s00227-005-0132-0 |
| Irigoien X, Obermuller B, Head R N, Harris R P, Rey C, Hansen B W, Hygum B H, Heath M R, Durbin E G, 2000. The effect of food on the determination of sex ratio in Calanus spp. : evidence from experimental studies and field data. ICES J. Mar. Sci., 57 (6) : 1752 –1763. |
| Iwasaki H, Katoh H, Fujiyama T, 1977. Cultivation of marine copepod, Acartia clausi Giesbrecht. I. Factors affecting the generation time and egg production. Bull. Plankton Soc. J a p a n, 24 (1) : 55 –61. |
| Johnson J K. 1974. The dynamics of an isolated population of Acartia tonsa dana (Copepoda) in Yaquina Bay, Oregon. Oregon State University, Corvallis, Oregon, USA. 97p. |
| Katona S K, 1970. Growth characteristics of the copepods Eurytemora affinis and E. h erdmani in laboratory cultures. Helg. wiss. Meeresunters., 20 (1) : 373 –384. |
| Kimoto K, Uye S I, Takashi O, 1986. Egg production of a brackish-water calanoid copepod Sinocalanus tenellus in relation to food abundance and temperature. Bull. Plankton Soc. J a p a n, 33 (2) : 133 –146. |
| Klein Breteler W C M, Fransz H G, Gonzalez S R, 1982. Growth and development of four calanoid copepod species under experimental and natural conditions. Netherlands. J. Sea. Res., 16 : 195 –207. Doi: 10.1016/0077-7579(82)90030-8 |
| Lance J, 1965. Respiration and osmotic behaviour of the copepod Acartia tonsa in diluted sea water. Comp. Biochem. Phys., 14 (1) : 155 –165. Doi: 10.1016/0010-406X(65)90016-2 |
| Matias-Peralta H, YusoffF M, ShariffM, Arshad A, 2005. Effects of some environmental parameters on the reproduction and development of a tropical marine harpacticoid copepod Nitocra affinis f. californica Lang. Mar. Pollut. Bull., 51 (8-12) : 722 –728. Doi: 10.1016/j.marpolbul.2005.02.047 |
| Mauchline J, 1998. The biology of calanoid copepods. Adv. Mar. Biol., 33 : 1 –13. Doi: 10.1016/S0065-2881(08)60234-5 |
| McKinnon A D, Duggan S, Nichol P D, Rimmer M A, Semmens G, Robino B, 2003. The potential of tropical paracalanid copepods as live feeds in aquaculture. Aquaculture, 223 (1-4) : 89 –106. Doi: 10.1016/S0044-8486(03)00161-3 |
| Milione M, Zeng C S, 2008. The effects of temperature and salinity on population growth and egg hatching success of the tropical calanoid copepod, Acartia sinjiensis. Aquaculture, 275 (1-4) : 116 –123. Doi: 10.1016/j.aquaculture.2007.12.010 |
| Miliou H, 1993. Temperature, salinity and light induced variations on larval survival and sex ratio of Tisbe holothuriae Humes (Copepods: harpacticoida). J. Exp. Mar. Biol. Ecol., 173 (1) : 95 –109. Doi: 10.1016/0022-0981(93)90209-7 |
| Ogle J, 1979. Adaptation of a brown water culture technique to the mass culture of the copepod Acartia tonsa. Gulf Res. Rep., 6 (3) : 291 –292. |
| Ohs C L, Rhyne A L, Grabe S W, DiMaggio M A, Stenn E, 2010. Effects of salinity on reproduction and survival of the calanoid copepod Pseudodiaptomus pelagicus. Aquaculture, 307 (1-2) : 219 –224. |
| Peck M A, Holste L, 2006. Effects of salinity, photoperiod and adult stocking density on egg production and egg hatching success in Acartia tonsa (Calanoida: copepoda): optimizing intensive cultures. Aquaculture, 255 (1-4) : 341 –350. Doi: 10.1016/j.aquaculture.2005.11.055 |
| Péqueux A, 1995. Osmotic regulation in crustaceans. J. Crustacean Biol., 15 (1) : 1 –60. Doi: 10.1163/193724095X00578 |
| Rey J R, Kain T, Crossman R A, Peterson M, Shaffer J, Vose F, 1991. Zooplankton of impounded marshes and shallow areas of a subtropical lagoon. Florida., Scientists, 54 (3-4) : 191 –203. |
| Rodriguez-Graoa L, Calliari D, Tiselius P, Hansen B W, Sköld H N, 2010. Gender-specific ageing and non-Mendelian inheritance of oxidative damage in marine copepods. Mar. Ecol. Prog. Ser., 401 : 1 –13. Doi: 10.3354/meps08459 |
| Rokneddine A, 2005. The influence of salinity and temperature on the growth of Arctodiaptomus salinus (DADAY, 1885) (Copepoda, Calanoida), from the temporary salt marsh, La Sebkha Zima, Morocco. Crustaceana, 77 (9) : 1025 –1044. |
| Sokal R R, Rohlf F J. 1981. Biometry. 2nd edn. W. H. Freeman and Company, New York, USA. 776p. |
| Sørensen T F, Drillet G, Engell-Sørensen K, Hansen B W, Ramløv H, 2007. Production and biochemical composition of eggs from neritic calanoid copepods reared in large outdoor tanks (Limfjord, Denmark). Aquaculture, 263 (1-4) : 84 –96. Doi: 10.1016/j.aquaculture.2006.12.001 |
| Støttrup J G, McEvoy L A. 2003. Live Feeds in Marine Aquaculture. Blackwell Science Ltd, Oxford, UK. 337p. |
| Stottrup J G, Richardson K, Kirkegaard E, Pihl N J, 1986. The cultivation of Acartia tonsa Dana for use as a live food source for marine fish larvae. Aquaculture, 52 (2) : 87 –96. Doi: 10.1016/0044-8486(86)90028-1 |
| Trujillo-Ortiz A, 1990. Hatching success, egg production and development time of Acartia califo r niensis Trinast (Copepoda: calanoida) under laboratory conditions. Cienc. Mar., 16 (1) : 1 –22. |
 2016, 34
2016, 34


