Institute of Oceanology, Chinese Academy of Sciences
Article Information
- Yunlong ZHANG(张云龙), Qiaowan WU(吴巧婉), Weihua HU(胡伟华), Fan WANG(汪帆), Weihan SHAO(邵韦涵), Chengming ZHANG(张诚明), Zhongbo ZHAO(赵忠波), Hui HE(何辉), Qixue FAN(樊启学), Zemao GU(顾泽茂)
- Morphological changes and allometric growth in hatcheryreared Chinese loach Paramisgurnus dabryanus (Dabry de Thiersant, 1872)
- Journal of Oceanology and Limnology, 34(4): 757-762
- http://dx.doi.org/10.1007/s00343-016-5079-0
Article History
- Received: Mar. 13, 2015
- Accepted: Jun. 4, 2015
Length-weight relationship (LWR) has many applications in stock assessment, monitoring fish stocks, ecological studies and speciesconservation programs (Froese, 2006; Vicentin et al., 2012) . LWR may also be used in estimating several components in models of fish population dynamics (Guibiani and da S Horlando, 2014) . The various ontogenic life stages of fish, especially the early life stages, have different LWR patterns; however, no LWR information is available on Chineseloach (Froese and Pauly, 2014) .
During the early life stages of fish species, a change in body shape, which results from the growth of its components at diff erent relative rates (allometric growth) , refl ects the close relationship between the ontogeny of morphology and function (Çoban et al., 2009) . Allometric growth can be used in fisheries, biological studies, and aquaculture to evaluate the developmental plasticity of a species (Gisbert and Doroshov, 2006) . During early developmental stages, fish undergo a change in shape, which increases their ability to perform vital biological functions (e.g. respiration, sensory functions and feeding) for survival (Russo et al., 2007; Peña and Dumas, 2009; Khemis et al., 2013) . Consequently, the body structure and specific organs/systems of fish species develop according to their importance for primary functions. As previously mentioned, knowledge of allometric growth patterns is crucial from an aquacultural point of view.
Chinese loach, Paramisgurnus dabryanus (Dabry de Thiersant, 1872) , an omnivorous freshwater fish, is one of the most commercially important cultured species in East Asia, especially in China and Korea (Zhang et al., 2015) . In recent years, the culture of Chinese loach has become more widespread with increasing market demand in China. However, the majority of Chinese loach larvae are obtained from the wild because of high rates of mortalityduring hatchery production of larvae.It is well known that wild larvaeare reluctant to accept an artificial compound diet and, thus, survivalis of wild-caught larva is inconsistent. This is one of the main constraints in the cultivation of Chinese loach.In addition, limited information is available on the Chineseloach’s early life history, which is also a limitingfactor for the aquaculture development of this species.Therefore, the aim of this study was to determine the allometric growth patternsduring the early life stages of the Chineseloach from hatchingto 60 days after hatching (DAH) and, thereby, improveour knowledge of its early life stages.
2 MATERIAL AND METHOD 2.1 Larval rearingP. dabryanus larvae were obtained from Hubei Wuyuan Agricultural Developmental Co. Ltd. (Jingzhou, China) . Spawners were obtained from a nearby market and the fertilizedeggs were obtained from a mix of females. Larvae were reared in three tanks (9 m×2 m×1 m) with a waterdepth of 0.5 m and arearing density of 1 000 larvae/m2. Water temperature, dissolved oxygen, and pH were monitored daily. Water temperature was raised to 24-25°C and kept constantusing a steam-heated iron pipeline installedon the bottom of the tanks. During the experiment, oxygen and pH rangedfrom 6.5-7.5 mg/L and 8.0-8.5, respectively.
Frominitial feeding at 4 DAH until 10 DAH, larvae werefed a rotifer diet composedof Brachiouns spp., A splanchnidae spp. and Filinia spp. at a density of 10 individuals/mL. From 10 to 35 DAH, the diet was composed of cladoceradominated by Diaphanosoma spp. and Moina spp. at a density of 5 individuals/mL. From 30 DAH until the end of the experiment, larvae were fed a commercial compound diet (crude protein 35%, crude lipid 7%) at a feeding rate of 5% per day.
2.2 Sample collectionTo monitor growth, 30 individuals were collected before food distribution each morning from hatching to 60 DAH. Total length (TL, mm) was measured individually with a digitalvernier calipers to the nearest 0.01 mm. Body mass (BM, mg) was also determined to the nearest 0.1 mg after drying with filter paper. Thereafter, the following morphometric characteristics were measured (to the nearest 0.1 mm) for each sampled individual (n=10-20) under a stereomicroscope (Nikon SMZ1500, Nikon Corporation, Japan) with a micrometric ocular (Pro-Microscan DP300) and a digital vernier calipers: total length (TL) , standardlength (SL) , head length (HL) , trunk length (TRL) , tail length (TAL) , eye diameter (ED) , head depth (HD) , body depth (BD) , tail depth (TD) , pectoralfin length (PFL) , tail fin length (TFL) , and the third pair of barbel length (BL) (Table 1) .

|
Curve estimates (createdwith SPSS 18.0, SPSS Inc., Chicago, IL, USA) were used to assess the best regression modelof the LWR. Allometric growth was analyzed using simple linearregression with the log-transformed data according to the equationlogY=loga+blogSL, where Y is the morphometric characteristic, a is the intercept, and b is the growth coeffcient.Growth was considered isometric when b=1; allometrically positive when b>1, and allometrically negativewhen b<1. Regression analysis via SPSS 18.0 was used to developthe allometricregression equations.
The inflection point of the growth curve was determined accordingto van Snik et al. (1997) and Gisbert (1999) .First, the SL-Y data set was sorted according to increasing SL. Then, two regressions lines were calculated: one for SLmin to SLintermediate and another for SLintermediate to SLmax, where SLintermediate varied iteratively from SLmin+2 to SLmax-2. A t-test was used to determine if b for SLmin-SLintermediate and SLintermediate-SLmax differed significantlyat the 0.05 level of significance. The SLintermediate value was utilized that resulted in the largest significant t as the inflection point.
3 RESULT 3.1 Length-weight relationshipIn the presentstudy, 1800 hatchery-reared Chinese loach individuals were examined. The best power model for LWR from curve estimates was a regression equation of BM=0.025×TL2.649 (Fig. 1) .
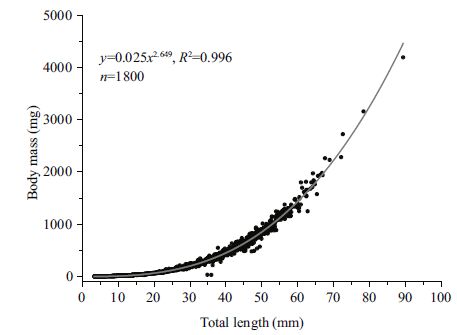
|
| Figure 1 Length-weight relationship of Chinese loach during the larval and juvenile stages |
Allometric growth equationsbetween the 10 measured morphometric characteristics and standard length duringthe larval development stage (1-60 DAH) are presented in Fig. 2. Growthof head length was allometically positive (b=2.60) until the inflection point at 5.04 mm standard length.Thereafter, the growth rate increased to being allometrically negative (b=0.69) . Head depth showed positiveallometric growth (b=2.44) until the inflectionpoint at 4.20 mm standard length. Thereafter, the growth rate increased to being allometrically negative (b=0.82) .

|
| Figure 2 Allometric growth equations between10 measured morphometric characteristics and standard lengthduring Chinese loach development |
Trunk length showedpositive allometric growth (b=1.51) until inflection point at 6.02 mm standard length, and decreased thereafter to being nearly isometric (b=1.05) . Growth of tail lengthwas allometrically positive (b=1.43) until the inflection point at 6.23 mm standard length. Although the growth coeffcient decreased after the inflection point, tail length growth remained allometically positive (b=1.14) . Pectoral finlengthand barbellength showed negative allometric growth ( b =0.84 and 0.81, respectively) until the end of the experiment.
A similar growth pattern was found with body depth and tail depth: allometrically negative growth (b=0.31 and 0.16, respectively) was observed until the inflection point at 7.07 mm standardlength, and increased thereafter to being nearlyisometric (b=1.06 and 1.08, respectively) .
Tail fin length showed negativeallometric growth (b=0.62) until theinflection pointat 4.20 mm standard length, then the growth coeffcient decreased to -3.29 until the inflectionpoint at 5.99 mm standardlength. Thereafter, tail fin length showed nearly isometric growth (b=1.08) . Eye diametershowed allometrically positive growth (b=2.26) until the inflection point at 21.09 mm standard length, and then decreased to being allometically negative (b=0.59) .
4 DISCUSSIONGrowth in fish is increasingly being linked with changes in husbandry parameters (Katsanevakis et al., 2007) .Allometric equations are the most common method used to analyze relative growth during early ontogeny in fish. In the present study, the growth coeffcientsdetermined for Chinese loach larvae and juveniles showed differentialgrowth of body ratios during the early life stages, which supports the hypothesis that a transitionin ontogenetic priorities occurs during development to enhance survival (Russo et al., 2007; Pea and Dumas 2009; Khemis et al., 2013) .
Positive allometricgrowth of the head length and head depth in Chineseloach, from hatchingto 60 DAH, is a commonfeature found in other fish species (Gisbert and Doroshov, 2006; Huysentruyt et al., 2009; Celik and Cirik, 2011; Çoban et al., 2009; Gao et al., 2014; Guimares-Cruz et al., 2014;Martínez-Montao et al., 2014; Nogueira et al., 2014) . The results of the present study supportsthe hypothesis that the development of the encephalon and other sensory, respiratory and feeding organs is a priority during the early life stages vis-à-visthe requirement to interactwith the environment (Russo et al., 2007; Pea and Dumas, 2009; Khemis et al., 2013) .Once theses organs have matured, the growth rate of the head decreased with a n isometrictendency, as has been reportedin the other Cobitidae species, such as Misgurnus anguillicaudatus (Gao et al., 2014) .
The positiveallometric growth of trunk length exhibited in the early life stages of Chineseloach was similar to that reportedin California halibut larvae (Paralichthys californicus) (Gisbert et al., 2002) . However, a large number of studies have reported negative allometric growth of trunklength in teleosts (Gisbert, 1999; Gisbert and Doroshov, 2006; Celik and Cirik, 2011; Gisbert et al., 2014; Kupren et al., 2014a, b; Martínez-Montao et al., 2014; Nogueira et al., 2014) . This difference might contribute to the rapid development of the accessory respiratory organ (posterior intestine) in Chinese loachlarvae. After the inflection points, both the trunklength and body depth displayed an isometric tendencyand did not change thereafter.
Positive allometric growth of tail length has also been reported in other species (Gisbert et al., 2014; Kupren et al., 2014a; Nogueira et al., 2014) , these authors suggested the resultswere related to the modifications in swimming performance (caudal fin) duringthe early life stages. However, negative allometric growth of swimming organs (caudaland pectoral fins) was observed in the presentstudy, which may resultfrom the demersal and adherentbehavior of Chinese loach.
The visual system associated with feeding (Shand et al., 2000; Uemura et al., 2000) and avoiding predation (Kunz et al., 1983) was allometrically positive in the early life stagesof Chinese loach, as reported previously for other fish species (Huysentruyt et al., 2009; Çoban et al., 2009, 2012; Lima et al., 2012; Gao et al., 2014; Gisbert et al., 2014; Kupren et al., 2014a;Martínez-Montao et al., 2014) . This is probably becausethis early development would enhance the probability of survival. The barbel, a sensory organ in Chinese loach, displayed allometrically negativegrowth in the early life stages, suggesting that, in this species, the barbel developed early and did not grow thereafter.
5 CONCLUSIONThe growth coeffcient resultsshow a clear and common tendencytowards isometry for all the measured body ratios during the early developmental stage of Chinese loach.Rapid head segment development associated with vital functions, such as feeding, sensingand breathing, is necessary for survival duringthe early life stages of Chinese loach. In addition, specific behaviorsof Chinese loach is probably related to its allometric growth patterns that differed from other teleosts, such as intestinal respiration and adherent behavior. Further research is warranted to evaluate morphological abnormalities resulting from the Chinese loach’s specific behavioral traits and biological performance during the larval stage. It is anticipated that this study will provide baseline information for further research.
| Celik P, Cirik S, 2011. Allometric growth in serpae tetra (Hyphessobrycon serpae) larvae. J. Anim. Vet. Adv., 10 (17) : 2267 –2270. Doi: 10.3923/javaa.2011.2267.2270 |
| Çoban D, Kamaci H O, Suzer C, Saka Ş, Firat K, 2009. Allometric growth in hatchery-reared gilthead seabream. N orth. Am. J. Aquacult., 71 (3) : 189 –196. Doi: 10.1577/A08-028.1 |
| Çoban D, Suzer C, Yıldırım Ş, Saka Ş, Firat K, 2012. Morphological development and allometric growth of sharpsnout seabream (Diplodus puntazzo) larvae. Turk. J. Fish. Aquat. Sci., 12 (4) : 883 –891. |
| Froese R, Pauly D. 2014. FishBase. 24 Dec 2014, http://www.fishbase.org. (accessed on 24 Dec 2014) |
| Froese R, 2006. Cube law, condition factor and weight-length relationships: history, meta-analysis and recommendations. J. Appl. Ichthyol., 22 (4) : 241 –253. Doi: 10.1111/jai.2006.22.issue-4 |
| Gao L, Duan M, Cheng F, Xie S G, 2014. Ontogenetic development in the morphology and behavior of loach (Misgurnus anguillicaudatus) during early life stages. Chin. J. Oceanol. Limnol., 32 (5) : 973 –981. Doi: 10.1007/s00343-014-3302-4 |
| Gisbert E, Asgari R, Rafiee Gh, Agh N, Eagderi S, Eshaghzadeh H, Alcaraz C, 2014. Early development and allometric growth patterns of beluga Huso huso (Linnaeus, 1758). J. Appl. Ichthyol, 30 (6) : 1264 –1272. Doi: 10.1111/jai.2014.30.issue-6 |
| Gisbert E, Doroshov S I, 2006. Allometric growth in green sturgeon larvae. J. Appl. Ichthyol., 22 (S1) : 202 –207. Doi: 10.1111/jai.2006.22.issue-s1 |
| Gisbert E, Merino G, Muguet J B, Bush D, Piedrahita R H, Conklin D E, 2002. Morphological development and allometric growth patterns in hatchery-reared California halibut larvae. J. Fish Biol., 61 (5) : 1217 –1229. Doi: 10.1111/jfb.2002.61.issue-5 |
| Gisbert E, 1999. Early development and allometric growth patterns in Siberian sturgeon and their ecological significance. J. Fish Biol., 54 (4) : 852 –862. Doi: 10.1111/jfb.1999.54.issue-4 |
| Guibiani É A, da S Horlando S, 2014. Length-weight and length-length relationships and length at first maturity for freshwater fish species of the Salto Santiago Reservoir, Iguaçu River Basin, Brazil. J. Appl. Ichthyol., 30 (5) : 1087 –1091. Doi: 10.1111/jai.2014.30.issue-5 |
| Guimarães-Cruz R J, Veloso-Júnior V C, Sales N G, Oliveira D A A, Santos J E, 2014. Allometric growth patterns in hatchery-reared larvae of the catfish Lophiosilurus alexandri (Pisces: Pseudopimelodidae). Arq. Bras. Med. Vet. Zoote., 66 (1) : 284 –288. Doi: 10.1590/S0102-09352014000100038 |
| Huysentruyt F, Moerkerke B, Devaere S, Adriaens D, 2009. Early development and allometric growth in the armoured catfish C o rydoras aeneus (Gill, 1858). Hydrobiologia, 627 (1) : 45 –54. Doi: 10.1007/s10750-009-9714-z |
| Katsanevakis S, Thessalou-Legaki M, Karlou-Riga C, Lefkaditou E, Dimitriou E, Verriopoulos G, 2007. Information-theory approach to allometric growth of marine organisms. Mar. Biol., 151 (3) : 949 –959. Doi: 10.1007/s00227-006-0529-4 |
| Khemis I B, Gisbert E, Alcaraz C, Zouiten D, Besbes R, Zouiten A, Masmoudi A S, Cahu C, 2013. Allometric growth patterns and development in larvae and juveniles of thick-lipped grey mullet Chelon labrosus reared in mesocosm conditions. Aquac. Res., 44 (12) : 1872 –1888. Doi: 10.1111/are.2013.44.issue-12 |
| Kunz Y W, Ennis S, Wise C, 1983. Ontogeny of the photoreceptors in the embryonic retina of the viviparous guppy, Poecilia reticulata P. (Teleostei): an electronmicroscopical study. Cell Tissue Res., 230 (3) : 469 –486. Doi: 10.1007/BF00216193 |
| Kupren K, Prusińska M, Żarski D, KrejszeffS, Kucharczyk D, 2014a. Early development and allometric growth in Nannacara anomala Regan, 1905 (Perciformes: Cichlidae) under laboratory conditions. Neotrop. Ichthyol., 12 (3) : 659 –665. |
| Kupren K, Trąbska I, Żarski D, KrejszeffS, Palińska-Żarska K, Kucharczyk D, 2014b. Early development and allometric growth patterns in burbot Lota lota L. Aquacult. Int., 22 (1) : 29 –39. Doi: 10.1007/s10499-013-9680-3 |
| Lima A R A, Barletta M, Dantas D V, Possato F E, Ramos J A A, Costa M F, 2012. Early development and allometric shifts during the ontogeny of a marine catfish (Cathorops spixii-Ariidae). J. Appl. Ichthyol., 28 (2) : 217 –225. Doi: 10.1111/jai.2012.28.issue-2 |
| Martínez-Montaño E, González-Álvarez K, Lazo J P, Audelo-Naranjo J M, Vélez-Medel A. 2014. Morphological development and allometric growth of yellowtail kingfish Seriola lalandi V. larvae under culture conditions. Aquac. Res., http://dx.doi.org/10.111/are.12587. |
| Nogueira L B, Godinho A L, Godinho H P, 2014. Early development and allometric growth in hatchery-reared characin Brycon orbignyanus. Aquac. Res., 45 (6) : 1004 –1011. Doi: 10.1111/are.2014.45.issue-6 |
| Peña R, Dumas S, 2009. Development and allometric growth patterns during early larval stages of the spotted sand bass Paralabrax maculatofasciatus (Percoidei: Serranidae). Sci. Mar., 73 (S1) : 183 –189. Doi: 10.3989/scimar.2009.73s1 |
| Russo T, Costa C, Cataudella S, 2007. Correspondence between shape and feeding habit changes throughout ontogeny of gilthead sea bream Sparus aurata L. J. Fish Biol., 71 (3) : 629 –656. Doi: 10.1111/jfb.2007.71.issue-3 |
| Shand J, Chin S M, Harman A M, Moore S, Collin S P, 2000. Variability in the location of the retinal ganglion cell area centralis is correlated with ontogenetic changes in feeding behavior in the black bream, Acanthopagrus butcheri (Sparidae, Teleostei). Brain Behav. Evol., 55 (4) : 176 –190. Doi: 10.1159/000006651 |
| Uemura M, Somiya H, Moku M, Kawaguchi K, 2000. Temporal and mosaic distribution of large ganglion cells in the retina of a daggertooth aulopiform deep-sea fish (Anotopterus pharao). Philos. Trans. R oy. Soc. Lon. B Biol. Sci., 355 (1401) : 1161 –1166. Doi: 10.1098/rstb.2000.0659 |
| van Snik G M J, van den Boogaart J G M, Osse J W M, 1997. Larval growth patterns in Cyprinus carpio and Clarias gariepinus with attention to the finfold. J. Fish Biol., 50 (6) : 1339 –1352. |
| Vicentin W, dos S Costa F E, Súarez Y R, 2012. Length-weight relationships and length at first maturity for fish species in the upper Miranda River, southern Pantanal wetland, Brazil. J. Appl. Ichthyol., 28 (1) : 143 –145. Doi: 10.1111/jai.2012.28.issue-1 |
| Zhang Y L, Hu W H, Wu Q W, Wang F, Zhao Z B, He H, Liu R P, Fan Q X, 2015. Ontogenetic changes in RNA, DNA and protein contents of Chinese loach, Paramisgurnus dabryanus (Dabry de Thiersant, 1872), larvae and juveniles. J. Appl. Ichthyol., 31 (5) : 876 –882. Doi: 10.1111/jai.12808 |
 2016, 34
2016, 34


