Institute of Oceanology, Chinese Academy of Sciences
Article Information
- Jianfeng NIU(牛建峰), Jianhua FENG(冯建华), Xiujun XIE(解修俊), Shan GAO(高山), Guangce WANG(王广策)
- Involvement of cyclic electron flow in irradiance stress responding and its potential regulation of the mechanisms in Pyropia yezoensis
- Journal of Oceanology and Limnology, 34(4): 730-739
- http://dx.doi.org/10.1007/s00343-016-4236-9
Article History
- Received: Oct. 31, 2014
- Accepted: May. 28, 2015
2. College of Marine Science and Engineering, Tianjin University of Science and Technology, Tianjin 300457, China;
3. Nantong Branch, Institute of Oceanology, Chinese Academy of Sciences, Nantong 226006, China;
4. Laboratory for Marine Biology and Biotechnology, Qingdao National Laboratory for Marine Science and Technology, Qingdao 266071, China
Light energy is absorbed by the photosynthetic apparatus and converted into chemical energy to fix CO2 in photoautotroph. However, beyonda certain light intensity, photosynthesis would become saturated and the extra energy absorbed by the photosystems may becomeharmful to plants.Most photoautotrophic organisms can switch the states of light-harvesting complexesin thylakoid membrane between effcient light harvesting and quenching forms, whichmeans that they can adapt to a wide range of light conditions (Brooks et al., 2013) . Various mechanisms are used to avoid the potential damage caused by reactive intermediates (Niyogi, 1999, 2000) . Among them, the cyclic electron flow (CEF) is thought to be a necessary componentof the photoprotective mechanisms. Through the CEF, electrons are recycled from NADPH which is derived from reducedferredoxin (Fd) , to plastoquinone (PQ) , and then to the cytochrome b6f complex (Cyt b6f) (Heber and Walker, 1992; Fork and Herbert, 1993; Ravenel et al., 1994; Bendall and Manasse, 1995; Endo and Asada, 1996; Joliot and Joliot, 2002) , acidificationof the thylakoid lumen increasesand thermal dissipation is induced (Müller et al., 2001) .
Photoprotection through thermal dissipation is believed to be a criticalmechanism for survivaland fitness of a higher plant.Several differentmechanisms, collectivelytermed “non-photochemical quenching” (NPQ) , have been reported (Melis, 1999; Niyogi, 1999; Li et al., 2009) . One of them is qE, which is known to function when there is a high pH gradient across the thylakoid membrane.In most plants and eukaryotic algae, this is a rapidly inducible quenching mechanism that dissipatesexcess photons absorbed by PS II (Horton et al., 1996) . A close relationship between zeaxanthin and qE has been demonstrated in a wide varietyof plants (Demmig-Adams and Adams, 1996; Porcar-Castell et al., 2008) . In addition to qE, another mechanism termed photoinhibitory quenching (qI) for dissipation of excess photons belongs to the sustained dissipation rather than rapidinduction. This mechanism usuallyleads to a decline in the size of light-harvesting antennae, the inhibition of genes associated withphotosynthesis (Escoubas et al., 1995; Maxwell et al., 1995;Pfannschmidt et al., 1999) , or degradation of existing light-harvesting proteins (Yang et al., 1998) . It seems that at least a part of qI has a similar mechanism to qE (Gilmore and Bjrkman, 1995; Ruban and Horton, 1995) , except that qE is dependenton the pH gradient across cell membrane (Verhoeven et al., 1998, 1999) . When the photosystems cannot utilize and dissipate all the photons absorbed, singlet oxygen speciescan accumulate, which are highlyharmful to proteinsand lipids of the photosynthetic apparatus. Therefore, some plants have evolved a quenching mechanism for 1O2 that uses carotenoids (Hideg et al., 1998) . The excited energy is transferred to the carotenoids and dissipated as heat (Foyer and Harbinson, 1999) . However, the totaldissipation via this pathway is quite small.
Other mechanisms to protect the photosynthetic apparatus from damage, apart from thermal dissipation, have been suggested (Niyogi, 1999) . These photoprotective pathways usually involve antioxidant reactions (Asada, 1999) , peroxidation repair (Baier and Dietz, 1999) and degradation of D1 protein in the PS II reaction centers (Melis, 1999) . In addition to these, electronsderived from PS II may also be transferred to O2 directly by PS I to generate O2-, which may subsequently be reduced to H2O2 by superoxide dismutase and then to H2O by ascorbate peroxidase. Through these reactions, the electrons derived from water are delivered to O2 to form water by PS I (Asada, 1999) . This water-water cycle runs when the photon intensityexceeds that needed for CO2- assimilation. As a rapid scavenging mechanism for O2- and H2O2 at the site of their generation, this pathway contributes to the generation of ∆pH and protects the organismfrom excess light.The cycle is believed to be widespread in plants (Long et al., 1994) and it has been reported that between 10% and 20% of the total linear electronflow (LEF) is throughthe water-water cycle (Lovelock and Winter, 1996) . It has alsobeen suggested that the water-water cycle is the main mechanismused to protect symbiotic alga in coral from photoinhibition and coral bleaching (Lesser, 1997) .
Chlororespiration is a type of electrontransport that transfers electronsfrom the plastoquinone pool to O2 and is mediatedby enzymes that are similar to those in the respiratory chain (Bennoun, 1982) . The existence of this pathway in Chlamydomonas reinhardtii and Arabidopsis has been demonstrated via the determination of plastoquinol oxidation (Cournac et al., 1997; Redding et al., 1999; Cournac et al., 2000) . The critical signi ficance of this pathway belongs to the mitigation of over-reduction of PS II acceptors in intense light (Peltier and Cournac, 2002) .
With the accumulation of literatures, it seems that the responsesof phototrophs to excessive irradiation vary in amplitude and in the way that they respond. Chlamydomonas has been foundto have a large state transition and qE is probably the most importantnon- photochemicalquenching method in higher plants (Finazzi et al., 2006) . Porphyra yezoensis in genus Pyropia (Sutherland et al., 2011) lives in the intertidal zone and can survive full sunlight at low tide.It has been suggested that the CEF around PS I in this alga plays an important role in the stress response (Lin et al., 2009) .In this study, we focused on analyzing the effects of different inhibitors on CEF and how the photosynthetic parameters are affected by different light intensities. Based on our analyses, we suggest that the electron back-pressure in the systemis the main factor regulating the involvement of different protective mechanisms against light stress in Py.yezoensis.
2 MATERIAL AND METHOD 2.1 Plant material preparationFresh Py. yezoensis blades were random collected from the intertidal zonesof Qingdao (36°3'0"N, 120°21'57"E) in China. After rinsing with plenty of seawater, the healthy thalli were cultured temporarily in Provasoli’s enrichedseawater medium at 15°C one day beforethe irradiation stresstreatment and photosynthesis determination. The medium inside the tanks was agitated by constant aeration and irradiated with 50 μmol photons/ (m2∙s) of cool-white lightin a 12 h light/12 h dark cycle.
2.2 Relationshipbetween photosynthetic activity and irradiance in Py. yezoensisAfter keepingthe sample in darkness for 10 min, the relativeelectron transport rate in PS II was recorded at differentphotosynthetic actinic irradiances (PAR 0, 1, 21, 56, 111, 146, 186, 231, 281, 336, 396, 461, and 531 μmol photons/ (m2∙s) ) using an Imaging PAM (HeinzWalz GmbH, Effeltrich, Germany) connected to a computer with WinControl software. This allowed us to construct the relationship between ETR (II) and irradiation intensity.
2.3 Determination of the photosynthetic parametersThe photosynthetic parameters of the samples treated with different irradiances, were determined at room temperature using aDual-PAM-100 measuring system (Heinz Walz, Effeltrich, Germany) connected to a PC. Experiments were carried out using the automated proceduresprovided by the Dual-PAM software at room temperature. The intrinsic minimum fluorescence (F0) of each samplewas detected under light at 7 μmol/ (m2∙s) and the maximum fluorescence (Fm) was obtained by using saturatingactinic light pulses (SP, intensity 6 000 μmol/ (m2∙s) , duration 0.8 s) . Variablefluorescence (Fv) was measuredby estimating the difference between Fm and F0. With repetitive application of saturation pulses, the fluorescenceand P700 parameters were derived automatically under the control of WinControl software. The relative photosynthetic electron transport rate (ETR) was applied to indicate the activity of the photosystem (Figueroa et al., 2003) . Non-photochemical quenching (NPQ) , which represents the dissipation of photosynthetic energy conversion (Schreiber et al., 1986, 1995) , was used to assess the stress levels occurred in Py. yezoensis. The non-photochemical quantum yield of PS I caused by donor side limitation, Y (ND) , was applied to indicate the oxidization of P700.
Before performance of the irradiation stress experiments, the algae were treated for different time (from half an hour to four hours) with 200 μmol photons/ (m2∙s) illumination firstly and the photosynthetic parameters were determined. The results revealed that the photosynthetic activityof the tested algaefluctuated greatlydue to various treatment duration (datanot shown) . When the treatmenttime of the algae was controlled at about 4 h, the profile of photosynthesis parameters showed a comparatively steady state with a low standarddeviation. Thus, all the samples in the following experiments were treated for 4 h with different light intensities, subjected to dark adaptation for 10 min, and then determined through a Dual-PAM-100 measuringsystem. All the data were expressed as the mean±standard deviation derived from threeindependent measurements. Statistical analysiswas performed using two way ANOVA and the values were deemed to be significantly different when P<0.05.
2.4 Effects of inhibitors on the Py. yezoensis photosynthetic parametersTo determine the possible importance of CEF in Py. yezoensis at different irradiance levels, rotenone, antimycin A, and N-ethyl-maleimide were applied to test the effects on the photosynthetic parameters. The sampleswere first irradiatedfor 4 h under the designed intensities. Then, the sampled algae was incubated with 100 μmol/L inhibitorin dark for 10 min and its photosyn thetic parameterswere determined using a Dual-PAM-100 apparatus (Heinz Walz, Effeltrich, Germany) . To analyzethe direct effects of each inhibitor on the CEF, the difference between ETR (I) and ETR (II) were determined and the inhibition ratios for ETR (I) were calculatedas [ETR (I) (treated by inhibitor) −ETR (I) (no inhibitor) ]/ETR (I) (no inhibitor) .
The values of ETR (II) , Y (NPQ) , Y (ND) and the relative value of CEF were presentedin mean±standard deviation derived from three independent measurements. Statistical analysis was performed using two way ANOVA and the values were deemed to be significantly different when P<0.05.
3 RESULT AND DISCUSSION 3.1 Determination of the ETR (II) under continual increased irradiation intensityThe relationship between electron transport through PS II and the irradiation intensity is shown in Fig. 1. ETR (II) increased as the radiationlevels rose and the slope of the line decreased significantly when the light intensity was approaching to 400 μmol photons/ (m2∙s) . Although the exact saturated irradiation intensity determined here through the rapid lightcurve was believedto be underestimated (Nitschke et al., 2012) , we suggested that the saturated irradiation intensity for Py. yezoensis was about 400 μmol photons/ (m2∙s) . Therefore, the following stress treatmentexperiments were designedbased on this irradiation level.
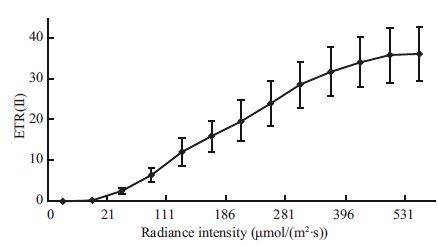
|
| Figure 1 P-I curvesof PSII (ETR (II) vs. PAR) of Py. yezoensis |
Compared to the control, ETR (II) decreased (Fig. 2a) significantly (P<0.001) as radiationintensity increased to 400 μmol photons/ (m2∙s) , but the relative value of CEF increased (Fig. 2b) . This suggested that the electron donors for PS I accumulated when the light reachednear the saturation point and that the CEF around PS I was the mechanism used to consume light energy which absorbed but could not be used. However, ETR (II) did not decreaseas irradiance increased from 400 to 1 200 μmol photons/ (m2∙s) (Fig. 2a) . Moreover, the non-photochemical quenching did not increaseaccordingly (Fig. 2c) . The results obtained here seemed to be not in line with the common modelof photosynthetic control, which suggested that the Benson-Calvin reactionwould be limited by increasing irradiance. It seemed that the increasing of light intensityfrom 400 to 1 200 μmol photons/ (m2∙s) , did not limit carbonassimilation as in most other photosynthetic organisms. This might contribution to the decreaseof the electron flow by restricting the reactionrate between PQ and the Cyt b6f complex and inhibition of photochemical quenching (Bendall, 1982) . Thus, Py. yezoensis contains some uniquepathways that enableit to avoid the loss of excessive energy throughcertain mechanisms.

|
| Figure 2 Effects of radiation intensityto the photosynthetic parameters in Py. yezoensis |
No matter antimycin A or rotenonehad distinct effects on the electron flow around PS I under different light intensities (Table 1) . In other words, both the Fd- dependent and the NAD (P) Hdehydrogenase complex (NDH-complex) dependent CEFs were involved in the stress responding, but did not always operate effciently during a high light stress response in Py.yezoensis. It is well-known that N-ethyl-maleimide inhibits ferredoxin: NADP+ reductase (FNR) , which leads to the suspension of linear electrontransportation and the inhibition of NDH-dependent CEF. As shown in Fig. 3a, ETR (II) stopped after the additionof N-ethyl-maleimide. The NDH-complex dependent CEF was inhibited and the Fd-dependent CEF should also be inhibiteddue to the reduced amountsof Fd. However, ETR (I) was still active.Therefore, there must be an alternative CEF pathway for operating, and it has been suggestedthat a rotenone-insensitive NAD (P) H: (PQ-acceptor) oxidoreductase might be involved in the reductionof the PQ pool. This enzyme has been reported in tobacco and it is a flavoprotein that catalyzesPQ reduction using NADH or NADPH as the electron donors (Corneille et al., 1998) . Our results reveal many cyclic electrontransport schemes in Py. yezoensis that enable it to inhabitin a place where light intensity changes dramatically.

|
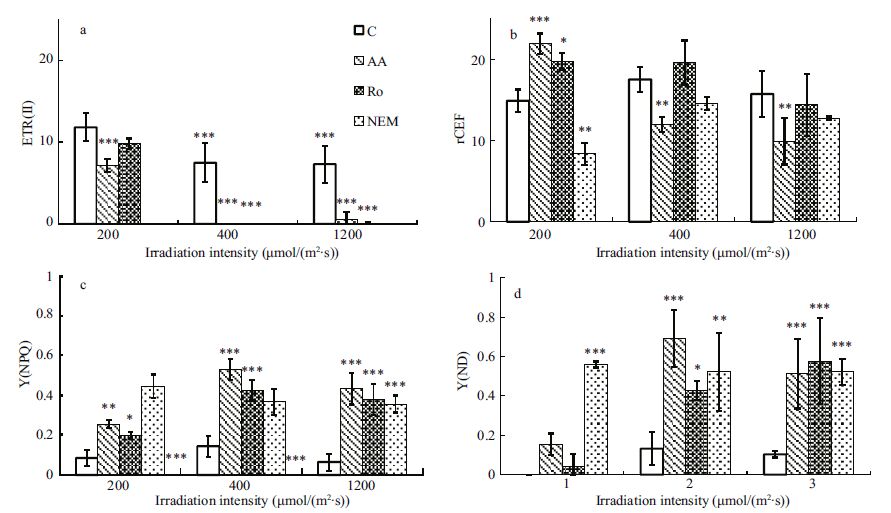
|
| Figure 3 Effects of different inhibitorsto the photosynthetic parameters in Py. yezoensis treated with different irradiations |
Antimycin A caused ETR (I) increase until it reached maximumat 200 μmol photons/ (m2∙s) (Table 1) . The additionof rotenone producedsimilar results as antimycin A (Table 1) , but produced a higher ETR (II) , a lower Y (ND) , and a lower quantum yield of regulated energy dissipation in PS II (Fig. 3a, d, c) . Moreover, the decreased ETR (II) and the increase in the relativevalue of CEF caused by the additionof antimycin A was larger than that in the algae treated with rotenoneat 200 μmol photons/ (m2∙s) . When the light intensity reached near the saturation point, the addition of an inhibitor usuallyabolished ETR (II) (Fig. 3a) . This indicatedthat the re dox state maintaining of electron transferchain was attributed to CEF, which was utmost important for the proper function of photosynthesis. The addition of rotenone increased the relative value of CEF slightly (Fig. 3b) , but antimycin A inhibited the relative value of CEF obviously (P<0.01) (Fig. 3b) . Moreover, the increases in Y (ND) and Y (NPQ) , due to the effects of antimycin A, were higher than that causedby rotenone (Fig. 3c, d) . This suggestedthat the Fd-dependent cyclic electron transfer around PS I was the main regulation mechanism when the light intensity was near or below saturation point. At this case, the electrontransfer intersystem chain was moderately reduceddue to the decrease in ETR (II) and the limited NADP+availability (Arnon and Chain, 1975) . The demand for NADPH in the dark reaction or by other photoprotective mechanisms limited the distribution of NADPH to the NDH-dependent CEF pathway. However, the accumulation of NADPH, due to the inhibition of the NDH-dependent CEF, may reduce Fd+ and thus increaseFd-dependent CEF levels.
Under severelight stress conditions, the total CEF in the samples was not significantly different from the CEFs recordednear the saturating light intensity treatment (Fig. 2b) . However, the addition of rotenone decreased the relative valueof CEF in the severelight stressed samples comparedto the increase seen near the saturated light intensity treatment (Fig. 3b) . Y (ND) in the samples treatedwith rotenone increased significantly as the light intensity rose from 400 to 1 200 μmol photons/ (m2∙s) (Fig 3. d) , which suggested that the contribution made by the NDH-complex to the total CEF became more and more important. At the same time, Y (ND) decreased as irradiation stress rose when the samples were treated with antimycin A. The Fd-dependent pathway may also have been weakened due to the over reductionor oxidation of the electron carriers (Bukhov and Carpentier, 2004) . This suggestedthat the Fd-dependent and NDH- dependent CEF were both important pathways under light stress conditions. The Fd-dependent CEF is probably the most importantbecause it is an effcient and fast response mechanismthat does not require de novoprotein synthesis, whereasthe NDH-complex dependent CEF mechanism dependslargely on the input of NAD (P) H from the stroma (Bukhov and Carpentier, 2004) . This is especially important in Py.yezoensis, which experiences extreme environmental variations in the intertidalzone (Bukhov and Carpentier, 2004) . Cooperation between these two pathways meansthat the stressresponse network becomes more flexible and is able to meet the ATP supply requirements.
N-ethyl-maleimide specifically inhibitsFNR (Ravenel et al., 1994) , which has been identified as a chloroplast reductase, catalyzing the electrontransfer from reduced Fd to NADP+ to produce assimilatory power during linear flow. In cyclic flow, the electron is usuallyrecycled from eitherreduced Fd or NADPH to PQ and then to the Cyt b6f complex (Heber and Walker, 1992; Fork and Herbert, 1993; Bendall and Manasse, 1995; Joliot and Joliot, 2002) . Thus, we inferred that the cyclicelectron transfer would be complemented by the other CEFs through the conversion between reduced Fd and NAD (P) H mediated by FNR when the preferredpathway was inhibited. That was the reason why the additionof antimycin A or rotenone did not cause a decrease in the relativevalue of CEF under controllight conditions.
However, the PQ pool is a common electron carrier in both the photosynthetic and chlororespiratory electron transport chain and chlororespiration could be inhibitedby antimycin A (Bennoun, 1982) . Molecular and biochemical investigation of chlororespiration revealed it involveda NADPH dehydrogenase complex (Peltier and Cournac, 2002) . Thus, the electronflow through this pathway could be inhibited by rotenone. The electrons that should have been transported through the chlororespiratory chain switched to CEF when antimycinA or rotenone wasused. The results here circumstantially indicatedwhy the CEF in the antimycinA or rotenone treated samples was significantlyhigher than that in the control algae (Fig. 3b) . It proved the existence of chlororespiration in Py. yezoensis under the light condition of 200 μmol photons/ (m2∙s) . Togetherwith the CEF, chlororespiration might play a role in the regulation of photosynthesis throughmodulating the electron flow around photosystem I. Under the radiation intensity of 400μmol photons/ (m2∙s) , antimycin A inhibitedthe relative value of CEF (P<0.01) significantly but rotenone made the value increased slightly (Fig. 3b) . The reason we suggested was photorespiration. Due to the inhibition of respiratory chain by rotenone (Foyer et al., 2009) , the electrons that should have been transported through photorespiration switched to CEF.
The additionof CEF inhibitors usually led to a significant increase in Y (NPQ) (Fig. 3c) , which implied that CEF was an essential ATP supply mechanism that used the energy absorbed by the photosynthetic apparatus, or it was involved in maintaining the oxidation reduction potential of certain enzymesthrough contributing electronsto some metabolic pathways during light stress response. However, it is unclear what the possiblemechanism to achieve this was or how the pathway protectedthe alga from excessiveirradiation damage in Py.yezoensis.
3.4 Analysis of the potential response mechanisms to light stress in Py. yezoensisMany thermal dissipation mechanismshave been recognized (Holt et al., 2005; Pascal et al., 2005) . Niyogi (2000) suggested that additional to the constitutive tripletdissipation of chlorophyll, inducible qE and qI sustained mechanisms and several oxygen-dependent electrontransport processes were also involvedin eliminating excessphotons in the chloroplasts. Previousstudies have shown that the modulation mechanisms for non-photochemical quenching differed, dependingon the plant species. State transition is thought the main method used by Chlamydomonas to dissipate the excess energy, while energy quenching by qE is probably the most common thermal dissipation mechanism in higherplants (Finazzi et al., 2006) . Furthermore, it has been suggested that the water-water cycle is the main mechanism when plants are subjected to rapidly fluctuating light intensities (Ruuska et al., 2000) .
To date, no reports have discussed thermal dissipation in Pyropia. It is not clear whether the xanthophyll cycle is the main way to eliminatethe excess photons absorbedby the photosynthetic apparatus or whetherqE is the main methodof thermal dissipation, as in higherplants. We analyzed the concentration of xanthophyll in Py. yezoensis cultured under different light intensities using HPLC. However, the pigment was not characterized in any group of treatments (data not shown) .Meanwhile, we noticed that the relativevalue of CEF level was high but the Y (NPQ) was low in light stressedPy. yezoensis. This suggested that Py. yezoensis has evolved a different strategyto avoid energy dissipation.The strategy is a sustainedenergy dissipation mechanismand CEF is an indispensable part of this process.When the electronsare recycled, ATP is producedand over-reduction of the electron transfer chain is avoided.
Chlororespiration is suggested to perform a protective function in photosynthesis due to the transportation of electrons to O2 in chloroplasts. It has been proposedas a mechanism to maintainATP syntheses in the active state in the dark or at very low irradiances (Peltier and Cournac, 2002) . As the results showed in our experiments, chlororespiration contributedsignificance when the light intensity at the controllevel but it declined with the increaseof light intensity. It is also related to the effcient biosynthesis of carotenoids (Josse et al., 2000) . Several previous papershave reported that carotenoids provide a safety release for excess electrons during light stress when PS I-dependent oxidationof PQ is less effcient (Carol et al., 1999; Wu et al., 1999) or when the repair of photodamaged PS II occurs (Trebst and Depka, 1997) . Althoughthe rate of electron transportation in chlororespiration is much lower compared to that in photosynthesis, it play a role in maintaining of proper redox state of the electron transport chain (Peltier and Cournac, 2002) . As presentation above, under the control light level, chlororespiration may be an alternative pathway to mitigate reductions in the electrontransfer chain. A relationship between chlororespiration and carotenoid biosynthesis has been proposed, especially during the early stagesof chloroplast development (Carol et al., 1999; Wu et al., 1999) .There have also been reports that chlororespiration might have a significant role in the repair of photodamaged PS II in fully green chloroplasts (Trebst and Depka, 1997) .As light intensity increases, 1Chl formed in the light harvesting complexes would exceed the amount that the PS II reaction centers could use and that would result in the accumulation of singlet excitation state molecules. If this occurs, 1Chl is converted into a long-lived triplet Chl through intersystem crossing. Finally, the excess energy is dissipated by transferring the energy from 3Chl to carotenoids (Niyogi, 2000) . It might be another constitutive mechanism that mediatesexcess energy dissipation, represents the strategy used by Py.yezoensis to deal with the stress of extremelyvariable light conditions.
The effcient non-photochemical quenching indicated that the de-excitation of 1Chl in the light harvesting complexeswas accompanied by the thermal dissipation of the excitation energy in PS II. However, as light levels increased, over reduction of the electrontransport chain occurredwhich led to the production of highly harmfuloxygen species such as O2- and H2O2 ( Niyogi, 1999; Foyer et al., 2009) . Thus, the activation of certain activeoxygen scavenging mechanisms is exceedingly importantif the photosystems are to be protected from light-induced damage. We suggestthat in Py. yezoensis, the water- water cycle is the pathwayused to remove these oxygen species, which is shown by the decrease in the relative value of CEF and constant ETR (II) when irradiance increased to 1 200 μmol photons/ (m2∙s) . Under the severe irradiance stress conditions, NAD (P) H and photoreduced Fd accumulated and the reduced flavodehydrogenases donatedelectrons to O2 directly, producing two molecules of O2- on the reducingside of PS I (Asada, 1999) . A similarresult was obtained when H2O2 was added to maize mesophyll chloroplasts (Neubauer and Yamamoto, 1992) . When this happens, activation of the water-water cyclemight be able to mitigate over reduction of the electrons carriers. We believe that there was competition betweenCEF and the water-water cycle over the supply of NAD (P) H+, which was not available due to the over reduction of the electron transferchain in the chloroplasts. This meant that O2- could be producedthrough autooxidation of the photoreduced Fd by the water-water cycle. This creates a proton gradient across the thylakoid membranes (Schreiber and Neubauer, 1990; Neubauer and Yamamoto, 1992, 1994) that leads to the production of ATP, makes ascorbate and glutathione remaining in reduced states in the chloroplasts. The active oxygen compounds, such as O2-and H2O2, are therefore scavenged before they interact with target molecules.
3.5 The role of CEF in photoprotectioncaused by excessive irradiationThe resultsfrom our inhibitor determination experiments showed that antimycin A and rotenone had different effects on the CEF. This implied that besides CEF, there were other mechanisms contribution to the protection of PS II from light- induced damage was different. In general, CEF played an important role in the formation of ∆pH acrossthe thylakoid membrane and in the production of the additional ATP needed for photosynthesis (Müller et al., 2001) . Secondly, the supply of electrons for chlororespiration was strongly related to CEF. Only the reduced PQ pool coulddonate electrons to an oxidase to produce ATP or to drive the biosynthesis of carotenoids, which directlycontrol excess 3Chl energy dissipation. Thirdly, the maintenance of a reducedPS I by CEF was a prerequisite for O2 reduction and the activation of scavenging enzymes, such as ascorbate and glutathione. Our results suggestthat the dissipation of excess energy throughdifferent mechanisms and the regulation of PS II activity are controlled by the oxidation-reduction stateof the electron transportcarriers and CEF may be the junction of these protection pathways in Py. yezoensis chloroplasts.
4 CONCLUSIONBased on our analyses, we suggest that the ability of Py. yezoensis to accommodate fluctuating irradiation levelsis based on a different mechanism than that found in most higher plants, which dissipate excessive photonsmainly through the thermal dissipation of qE. CEF controlledby electron back- pressure, seems to be the main factor that regulates the light stress protective mechanisms in Py.yezoensis. When the light stress was lower than the saturation intensity, chlorore spiration and CEF around PS I were the main mechanisms used to maintainthe proper ratio of ATP/NAD (P) H. The Fd-dependent pathway is a form of sustainedCEF that supplies additional ATP. While the contribution of CEF mediated with NDH-complex to total CEF increased continually with the increaseof light intensity. CEF began to decrease under the severe light stress conditions, which implied that the NADP+ pool or PQ+ pool was restrictedto very small size and that the electrons were transferred from the reducedPS I to O2. This activatedthe scavenging enzymesand the water-watercycle is probablythe mechanism used to remove the active oxygen compounds createdin the highly light stressed Py. yezoensis.
| Arnon D I, Chain R K, 1975. Regulation of ferredoxincatalyzed photosynthetic phosphorylations. Proceeding of the National Academy of Sciences of the United States of America, 72 (12) : 4961 –4965. Doi: 10.1073/pnas.72.12.4961 |
| Asada K, 1999. The water-water cycle in Chloroplasts: scavenging of active oxygens and dissipation of excess photons. Annual Review of Plant Physiology and Plant Molecular Biology, 50 : 601 –639. Doi: 10.1146/annurev.arplant.50.1.601 |
| Baier M, Dietz K J, 1999. Alkyl hydroperoxide reductases: the way out of the oxidative breakdown of lipids in chloroplasts. Trends in P lant S cience, 4 (5) : 166 –168. Doi: 10.1016/S1360-1385(99)01398-9 |
| Bendall D S, 1982. Photosynthetic cytochromes of oxygenic organisms. Biochimica et Biophysica Acta (BBA)-Reviews on Bioenergetics, 683 (2) : 119 –151. |
| Bendall D S, Manasse R S, 1995. Cyclic photophosphorylation and electron transport. Biochimica et Biophysica Acta (BBA)-Bioenergetics, 1229 (1) : 23 –38. Doi: 10.1016/0005-2728(94)00195-B |
| Bennoun P, 1982. Evidence for a respiratory chain in the chloroplast. Proceeding of the National Academy of Sciences of the United States of America, 79 (14) : 4352 –4356. Doi: 10.1073/pnas.79.14.4352 |
| Brooks M D, Sylak-Glassman E J, Fleming G R, Niyogi K K, 2013. A thioredoxin-like/β-propeller protein maintains the efficiency of light harvesting in Arabidopsis. Proceeding of the National Academy of Sciences of the United States of America, 110 (29) : E2733 –E2740. Doi: 10.1073/pnas.1305443110 |
| Bukhov N, Carpentier R, 2004. Alternative photosystem I-driven electron transport routes: mechanisms and functions. Photosynthesis Research, 82 (1) : 17 –33. Doi: 10.1023/B:PRES.0000040442.59311.72 |
| Carol P, Stevenson D, Bisanz C, Breitenbach J, Sandmann G, Mache R, Coupland G, Kuntz M, 1999. Mutations in the Arabidopsis gene IMMUTANS cause a variegated phenotype by inactivating a chloroplast terminal oxidase associated with phytoene desaturation. The Plant Cell, 11 (1) : 57 –68. Doi: 10.1105/tpc.11.1.57 |
| Corneille S, Cournac L, Guedeney G, Havaux M, Peltier G, 1998. Reduction of the plastoquinone pool by exogenous NADH and NADPH in higher plant chloroplasts: characterization of a NAD(P)H-plastoquinone oxidoreductase activity. Biochimica et Biophysica Acta (BBA)-Bioenergetics, 1363 (1) : 59 –69. Doi: 10.1016/S0005-2728(97)00074-1 |
| Cournac L, Redding K, Bennoun P, Peltier G, 1997. Limited photosynthetic electron flow but no CO 2 fixation in Chlamydomonas mutants lacking photosystem I. FEBS Let ters, 416 (1) : 65 –68. Doi: 10.1016/S0014-5793(97)01170-8 |
| Cournac L, Redding K, Ravenel J, Rumeau D, Josse E M, Kuntz M, Peltier G, 2000. Electron flow between photosystem II and oxygen in chloroplasts of photosystem I-deficient algae is mediated by a quinol oxidase involved in chlororespiration. J ournal of B iological C hemistry, 275 (23) : 17256 –17262. |
| Demmig-Adams B, Adams W W III, 1996. Xanthophyll cycle and light stress in nature: uniform response to excess direct sunlight among higher plant species. Planta, 198 (3) : 460 –470. Doi: 10.1007/BF00620064 |
| Endo T, Asada K, 1996. Dark induction of the nonphotochemical quenching of chlorophyll fluorescence by acetate in Chlamydomonas reinhardtii. Plant and Cell Physiology, 37 (4) : 551 –555. Doi: 10.1093/oxfordjournals.pcp.a028979 |
| Escoubas J M, Lomas M, LaRoche J, Falkowski P G, 1995. Light intensity regulation of cab gene transcription is signaled by the redox state of the plastoquinone pool. Proceeding of the National Academy of Sciences of the United States of America, 92 (22) : 10237 –10241. Doi: 10.1073/pnas.92.22.10237 |
| Figueroa F L, Conde-Álvarez R, Gómez I, 2003. Relations between electron transport rates determined by pulse amplitude modulated chlorophyll fluorescence and oxygen evolution in macroalgae under different light conditions. Photosynthesis Research, 75 (3) : 259 –275. Doi: 10.1023/A:1023936313544 |
| Finazzi G, Johnson G N, Dall'Osto L, Zito F, Bonente G, Bassi R, Wollman F A, 2006. Nonphotochemical quenching of chlorophyll fluorescence in Chlamydomonas reinhardtii. Biochemistry, 45 (5) : 1490 –1498. Doi: 10.1021/bi0521588 |
| Fork D C, Herbert S K, 1993. Electron transport and photophosphorylation by photosystem I in vivo in plants and cyanobacteria. Photosynthesis Research, 36 (3) : 149 –168. Doi: 10.1007/BF00033035 |
| Foyer C H, Harbinson J. 1999. Relationships between antioxidant metabolism and carotenoids in the regulation of photosynthesis. In: Frank H A, Young A J, Britton G, Cogdell R J eds. The Photochemistry of Carotenoids. Kluwer Academic Publishers, Dordrecht. p.305-325. |
| Foyer C H, Bloom A J, Queval G, Noctor G, 2009. Photorespiratory metabolism: genes, mutants, energetics, and redox signaling. Annual Review of Plant Biology, 60 : 455 –484. Doi: 10.1146/annurev.arplant.043008.091948 |
| Gilmore A M, Björkman O, 1995. Temperature-sensitive coupling and uncoupling of ATPase-mediated, nonradiative energy dissipation: similarities between chloroplasts and leaves. Planta, 197 (4) : 646 –654. |
| Heber U, Walker D, 1992. Concerning a dual function of coupled cyclic electron transport in leaves. P lant P hysiology, 100 (4) : 1621 –1626. |
| Hideg É, Kálai T, Hideg K, Vass I, 1998. Photoinhibition of photosynthesis in vivo results in singlet oxygen production detection via nitroxide-induced fluorescence quenching in broad bean leaves. Biochemistry, 37 (33) : 11405 –11411. Doi: 10.1021/bi972890+ |
| Holt N E, Zigmantas D, Valkunas L, Li X P, Niyogi K K, Fleming G R, 2005. Carotenoid cation formation and the regulation of photosynthetic light harvesting. Science, 307 (5708) : 433 –436. Doi: 10.1126/science.1105833 |
| Horton P, Ruban A V, Walters R G, 1996. Regulation of light harvesting in green plants. Annual Review of Plant Physiology and Plant Molecular Biology, 47 : 655 –684. Doi: 10.1146/annurev.arplant.47.1.655 |
| Joliot P, Joliot A, 2002. Cyclic electron transfer in plant leaf. Proceeding of the National Academy of Sciences of the United States of America, 99 (15) : 10209 –10214. Doi: 10.1073/pnas.102306999 |
| Josse E M, Simkin A J, Gaffé J, Labouré A M, Kuntz M, Carol P, 2000. A plastid terminal oxidase associated with carotenoid desaturation during chromoplast differentiation. Plant Physiol ogy, 123 (4) : 1427 –1436. Doi: 10.1104/pp.123.4.1427 |
| Lesser M P, 1997. Oxidative stress causes coral bleaching during exposure to elevated temperatures. Coral Reefs, 16 (3) : 187 –192. Doi: 10.1007/s003380050073 |
| Li Z R, Wakao S, Fischer B B, Niyogi K K, 2009. Sensing and responding to excess light. A nnual Review of P lant B iology, 60 : 239 –260. |
| Lin A P, Wang G C, Yang F, Pan G H, 2009. Photosynthetic parameters of sexually different parts of Porphyra katadai var. hemiphylla (Bangiales, Rhodophyta) during dehydration and re-hydration. Planta, 229 (4) : 803 –810. |
| Long S L, Humphries S, Falkowski P G, 1994. Photoinhibition of photosynthesis in nature. Annual Review of Plant Physiology and Plant Molecular Biology, 45 : 633 –662. Doi: 10.1146/annurev.pp.45.060194.003221 |
| Lovelock C E, Winter K, 1996. Oxygen-dependent electron transport and protection from photoinhibition in leaves of tropical tree species. Planta, 198 (4) : 580 –587. |
| Maxwell D P, Laudenbach D E, Huner N P A, 1995. Redox regulation of light harvesting complex II and cab mRNA abundance in Dunaliella salina. P lant P hysiology, 109 (3) : 787 –795. |
| Melis A, 1999. Photosystem-II damage and repair cycle in chloroplasts: what modulates the rate of photodamage in vivo ?. T rends in P lant S cience, 4 (4) : 130 –135. |
| Müller P, Li X P, Niyogi K K, 2001. Non-photochemical quenching. A response to excess light energy. Plant Physiology, 125 (4) : 1558 –1566. |
| Neubauer C, Yamamoto H Y, 1992. Mehler-peroxidase reaction mediates zeaxanthin formation and zeaxathine-related fluorescence quenching in intact chloroplasts. Plant Physiology, 99 (4) : 1354 –1361. Doi: 10.1104/pp.99.4.1354 |
| Neubauer C, Yamamoto H Y, 1994. Membrane barriers and Mehler-peroxidase reaction limit the ascorbate available for violaxanthin de-epoxidase activity in intact chloroplasts. P hotosynthesis R esearch, 39 (2) : 139 –147. |
| Nitschke U, Connan S, Stengel D B, 2012. Chlorophyll a fluorescence responses of temperate Phaeophyceae under submersion and emersion regimes: a comparison of rapid and steady-state light curves. P hotosynthesis R esearch, 114 (1) : 29 –42. |
| Niyogi K K, 1999. Photoprotection revisited: genetic and molecular approaches. Annual Review of Plant Physiology and Plant Molecular Biology, 50 : 333 –359. Doi: 10.1146/annurev.arplant.50.1.333 |
| Niyogi K K, 2000. Safety valves for photosynthesis. Current Opinion in Plant Biology, 3 (6) : 455 –460. Doi: 10.1016/S1369-5266(00)00113-8 |
| Pascal A A, Liu Z F, Broess K, van Oort B, van Amerongen H, Wang C, Horton P, Robert B, Chang W R, Ruban A, 2005. Molecular basis of photoprotection and control of photosynthetic light-harvesting. Nature, 436 (7047) : 134 –137. Doi: 10.1038/nature03795 |
| Peltier G, Cournac L, 2002. Chlororespiration. Annual Review of Plant Biology, 53 : 523 –550. Doi: 10.1146/annurev.arplant.53.100301.135242 |
| Pfannschmidt T, Nilsson A, Allen J F, 1999. Photosynthetic control of chloroplast gene expression. Nature, 397 (6720) : 625 –628. Doi: 10.1038/17624 |
| Porcar-Castell A, Juurola E, Nikinmaa E, Berninger F, Ensminger I, Hari P, 2008. Seasonal acclimation of photosystem II in Pinus sylvestris. I. Estimating the rate constants of sustained thermal energy dissipation and photochemistry. Tree Physiol ogy, 28 (10) : 1475 –1482. |
| Ravenel J, Peltier G, Havaux M, 1994. The cyclic electron pathways around photosystem I in Chlamydomonas reinhardtii as determined in vivo by photoacoustic measurements of energy storage. Planta, 193 (2) : 251 –259. |
| Redding K, Cournac L, Vassiliev I R, Golbeck J H, Peltier G, Rochaix J D, 1999. Photosystem I is indispensable for photoautotrophic growth, CO2 fixation, and H2 photoproduction in Chlamydomonas reinhardtii. J ournal of B iological C hemistry, 274 (15) : 10466 –10473. |
| Ruban A V, Horton P, 1995. An investigation of the sustained component of nonphotochemical quenching of chlorophyll fluorescence in isolated chloroplasts and leaves of spinach. Plant Physio logy, 108 (2) : 721 –726. |
| Ruuska S A, Badger M R, Andrews T J, von Caemmerer S, 2000. Photosynthetic electron sinks in transgenic tobacco with reduced amounts of Rubisco: little evidence for significant Mehler reaction. J ournal of E xperimental B otany, 51 (Suppl. 1) : 357 –368. |
| Schreiber U, Schliwa U, Bilger W, 1986. Continuous recording of photochemical and non-photochemical chlorophyll fluorescence quenching with a new type of modulation fluorometer. Photosynthesis Research, 10 (1-2) : 51 –62. Doi: 10.1007/BF00024185 |
| Schreiber U, Neubauer C, 1990. O 2-dependent electron flow, membrane energization and the mechanism of nonphotochemical quenching of chlorophyll fluorescence. P hotosynthesis R esearch, 25 (3) : 279 –293. |
| Schreiber U, Endo T, Mi H L, Asada K, 1995. Quenching analysis of chlorophyll fluorescence by saturation pulse method: particular aspects relating to the study of eukaryotic algae and cyanobacteria. Plant and Cell Physiology, 36 (5) : 873 –882. |
| Sutherland J E, Lindstrom S C, Nelson W A, Brodie J, Lynch M D J, Hwang M S, Choi H G, Miyata M, Kikuchi N, Oliveira M C, Farr T, Neefus C, Mols-Mortensen A, Milstein D, Müller K M, 2011. A new look at an ancient order: generic revision of the Bangiales (Rhodophyta). Journal of Phycology, 47 (5) : 1131 –1151. Doi: 10.1111/j.1529-8817.2011.01052.x |
| Trebst A, Depka B, 1997. Role of carotene in the rapid turnover and assembly of photosystem II in Chlamydomonas reinhardtii. FEBS Lett ers, 400 (3) : 359 –362. Doi: 10.1016/S0014-5793(96)01419-6 |
| Verhoeven A S, Adams W W III, Demmig-Adams B, 1998. Two forms of sustained xanthophyll cycle-dependent energy dissipation in overwintering Euonymus kiautschovicus. P lant C ell and E nvironment, 21 (9) : 893 –903. |
| Verhoeven A S, Adams W W III, Demmig-Adams B, 1999. The xanthophyll cycle and acclimation of Pinus ponderosa and Malva neglecta to winter stress. Oecologia, 118 (3) : 277 –287. Doi: 10.1007/s004420050728 |
| Wu D Y, Wright D A, Wetzel C, Voytas D F, Rodermel S, 1999. The IMMUTANS variegation locus of Arabidopsis defines a mitochondrial alternative oxidase homolog that functions during early chloroplast biogenesis. Plant Cell, 11 (1) : 43 –55. Doi: 10.1105/tpc.11.1.43 |
| Yang D H, Webster J, Adam Z, Lindahl M, Andersson B, 1998. Induction of acclimative proteolysis of the light-harvesting chlorophyll a/b protein of photosystem II in response to elevated light intensities. P lant P hysiology, 118 (3) : 827 –834. |
 2016, 34
2016, 34


