Institute of Oceanology, Chinese Academy of Sciences
Article Information
- Chengxu ZHOU(周成旭), Jie LUO(罗杰), Yangfang YE(叶央芳), Xiaojun YAN(严小军), Baoning LIU(刘宝宁), Xin WEN(文欣)
- The metabolite profiling of coastal coccolithophorid species Pleurochrysis carterae (Haptophyta)
- Journal of Oceanology and Limnology, 34(4): 749-756
- http://dx.doi.org/10.1007/s00343-016-5042-0
Article History
- Received: Feb. 3, 2015
- Accepted: May. 22, 2015
Coccolithophores are widely distributed autotrophy phytoplankton in the ocean.They are the main contributors of the oceaniccalcite by producing extracellular intricatecalcium carbonates plates (coccolith) and play important roles in carbonand sulfur elemental biogeochemical cycles (Holligan et al., 1993; Sukhanova and Flint, 1998; Thierstein and Young, 2004) .That’s why coccolithophores are the study aims in multipledisciplines. Pleurochrysis carterae and Emiliania huxley are two most studied coccolithophorid species (Thierstein and Young, 2004) . P. carterae is a typical coccolithophorid species thatdistributes and bloomsin coastal areas.E. huxleyi is the most significant global distributing and blooming coccolithophorid speciesin the open ocean. Up to now, the metabolites involved in specific metabolicpathway in E. huxleyi have been widely studied. For example, Fernándezet al. (1994) analyzed the carbon flow in the metabolic path ways in E. huxleyi, and found the significance of the rapid neutral lipid synthesis in the population dynamics. Lipid composition of E. huxleyi was also analyzed and comparedwith phytoplankton of other division (Maxwell et al., 1980; Viso and Marty, 1993) . Recently, Mausz and Pohnert (2015) , by using the method of GC-MS, compared the metabolites differenceof E. huxleyi between the cells of two ploidy level, namely haploidand diploid, and among differentpopulation growth phase.
However, little has been carried out on the metabolites of P. carterae, which is a harmful coccolithophoride species leading to long -lasting bloom with notorious foams in coastalarea, brackish water or marine aquaculture ponds. Moreover, those related studies focused merely on some selected metabolites withoutconsidering other metabolites simultaneously. For example, on considering of the calcificationmechanism of coccolithophores, three acidic polysaccharides that play fundamental roles in coccolith formation, namely PS-1, PS-2 and PS-3, were characterized in the organic base of coccolith of P. carterae (Marsh, 1994; 1996; Marsh and Dickson, 1997) . The polysaccharides are the polymerof residues such as galacturonic acid, glucuronate, tartrate and glyoxylate (Marsh et al., 1992) . The compositions of coccolith-related polysaccharides are different from that of E. huxleyi according to the study of Fichtinger-Schepman et al. (1979) , as no glyoxylate was detectedin the calcified E. huxleyi cells in their study. However, Rokitta et al. (2012) found that glyoxylate cycle related enzymes were up expressed significantly in E. huxleyi non-calcify haploid cell when exposed to ocean acidification.The difference of this coccolith related metabolite suggested that, there are different metabolismpathways in the two species and in their different life cycle stages which are closely related to calcification process under different environmental conditions (Rokitta et al., 2014) . So the metabolomic study is extremely important for fundamental biochemical inf ormation. For the time being, however, there was no holistic metabolomic study done in P. carterae.
Nuclear Magnetic Resonance (NMR) spectroscopy is an alternative for reliable and high-throughput metabolomic analysiswith many successful applications in algae (Jamers et al., 2013; Zhang et al., 2014) . Untargeted metabolite profiling is a popular method to investigate the fundamental biochemical information of differentorganisms. NMR is intrinsically unbiasedand non-destructive in detecting metabolites with no need for the separation processes or chemical derivatization. It is effective both in quantitation and structural assignment for abundant of primary and secondary metabolites. Metabolomic data give snapshotof a system view of the metabolism and hints on what is happeningin the cell in response to environmental conditions. However, little has been reported on the algal metabolomic analysis using NMR spectroscopy. There also has been a lack of NMR data for algal metabolome, includingthe structural data for its metabolites.
In this study, we detected and assigned the metabolites of P. carterae strain isolated from aquaculture pool near the East China Sea coastalarea, by using NMR methods. The aim of this work is to profile species-specific biochemical information of P.carterae while applying the method of NMR in coccolithophorid metabolome.
2 MATERIAL AND METHOD 2.1 Algae strain and maintaining conditionsP. carterae (strain NMBjih026 in the Microalgae Collection in Ningbo University, China; EF208116 in GenBank) was isolated from a shrimppool in Zhejiang Province, China, in 2005, when a foaming bloom of P. carterae occurred in the pool and caused lethal effects on the aquaculture organism (crab) . This strain was activelymaintained in natural seawater of salinity of 23-25, enriched with f/2-Siculture medium, at 20℃ in light densityof 60 μmol/ (m2∙s) with a light/ dark cycle of 12 h:12 h.
2.2 Apparatus and reagentsThe NMR: The analysis were performed on a Bruker Avance III 400 MHz (Bruker Biospin, Germany) .
Reagents: Analytical grade of the reagents were purchased from different traders. Acetonitrile, NaCl, NaH2PO4·2H2O and K2HPO4·3H2O were purchased from SinopharmCo. Ltd. (Shanghai, China) ; NaN3was purchased from Sigma-Aldrich (Shanghai) Trading Co. Ltd. (Shanghai, China) ; Deuterated water (D2O, 99.9% D) and sodium 3-trimethylsilyl [2, 2, 3, 3-2D4] propionate (TSP) were purchasedfrom Cambridge Isotope Laboratories Inc. (Andover, MA, USA) .
Buffer and extraction solvent: Na+-K+ phosphate buffer (0.1 mol/L NaH2PO4/K2HPO4, pH 7.4, containing 10% D2O, 0.1% NaN3 and 0.004% TSP) was preparedin redistilled water. Metabolites extraction solvent was preparedby mixing equal volumes of acetonitrile and the above prepared buffer.
2.3 Culture growing, sampleharvesting for metabolic analysisP. carterae culture in exponential phase with the cell densityof 3×105 cells/mL was grown under the conditions as mentioned above except that the light density was changed to 80 μmol/ (m2∙s) to make the culture grow faster. Six aliquots of the culture were inoculated into 2 000 mL freshly preparedf/2-Si medium at the initial densityof 2×104 cells/mL and grewunder the same conditions. When the culture grew to the early stationary phase with the cell density of 3.6×105 cells/mL, all the cultures were harvested by centrifugation at 6 000 r/min at 4℃ after simultaneously being put on ice for a few minutestill be centrifuged. All the algal pellets were lyophilized and storedat -20℃ till analysis.
2.4 Sample preparation and NMR spectroscopyThe lyophilized powdersamples (50 mg) were extracted twice with 600 μL of 50% aqueous acetonitrile by intermittent sonication (i.e., 30 s sonication with 30 s break) for 10 min in an ice bath. After 10 min centrifugation at 12 000 r/min and 4℃, the combined supernatants were lyophilized after removal of acetonitrile under vacuum. The extracts were then reconstituted in 550 μL of phosphate buffer (0.1 mol/L, pH7.4, NaN3 0.1%, TSP 0.004%, K2HPO4/NaH2PO4=4:1) . A total of 500 μL of supernatant of each sample was then transferred into 5 mm NMR tube for NMR analysis.
One-dimensional 1H NMR spectra of algal samples were acquired at 298 K on a Bruker Avance III 400 MHz NMR spectrometer (operating at 400.13 MHz for 1H) , equipped with an inverse detection probe (Bruker, Biospin, Germany) .A total of 64 transients were collected using a standardpre-saturation pulse sequence (90°-3 μs -90°-tm-90°-acquire) with irradiation during a 2 s relaxation delay and during a 100 ms mixing time. For assignment purposes, a range of two-dimensional NMR spectra, including 1H J-resolved, 1H-1H COSY, 1H-1H TOCSY, 1H-13C HSQC, and 1H-13C HMBC (Aue et al., 1976a, b; Braunschweiler and Ernst, 1983) , were also acquired for selected samples.
2.5 NMR spectral processes and analysisAll of 1H NMR spectrawere preprocessed routinely as previous described (Fan, 1996) . In brief, all free induction decays were multiplied by an exponential function with a 1 Hz line-broadening factor prior to Fourier transformation. The spectra were manually adjusted for phase and baseline distortion and referenced to TSP at δ0.00×10-6.
2.6 Quantitative analysis of metabolitesMetabolite concentrations of algal sampleswere calculated from the integrals of selected metabolite NMR signals (non-overlapping ones) relative to that of TSP, an internal reference.
3 RESULT 3.1 Metabolic profiles of P. carteraeTypical 1H NMR spectrum of the aqueous acetonitrile extractsfrom algae is shown in Fig. 1. The metabolite resonances were assigned according to the connectivity between proton and carbon in 2D NMR spectra. For example, COSY spectrum providesthe neighboringprotons (usually up to four bonds) . TOCSY spectrumis useful for creating correlations between all protons with a given spin system. HSQC spectrum reveals the direct one-bond 1H-13C correlations whereasHMBC spectrum shows the long-range 1H and 13C correlations. The atomic connectivities (i.e., planar structures) for metabolites can be determined accordingto these homonuclear or heteronuclearcorrelations, literature data (Fan, 1996; Fan and Lane, 2008) and in-housestandard sample databases. For instance, a scalar couplingconnectivity between a doublet (d1.49, 7.1 Hz) and a quartet (d3.79, 7.2 Hz) was obtainedin COSY spectrum of algal extracts.No more signals of the entire 1H spin network of this metabolite were observed based on TOCSY spectrum.However, HSQC spectrum further revealed that the proton signal at d1.49 belonged to methyl (CH3) group whereas the proton signal at d3.79 was a methine (CH) moiety. In addition, HMBC spectrum provided the long-range correlations among the methyl group, the methine group and the carboxylic group.Thus, this metabolite was assigned to alanine after compared with the NMR data of the metabolite in literature data. Such a way of structural elucidation was also used for other NMR signal assignments. However, in most cases, seriously overlapping signalsand week signalintensity caused uncompleted NMR data. For example, only a correlation between 7.19 (m) and 6.90 (m) was observed in COSY spectrum. However, HMBC spectrum revealedthat these two signals belongedto the phenyl ring protons. Following comparison with the well-established characteristic signals of tyrosine, the structureassignment of tyrosinewas confirmed.
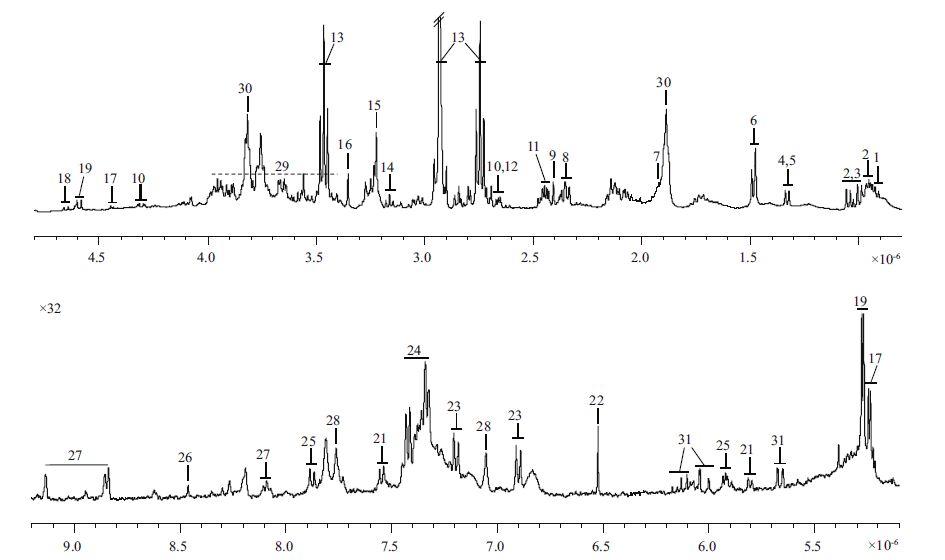
|
| Figure 1 Typical 400 MHz 1H NMR spectrumof Pleurochrysis carteraeextracts |
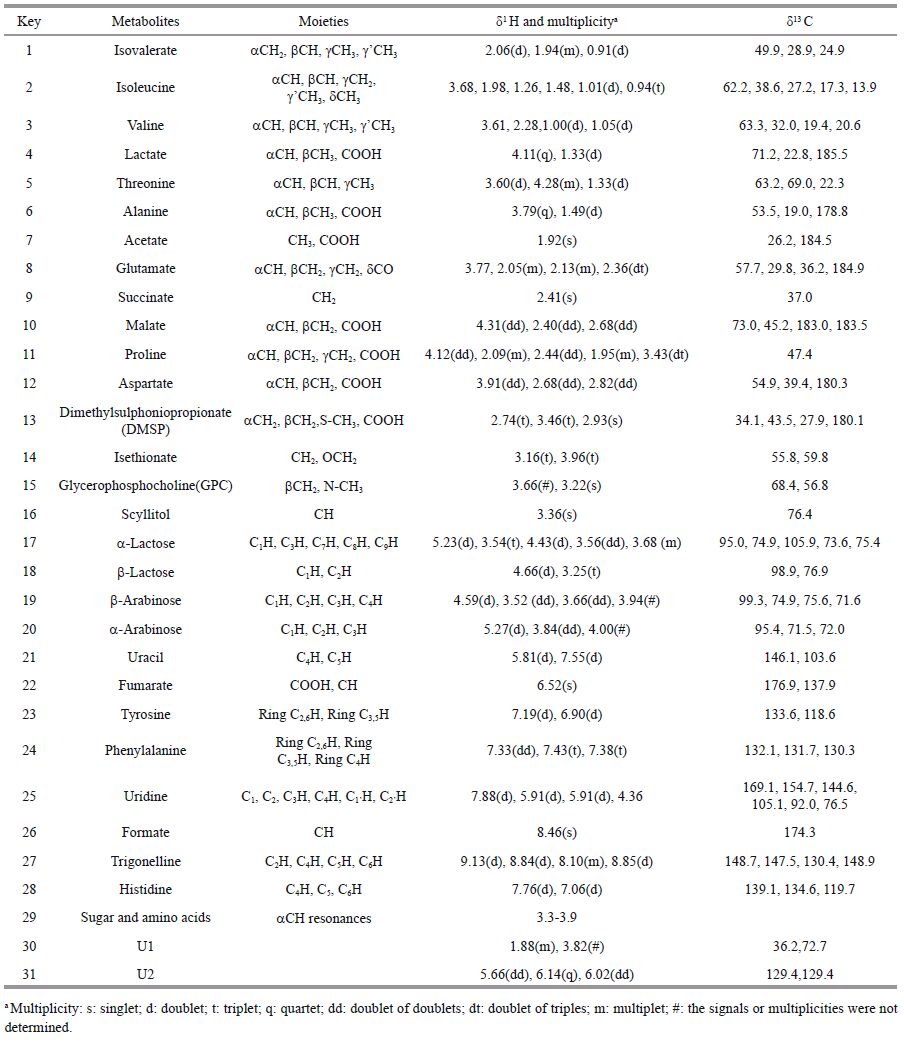
In this study, a total of 26 metabolites from algal extracts were assigned, as tabulated in Table 1 together with the corresponding 1H and 13C NMR biochemical shifts and signalmultiplicities. It is apparent that the algal metabolome is dominated by 10 amino acids, 2 carbohydrates, 8 organic acids/amines, 2 nucleotide derivatives, GPC, DMSP, scyllitol, trigonelline, and 2 unknown metabolites. To the best of our knowledge, this is the relatively com prehensive metabolite assignments for algal extract using NMR method which has not been reported previously.
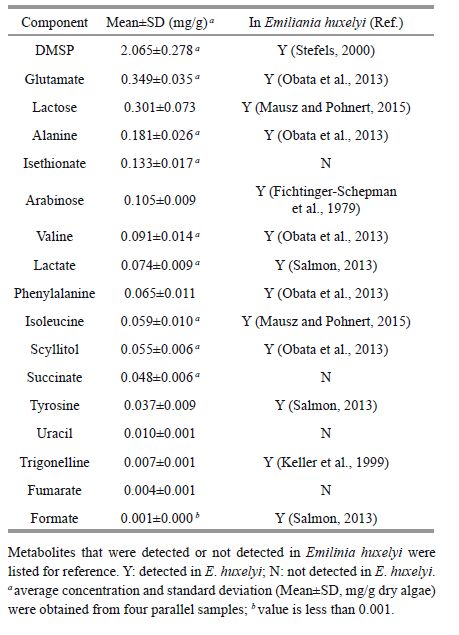
Among all of detectedmetabolites, the content of DMSP was the highest, up to 2.065±0.278 mg/g dry algae (Table 2) . The contentsof glutamate, lactose alanine, isethionate, and arabinose were between 0.1-0.4 mg/g dry algae. Isethionate was first detected in coccolithophorid species.Other detected metabolites presented very low contentswith less than 0.1 mg/g dry algae. They were valine, phenylalanine, isoleucine, tyrosine, lactate, succinate, scyllitol and uracil. Trigonelline, fumarate and formate were detected in very low content (thousandths of 1 mg per gram) . The content of formate was only 0.001 mg/g dry algaein the algal extracts. Same metabolites that were detected or not detected in Emiliania huxelyiin other studieswere listed in Table 2 for the purposeof comparison.
P. carterae is a coccolithophorid species that mainly distributes in the littoral areas. It is another well studiedcoccolithophorid species other than E.huxleyi. In laboratory studies, P. carterae has mechanicallyunclear lethal effects on Artemia salina (Houdan et al., 2004; Jiang et al., 2009) . Metabolites of a particular organism ser vedas excellent probesto reflect the metabolismpathway and give hints of characteristics of the organism in response to particular environmental or physiological conditions. In this study, we detected26 and several unknown metabolites in the cell of P. carterae by the way of NMR. The metabolites were extracted from lyophilized algal cells harvested from optimally growing cultures at early stationary phase. The metabolites, involvedin many metabolic pathways, are mainly low molecularweight chemicals such as organic acids, amino acids, sugars and nucleic aides. Not only some basic metabolites were detected in our studies, some species specific characteristics of metaboliteswere found in this species.
The most abundant metabolite we detected in P. carterae was DMSP. By using the NMR methods, DMSP can be analyzeddirectly without the need to degrade the samples to detect its breakdown product DMS as some other studies (Kiene, 1996; Zhou et al., 2009) or the need of variousinternal standards (Gebser and Pohnert, 2013) . DMSP was found to be abundant in a wide range of taxa of microalgae (Malin and Erst, 1997) . It is thoughtto play important roles in the phytoplankton physiological functions, such as osmotic solute (Keller, 1989; Stefels, 2000) , anti-predation (Wolfe and Steinke, 1996; Wolfe et al., 1997) , infochemical substances (Nevitt and Bonadonna, 2005; Strom and Wolfe, 2009; Seymour et al., 2010) , antioxidant substance (Sunda et al., 2002) , and overflow system (Stefels, 2000) . In this study, we did not check the final weight of the total P.carterae cells when frozen dried the cells and was unable to calculate the weight per frozen dried cell in this study. Becausethat most of other studies addressed the cellular contentof DMSP were calculatedper cell, therelacks the resultswith which our resultsin this studycan be compared. However, the cellularparticle DMSP (DMSPp) content of the same strain of P. carterae was detected to be 13.8±0.9 pg per cell in the study of Zhou et al. (2009) , it was at the same level with that of E. huxelyi in the study of Wiesemeier and Pohnert (2007) , i.e. 10.8±3.3 pg per cell. From our study, the cellularabundance of DMSP in P. carterae was demonstrated.
In addition to DMSP, lactose and isethionate were also detected be abundant metabolites in P. carterae.These biochemical molecules serve as the compatible osmotic solutes as well (Yancey, 2005) . Isethionate is an anionic organosulfur compound. It was first detected in P. carterae in this study. Thereis no report of isethionate detectedin E. huxleyi or other coccolithophores. However, proline, another effective organicosmolyte (Kirst, 1996) , was presentedin both P. carterae and E. huxleyi (Mausz and Pohnert, 2015) . E. huxleyi is a typical cosmopolitan coccolithophore distributing in the open ocean and in the coastal area (Winter et al., 2013) . The ability of constant adjustment of osmosis in these nichesis required for the algae.
In this study, trigonelline was detected in P.carterae even the contentwas low. Trigonelline is an organiczwitterionic osmolyte occurring in marine algae (Keller et al., 1999; Blunden et al., 2012) . It was also detectedin low amount in strains of E. huxleyi (Keller et al., 1999; Gebser and Pohnert, 2013) . By using the method of HPLC-MS, Gebser and Pohnert (2013) found that trigonelline decreased with increase salinity and proposed that trigonelline had no use in osmoadaptation for E. huxleyi. However, they found that trigonelline was the second most abundant metabolite in Prorocentrum minimum, a widely distributed toxic dinoflagellate, when exposed to low salinity. Unlike E. huxleyi, which changes the cell size to different salinity, P. minimum did not change cell size but the composition of organicosmolytes changed to theambient salinity variation. The osmotic pressure adaptationobviously was different from different species. Marine algae regulate intracellular osmotic pressureusing both inorganic ions and low molecular weight organic solutes (Kobayashi et al., 2007) . Having more than one compatible solute may allow the organismsto adapt to environmental change, especially in halotolerant species (Hanson et al., 1994) . P . carterae is typical cosmopolitan coastal and brackish water coccolithophore. Dominantof P.carterae in these areas reflects its ability to adapt to varied environmental conditions, especially the changing osmoticpressure.
5 CONCLUSIONThe unbiased NMR spectroscopic method is practical in the analysisof holistic metabolites of phytoplankton. By applying the method of NMR in the analysisof metabolites of phytoplankton for the first time, we detected and assigned 26 metabolites that closely related to primary and secondary metabolism pathway in the costal blooming coccolithophorid speciesP. carterae. DMSP was the most abundantmetabolite in P. carterae. Metabolites that serve as compatible osmotic solutes, such as lactose, prolineand isethionate, werealso abundant in P. carterae. This characteristic of the metabolic composition possibly reflect the active ability of P.carterae to adapt to the versatilecoastal niche.
6 ACKNOWLEDGEMENTThanks to Ms. JIANG Ying for maintainthe cultures and Prof. LUO Qijunfor collecting the microalgae strainsin the field.
| Aue W P, Bartholdi E, Ernst R R, 1976a. Two-dimensional spectroscopy. Application to nuclear magnetic-resonance. J. Chem. Phys., 64 (5) : 2229 –2246. |
| Aue W P, Karhan J, Ernst R R, 1976b. Homonuclear broad band decoupling and two-dimensional J-resolved NMR spectroscopy. J. Chem. Phys., 64 (10) : 4226 –4227. Doi: 10.1063/1.431994 |
| Blunden G, Guiry M D, Druehl L D, Kogame K, Kawai H, 2012. Trigonelline and other betaines in species of laminariales. Nat. Prod. Commun., 7 (7) : 863 –865. |
| Braunschweiler L, Ernst R R, 1983. Coherence transfer by isotropic mixing: application to proton correlation spectroscopy. J. Magn. Reson., 53 (3) : 521 –528. |
| Fan T W M, Lane A N, 2008. Structure-based profiling of metabolites and isotopomers by NMR. Prog. Nucl. Magn. Reson. Spectrosc., 52 (2-3) : 69 –117. Doi: 10.1016/j.pnmrs.2007.03.002 |
| Fan T W M, 1996. Metabolite profiling by one-and twodimensional NMR analysis of complex mixtures. Prog. Nucl. Magn. Reson. Spectrosc., 28 (2) : 161 –219. Doi: 10.1016/0079-6565(95)01017-3 |
| Fernández E, Balch W M, Marãnón E, Holligan P M, 1994. High rates of lipid biosynthesis in cultured, mesocosm and coastal populations of the cocco-lithophore Emiliania huxleyi. Mar. Ecol. Prog. Ser., 114 : 13 –22. Doi: 10.3354/meps114013 |
| Fichtinger-Schepman A M J, Kamerling J P, Vliegenthart J F G, De Jong E W, Bosch L, Westbroek P, 1979. Composition of a methylated, acidic polysaccharide associated with coccoliths of Emiliania huxleyi (Lohmann) Kamptner. Carbohydrate Research, 69 (1) : 181 –189. Doi: 10.1016/S0008-6215(00)85763-8 |
| Gebser B, Pohnert G, 2013. Synchronized regulation of different zwitterionic metabolites in the osmoadaption of phytoplankton. Mar. Drugs, 11 (6) : 2168 –2182. Doi: 10.3390/md11062168 |
| Hanson A D, Rivoal J, Paquet L, Cage D A, 1994. Biosynthesis of 3-dimethylsulfoniopropionate in Wollastonia biflora (L. ) DC. Evidence that S-methylmethionine is an intermediate. Plant Physiol., 105 (1) : 103 –110. |
| Holligan P M, Fernández E, Aiken J, Balch W M, Burkill P H, Finch M, Groom S B, Malin G, Muller K, Purdie D A, Robinson C, Trees C C, Turner S M, Van der Wal P, 1993. A biogeochemical study of the coccolithophore Emiliania huxleyi, in the north Atlantic. Global Biogeochem. Cy., 7 (4) : 879 –900. Doi: 10.1029/93GB01731 |
| Houdan A, Bonnard A, Fresnel J, Fouchard S, Billard C, Probert I, 2004. Toxicity of coastal coccolithophores (Prymnesiophyceae, Haptophyta). J. Plankton Res., 26 (8) : 875 –883. Doi: 10.1093/plankt/fbh079 |
| Jamers A, Blust R, De Coen W, Griffin J L, Jones O A H, 2013. An omics based assessment of cadmium toxicity in the green alga Chlamydomonas reinhardtii. Aquat. Toxicol., 126 : 355 –364. Doi: 10.1016/j.aquatox.2012.09.007 |
| Jiang Y, Zhou C X, Luo Q J, Ma B, 2009. Lethal effects of different Pleurochrysis carterae cells on brine shrimp. Asian Journal of Ecotoxicology, 4 (4) : 561 –568. |
| Keller M D, Kiene R P, Matrai P A, Bellows W K, 1999. Production of glycine betaine and dimethylsulfoniopropionate in marine phytoplankton. I. Batch cultures. Mar. Biol., 135 (2) : 237 –248. |
| Keller M D, 1989. Dimethyl sulfide production and marine phytoplankton: the importance of species composition and cell size. Biol. Oceanogr., 6 (5-6) : 375 –382. |
| Kiene R P, 1996. Production of methanethiol from dimethylsulfoniopropionate in marine surface waters. Mar. Chem., 54 (1-2) : 69 –83. Doi: 10.1016/0304-4203(96)00006-0 |
| Kirst G O. 1996. Osmotic adjustment in phytoplankton and MacroAlgae. The use of Dimethylsulfoniopropionate (DMSP). In: Kiene R P, Visscher P T, Keller M D, Kirst G O eds. Biological and Environmental Chemistry of DMSP and Related Sulfonium Compounds. Plenum Press, New York, USA. p.121-129. |
| Kobayashi Y, Torii A, Kato M, Adachi K, 2007. Accumulation of cyclitols functioning as compatible solutes in the Haptophyte alga Pavlova sp. Phycological Research, 55 (2) : 81 –90. Doi: 10.1111/pre.2007.55.issue-2 |
| Malin G, Erst G O, 1997. Algal production of dimethyl sulfide and its atmospheric role. J. Phycol., 33 (6) : 889 –896. Doi: 10.1111/j.0022-3646.1997.00889.x |
| Marsh M E, Chang D K, King G C, 1992. Isolation and characterization of a novel acidic polysaccharide containing tartrate and glyoxylate residues from the mineralized scales of a unicellular coccolithophorid alga Pleurochrysis carterae. The Journal of Biological Chemistry, 267 (28) : 20507 –20512. |
| Marsh M E, Dickinson D P, 1997. Polyanion-mediated mineralization-mineralization in coccolithophore (Pleurochrysis carterae) variants which do not express PS2, the most abundant and acidic mineral-associated polyanion in wild-type cells. Protoplasma, 199 (1-2) : 9 –17. Doi: 10.1007/BF02539801 |
| Marsh M E, 1994. Polyanion-mediated mineralizationassembly and reorganization of acidic polysaccharides in the Golgi system of a coccolithophorid alga during mineral deposition. Protoplasma, 177 (3-4) : 108 –122. Doi: 10.1007/BF01378985 |
| Marsh M E, 1996. Polyanion-mediated mineralization-a kinetic analysis of the calcium-carrier hypothesis in the phytoflagellate Pleurochrysis carterae. Protoplasma, 190 (3) : 181 –188. |
| Mausz M A, Pohnert G, 2015. Phenotypic diversity of diploid and haploid Emiliania huxleyi cells and of cells in different growth phases revealed by comparative metabolomics. J. Plant Physiol., 172 : 137 –148. Doi: 10.1016/j.jplph.2014.05.014 |
| Maxwell J R, Mackenzie A S, Volkman J K, 1980. Configuration at C-24 in steranes and sterols. Nature, 286 (5774) : 694 –697. Doi: 10.1038/286694a0 |
| Nevitt G A, Bonadonna F, 2005. Sensitivity to dimethyl sulphide suggests a mechanism for olfactory navigation by seabirds. Biology Letters, 1 (3) : 303 –305. Doi: 10.1098/rsbl.2005.0350 |
| Obata T, Schoenefeld S, Krahnert I, Bergmann S, Scheffel A, Fernie A R, 2013. Gas-Chromatography Mass-Spectrometry (GC-MS) based metabolite profiling reveals mannitol as a major storage carbohydrate in the coccolithophorid alga Emiliania huxleyi. Metabolites, 3 (1) : 168 –184. Doi: 10.3390/metabo3010168 |
| Rokitta S D, John U, Rost B, 2012. Ocean acidification affects redox-balance and ion-homeostasis in the life-cycle stages of Emiliania huxleyi. PLoS One, 7 (12) : e52212 . Doi: 10.1371/journal.pone.0052212 |
| Rokitta S D, von Dassow P, Rost B, John U, 2014. Emiliania huxleyi endures N-limitation with an efficient metabolic budgeting and effective ATP synthesis. BMC Genomics, 15 : 1051 . Doi: 10.1186/1471-2164-15-1051 |
| Seymour J R, Simó R, Ahmed T, Stocker R, 2010. Chemoattraction to dimethylsulfoniopropionate throughout the marine microbial food web. Science, 329 (5989) : 342 –345. Doi: 10.1126/science.1188418 |
| Stefels J, 2000. Physiological aspects of the production and conversion of DMSP in marine algae and higher plants. Journal of Sea Research, 43 (3-4) : 183 –197. Doi: 10.1016/S1385-1101(00)00030-7 |
| Strom S L, Bright K J, 2009. Inter-strain differences in nitrogen use by the coccolithophore Emiliania huxleyi, and consequences for predation by a planktonic ciliate. Harmful Algae, 8 (5) : 811 –816. Doi: 10.1016/j.hal.2007.10.005 |
| Sukhanova I N, Flint M V, 1998. Anomalous blooming of coccolithophorids over the eastern Bering Sea shelf. Oceanology, 38 (4) : 502 –505. |
| Sunda W, Kieber D J, Kiene R P, Huntsman S, 2002. An antioxidant function for DMSP and DMS in marine algae. Nature, 418 (6895) : 317 –320. Doi: 10.1038/nature00851 |
| Thierstein H R, Young J R. 2004. Coccolithophores: From Molecular Processes to Global Impact. Springer-Verlag, Berlin Heidelberg, Germany. |
| Viso A C, Marty J C, 1993. Fatty acids from 28 marine microalgae. Phytochemistry, 34 (6) : 1521 –1533. Doi: 10.1016/S0031-9422(00)90839-2 |
| Wiesemeier T, Pohnert G, 2007. Direct quantification of dimethylsulfoniopropionate (DMSP) in marine microand macroalgae using HPLC or UPLC/MS. J ournal of Chromatogr aphy B, 850 (1-2) : 493 –498. |
| Winter A, Henderiks J, Beaufort L, Rickaby R E M, Brown C W, 2013. Poleward expansion of the coccolithophore Emiliania huxleyi. J. Plankton Res., 36 (2) : 316 –325. |
| Wolfe G V, Steinke M, Kirst G O, 1997. Grazing-activated chemical defence in a unicellular marine alga. Nature, 387 (6636) : 894 –897. Doi: 10.1038/43168 |
| Wolfe G V, Steinke M, 1996. Grazing-activated production of dimethyl sulfide (DMS) by two clones of Emiliania huxleyi. Limnology and Oceanography, 41 (6) : 1151 –1160. Doi: 10.4319/lo.1996.41.6.1151 |
| Yancey P H, 2005. Organic osmolytes as compatible, metabolic and counteracting cytoprotectants in high osmolarity and other stresses. The Journal of Experimental Biology, 208 (15) : 2819 –2830. Doi: 10.1242/jeb.01730 |
| Zhang W L, Tan N G J, Li S F Y, 2014. NMR-based metabolomics and LC-MS/MS quantification reveal metal-specific tolerance and redox homeostasis in Chlorella vulgaris. Mol. BioSyst., 10 (1) : 149 –160. Doi: 10.1039/C3MB70425D |
| Zhou C X, Xu J L, Yan X J, Hou Y D, Jiang Y, 2009. Analysis of dimethylsulfide and dimethylsulfoniopropionate in marine microalgae culture. Chin. J. Anal. Chem., 37 (9) : 1308 –1312. Doi: 10.1016/S1872-2040(08)60129-2 |
 2016, 34
2016, 34