Institute of Oceanology, Chinese Academy of Sciences
Article Information
- Zhiqiang CHANG(常志强), Fei LIU(柳飞), Chun'ang LIAN(连春盎), Qianqian ZHAI(翟倩倩), Jian LI(李健)
- Pharmacokinetics and acetylation of sulfamethoxazole in turbot Scophthalmus maximus after intravascular administration
- Journal of Oceanology and Limnology, 34(4): 789-794
- http://dx.doi.org/10.1007/s00343-016-4310-3
Article History
- Received: Dec. 30, 2014
- Accepted: May. 8, 2015
2. College of Fisheries and Life Sciences, Shanghai Ocean University, Shanghai 201306, China
As a group of synthetic broad spectrum antimicrobial drugs, sulfonamides have been used to treat and prevent bacterial infections in humans and various food producing animals for a long time. They have also been used in aquaculture worldwide because of their activity against various fish disease pathogens, such as vibriosis and furunculosis . They are also used to control aquatic red pest disease(Uno et al., 1993). A combination of sulfadimethoxine(SDM)and ormetoprim(under the brand name Romet-30), is approved by the U.S. FDA for use in aquaculture.
Overuse or misuse of sulfonamides in aquaculture may not only lead to drug residues in food of animal origin, transmission of resistance elements from aquatic environment also poses public health challenges(Landers et al., 2012)and has potential adverse effects on the ecosystem(Kümmerer, 2009; Li et al., 2012). Recently, several investigations have identified sulfamethoxazole(SMX)as the dominant antibiotic contaminant in the offshore waters of the Bohai Sea, the Yellow Sea, the Beibu Gulf of China, and in Vietnam’s Red River Delta(Le et al., 2005; Managaki et al., 2007; Zheng et al., 2012). This can be indirectly linked to the widespread use of SMX in these areas; although there is no exact statistical data on its consumption in aquaculture at present.
Generally, a thorough understanding of antimicrobial drug pharmacokinetic profiles is essential for optimizing their doses and dosing regimens in preclinical studies(Toutain and Lees, 2004), which can ensure the therapeutic effects of a drug in clinical practice. Acetylation of the paraamino group is the major metabolic pathway for sulfonamides in mammals. It is reversible and equilibrium of the acetylation-deacetylation process has a significant influence on the pharmacokinetic and residue profiles of a drug(Yuan, 2001). The magnitude of acetylation and deacetylation varies greatly not only among sulfonamides, but also among animal species(Nouws et al., 1983; Kleinow et al., 1992; Samuelsen et al., 1995; Uno et al., 1997; Samuelsen et al., 1997). Although SMX pharmacokinetics following oral administration have been studied in perch(Wang et al., 2001), carp(Ai et al., 2004), and turbot(Sun et al., 2009), there are few reports on SMX acetylation in aquaculture animals. Turbot is a fast-growing aquaculture species of high commercial value that is mainly reared in indoor intensive culture in China. In the present study, we investigated the SMX acetylation-deacetylation process in turbot to gain useful information for optimal SMX dosage regimen designs in turbot aquaculture.
2 MATERIAL AND METHOD 2.1 ChemicalsSMX(purity 98%)was purchased from Aladdin Industrial Inc.(Shanghai, China), and the reference material SMX(purity>99%)and N 4 - acetylsulfamethoxazole(AcSMX, purity>99%)were purchased from Dr. Ehrenstorfer GmbH(Augsburg, Germany). Other chemicals used in the study were analytical or HPLC grade unless specifically described.
2.2 AnimalsOne hundred and twenty healthy turbot weighing 100±8 g(mean±SD)were obtained from the Haiyang Yellow Sea Fisheries Co. Ltd., and then assigned into 10 fiberglass reinforced plastic tanks(1 m3)with aerated seawater. The seawater temperature was 18±1℃ and salinity was 31.7. The turbot were acclimatized for 2 weeks before the experiment and fed with commercial feed pellets. No SMX or AcSMX were found in the turbot serum. The study was approved by the Animal Ethics Committee of Yellow Sea Fisheries Research Institute, Chinese Academy of Fishery Sciences.
2.3 Drug administration and samplingThe turbot were randomly divided into two groups, one group was treated with SMX intravascularly(i.v.)by injecting the drug as a single bolus into the caudal vein at a dose of 50 mg/kg body weight, and the other group was treated with AcSMX by the same route and dosage. To restrain the animals for drug administration, they were covered with a wet towel and gently held down. The sterile 100 mg/mL SMX and AcSMX injections were initially prepared using PBS with sodium hydroxide, and i.v. administration was first confirmed by drawing blood into the syringe from the caudal vein prior to the injection.
A volume of blood, 500 μL, was taken from the caudal vein using a 1 mL syringe at 0.167, 0.5, 1, 2, 4, 6, 8, 10, 12, and 24 h, following drug administration, and six turbot were sampled at each time point. Serum was obtained by centrifugation at 3 000× g for 10 min and was stored at -20℃ until analysis.
2.4 Drug analysisThe SMX and AcSMX contents in the serum samples were simultaneously measured by highperformance liquid chromatography(HPLC), using an Agilent 1200 HPLC system that comprises a quaternary pump, a 5-μm Hypersil ODS column(250 mm×4.6 mm), an automatic injector, and an Agilent G1314A UV detector(Agilent Technologies, Waldbronn, Germany). The mobile phase consists of acetonitrile at 23% and 0.017 mol/L phosphoric acid solution at 77%. The flow rate was set at 1 mL/min and the UV absorbance was 270 nm. After being thawed at room temperature, 400 μL serum was deproteinized with 1 mL acetonitrile(containing 1% acetic acid)and then centrifuged at 5 000× g for 10 min. The supernatant was transferred into a clean tube and the residue was re-extracted once using acetonitrile with 1% acetic acid. The combined supernatants were dried under nitrogen gas at 40℃, and then re-dissolved in 400 μL mobile phase. Hexane was added to remove fat in the sample, and 20 μL of the lower layer was injected into HPLC system.
2.5 Kinetics analysisThe concentration vs time data of SMX and AcSMX in turbot serum following i.v. administration was analyzed using the intelligent analysis program DAS 2.0(Beijing, China). The selection of compartment model(i.e., one-, two-, or threecompartment)was judged by comparing minimum Akaike’s information criterion(AIC), and the elimination of both SMX and AcSMX from turbot were best fitted to a two-compartment model described by the following equation:
Ct=Ae-αt+Be-βt,
where,Ct is the concentration in serum at time t, A and B are zero-time serum drug concentration intercepts of biphasic disposition curves, and α and β are values related to the slopes of distribution and elimination phases.
The parameter terminal half-life(t 1/2), apparent volume of distribution(V d), body clearance(CL), and area under the concentration-time curves(AUC)were calculated by the method of Gibaldi and Perrier(1975), whereas the mean residue time(MRT)was calculated by a non-compartmental analysis. The SMX acetylation and AcSMX deacetylation following i.v. administration were calculated by the following equation:
Acetylation(%)=AUC AcSMX /(AUC SMX +AUC AcSMX)×100,
Deacetylation(%)=AUC SMX /(AUC AcSMX +AUC SMX)×100.
In the acetylation equation the AUCs were calculated after SMX administration while in the deacetylation equation they were calculated after AcSMX administration.
3 RESULTTo simultaneously detect SMX and its main metabolite(AcSMX)in the turbot serum, we optimized the standard HPLC method for animal derived foods by adjusting the mobile phase composition. It has been found that the method used was simple and sensitive for measuring the concentration of SMX and AcSMX(Fig. 1). The retention time of both compounds were longer than 10 min, which was necessary to avoid interference from plasma components, and their responses were linear within the concentration range of 0.5– 200 μg/mL with high correlation coefficients R 2 >0.999. The limit of detection was 0.01 μg/mL.
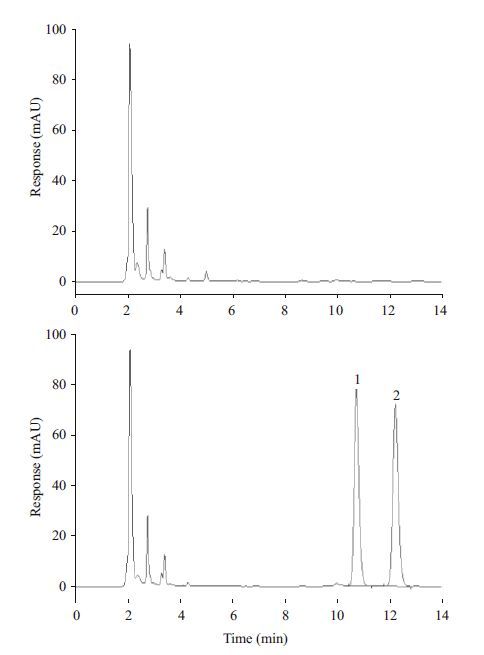
|
| Figure 1 HPLC chromatogram of the blank serum(upper panel)and the serum simultaneously spiked with sulfamethoxazole(peak 1)and N 4 - acetylsulfamethoxazole(peak 2)at 10 μg/mL(lower panel) |
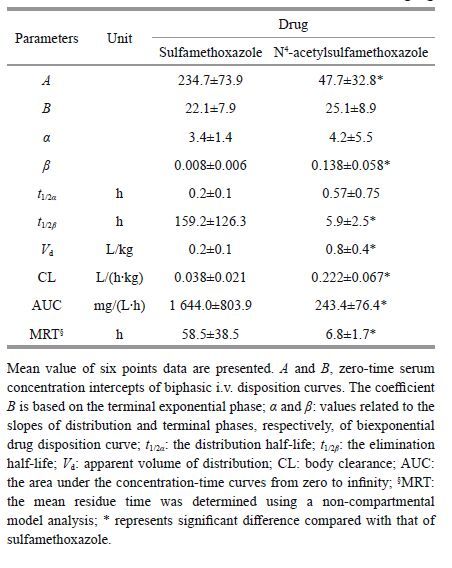
To clarify the acetylation process, SMX was selected as the parent drug and administrated to turbot at 50 mg/kg BW through a single i.v. route. The SMX serum concentration was 152.9 μg/mL at the first sampling time(10 min), and decreased sharply within 2 h. The drug concentrations were then maintained at 14.5 μg/mL until 24 h had passed and peaked at 8 h(Fig. 2a). Meanwhile, AcSMX, the main metabolite of SMX, was also found in the serum. It increased to 2μg/mL within 1 h and remained there at 4 h, and then decreased gradually to 0.9 μg/mL after 24 h. The SMX serum concentration over time data in turbot was best fitted with a two-compartment model, for which the equation was C SMX =234.7 e -3.4 t +22.1 e -0.008 t, and the corresponding pharmacokinetic parameters are shown in Table 1.
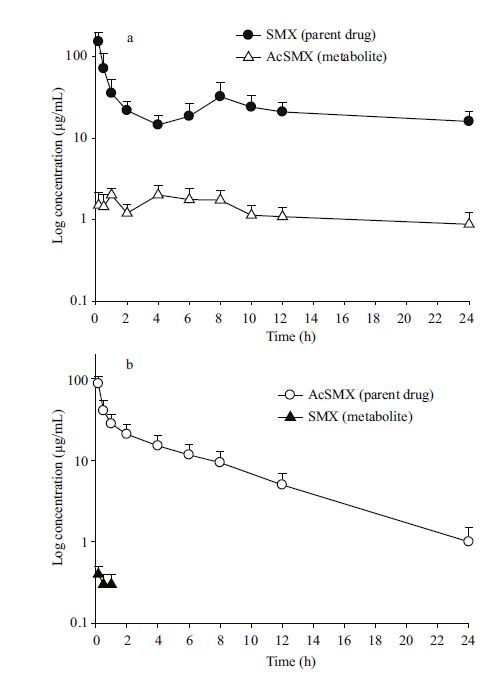
|
| Figure 2 Serum concentration of the parent drugs(circle)and their metabolites(triangle)in turbot |
|
To clarify the deacetylation process, AcSMX was then solely administrated to turbot by the same route and dosage as the parent drug. The AcSMX serum concentration was 87.4 μg/mL at the first sampling time(10 min), decreased sharply within 2 h, and then decreased gradually to 1.0 μg/mL after 24 h(Fig. 2b). However, the deacetylated metabolite of AcSMX(i.e., SMX)was not detected at most of the sampling times, except for 1 h, and those concentrations were relatively low( <0.4 μg/mL). The AcSMX serum concentration over time data in turbot was also best fitted with a two-compartment model, for which the equation was C AcSMX =47.7 e -4.2 t +25.1 e -0.138 t, and the corresponding pharmacokinetic parameters are shown in Table 1. The AcSMX t 1/2 β and AUC were significantly lower(P <0.05)than those of SMX, but the V d and CL were significantly higher(P <0.05).
By comparing the AUC of the parent drug and its metabolite according to the equation shown in the methods section, the SMX acetylation in turbot was calculated to be 2.8%, and AcSMX deacetylation was 0.2%.
4 DISCUSSIONThe mean elimination half-life(t 1/2 β)of SMX in turbot following an i.v. administration was estimated to be 159.2 h, which was much longer than that of AcSMX(5.9 h)in the current study. Previous studies have reported a mean SMX elimination half-life(t 1/2 β)in carp(12.64 h)and perch(38.30 h)following oral administration(Wang et al., 2001; Ai et al., 2004). We hypothesize that it is harder for turbot to eliminate SMX residues than it is for other fish species. This might be because of a higher binding ratio of SMX to the membrane protein in turbot tissues. Interestingly, a peak was also found in the SMX serum concentrationtime curve at 8 h post drug administration, but this was not in the case with AcSMX. Multiple peaking phenomena have been occasionally encountered in some pharmacokinetic studies of mammals, poultry, and even fish(Sumano et al., 2001; Davies et al., 2010; Li et al., 2013; Yang et al., 2014). Various mechanisms related to the physiological makeup of the gastrointestinal tract, such as enterohepatic recycling, gastric emptying and intestinal transit time, site-specific absorption, and gastric secretion-enteral reabsorption, have been used to explain such phenomena(Shepard et al., 1985). In the present study, however, these mechanisms clearly do not explain the occurrence of the SMX peak, because an absorption phase was absent in the intravascular administration of SMX. This is likely the result of recirculation of SMX from other tissues.
Acetylation is the major metabolic pathway for sulfonamides in various mammals, such as humans(Vree et al., 1986), pig(Nouws et al., 1989)and rabbits(Yuan and Fung, 1990). It is synergistically catalyzed by N-acetyltransferase and acetyl coenzyme A. The metabolite N 4 -acetylsulfonamide, however, can also be deacetylated to its parent drug(Yuan, 2001). Because the N 4 -acetyl metabolite has lost the antibacterial action and become less water-soluble than the parent, the equilibrium of acetylationdeacetylation should be considered in sulfonamide dosing regimen designs. In aquatic animals, sulfonamide acetylation has been reported in carp, rainbow trout, yellowtail, and Atlantic halibut, but the degree of acetylation differs among species. The acetylation ratio of sulfamonomethoxine(SMM)is 23%, close to that of SDM, in rainbow trout, but 64% in yellowtail(Uno et al., 1997). Samuelsen et al.(1997)compared the metabolism of several sulfonamides in Atlantic halibut, and the results revealed that after bath-administration for 72 h, approximately 90% of SDM and SMX present in tissues was the N 4 -acetylated metabolite, whereas for sulfadimidine and sulfaguanidine, the N 4 -acetylations ranged from 9% to 23%. In the present study, only 2.8% of SMX was found as N 4 -acetylated metabolites in turbot blood, indicating a relatively low level of acetylation.
However, the presence of acetylation for sulfonamides should not be judged simply by measuring the proportion of N 4 -acetylated metabolites in animal tissues, because equilibrium of the acetylation-deacetylation process is the result of a complex function of formation, deacetylation, and elimination of N 4 -acetylsulfonamide. The position of equilibrium is not affected by drug dosage, but strongly depends upon the structure of the N 1 - substituent and the acetylating-deacetylating enzyme composition(Nouws et al., 1983). In humans, rats, monkeys, rabbits, and goats, deacetylation proceeds more slowly than acetylation, which results in large amounts of acetylated products in the blood, whereas in pigs, cattle, and horses, the equilibrium tend to deacetylation reaction, which results in low levels of acetylated products in the blood(Vree et al., 1983). It is worth noting that dogs were previously thought unable to acetylate sulfonamides because no acetyl derivatives were detectable in the blood following sulfonamide administration. However, dogs can equilibrate acetylation-deacetylation, and their deacetylation rate is much faster than their acetylation rate(Vree et al., 1983). Therefore, in the present study we also checked the AcSMX and SMX serum concentrations in turbot following an intravascular treatment with AcSMX to investigate whether or not the AcSMX deacetylation process is as fast in turbot as it is in dogs. We found that AcSMX was eliminated quickly in turbot, but only very low levels of SMX were detected.
Additionally, the serum protein binding of a drug determines how much drug is in the free form and available for tissue distribution and therapeutic action(Reed et al., 2004). Although in sulfonamides, such as SMM, protein binding is very low in fish compared with that in pigs and humans, and acetylated metabolite protein binding is relatively higher than that of the parent drug(Uno et al., 1997). The free or unbound drug in the serum should also be considered in determining the dose and dosing regimen of a drug. However, we did not calculate the unbound drug in the serum because it was not within the scope of this study.
For an antimicrobial drug, in vitro pharmacodynamic measurements, such as minimum inhibitory concentration(MIC), are traditionally considered in parallel in drug dosing regimen design(Toutain, 2002). We have previously examined the MIC of several sulfonamides on commonly occurring vibrio pathogens in mariculture, and SMX was one of the most effective sulfonamides with MICs of 64, 128, 256, 32, 64 μg/mL in V . anguillarum W-1, V . parahaemolyticus 2164, V . parahaemolyticus 1614, V . alginolyticus 1833, and V . harvey 1593(unpublished data). The combination of pharmacokinetic data for various fish species is useful in determining SMX dosing regimens in mariculture.
5 CONCLUSIONOur results have shown that SMX was metabolized to AcSMX in turbot; however, the metabolic rate was relatively low, with an acetylation rate of 2.8% and a deacetylation rate of 0.2%. Additionally, the elimination of SMX in turbot was much slower than that of AcSMX following intravascular administration, which presented typical multiple peaking phenomena. The pharmacokinetic profiles are necessary in optimizing SMX dosage regimens in turbot mariculture, and it may also provide a useful reference for the use of SMX in other flatfish.
| Ai X H, Liu C Z, Liu Y P, 2004. Pharmacokinetics of SMZTMP in Ctenopharyngodonidellus and its elimination in vivo. Journal of Fisheries of China, 28 (Suppl) : 53 –57. |
| Davies N M, Takemoto J K, Brocks D R, Yáñez J A, 2010. Multiple peaking phenomena in pharmacokinetic disposition. Clinical Pharmacokinetics, 49 (6) : 351 –377. Doi: 10.2165/11319320-000000000-00000 |
| Gibaldi M, Perrier D, 1975. Marcel Dekker Inc., New York. p.281-292. |
| Kleinow K M, Beilfuss W L, Jarboe H H, Droy B F, Lech J J, 1992. Pharmacokinetics, bioavailability, distribution, and metabolism of sulfadimethoxine in the rainbow trout (Oncorhynchus mykiss). Canadian Journal of Fisheries and Aquatic Sciences, 49 (5) : 1070 –1077. Doi: 10.1139/f92-118 |
| Kümmerer K, 2009. Antibiotics in the aquatic environment-A review-Part II. Chemosphere, 75 (4) : 435 –441. Doi: 10.1016/j.chemosphere.2008.12.006 |
| Landers T F, Cohen B, Wittum T E, Larson E L, 2012. A review of antibiotic use in food animals: perspective, policy, and potential. Public Health Reports, 127 (1) : 4 –22. |
| Le T X, Munekage Y, Kato S, 2005. Antibiotic resistance in bacteria from shrimp farming in mangrove areas. Science of the Total Environment, 349 (1-3) : 95 –105. Doi: 10.1016/j.scitotenv.2005.01.006 |
| Li S Q, Dong S, Su Z H, Zhang H W, Peng J B, Yu C Y, Zou Z M, 2013. Comparative pharmacokinetics of naringin in rat after oral administration of Chaihu-Shu-Gan-San aqueous extract and naringin alone. Metabolites, 3 (4) : 867 –880. Doi: 10.3390/metabo3040867 |
| Li W H, Shi Y L, Gao L H, Liu J M, Cai Y Q, 2012. Occurrence of antibiotics in water, sediments, aquatic plants, and animals from Baiyangdian Lake in North China. Chemosphere, 89 (11) : 1307 –1315. Doi: 10.1016/j.chemosphere.2012.05.079 |
| Managaki S, Murata A, Takada H, Tuyen B C, Chiem N H, 2007. Distribution of macrolides, sulfonamides, and trimethoprim in tropical waters: ubiquitous occurrence of veterinary antibiotics in the Mekong delta. Environmental Science & Technology, 41 (23) : 8004 –8010. |
| Nouws J F M, Mevius D, Vree T B, Degen M, 1989. Pharmacokinetics and renal clearance of sulphadimidine, sulphamerazine and sulphadiazine and their N4-acetyl and hydroxy metabolites in pigs. Veterinary Quarterly, 11 (2) : 78 –86. Doi: 10.1080/01652176.1989.9694203 |
| Nouws J F M, Vree T B, Tijhuis M, Baakman M, 1983. The acetylation-deacetylation equilibrium of sulfadimidine in ruminant calves. Veterinary Quarterly, 5 (1) : 41 –48. Doi: 10.1080/01652176.1983.9693871 |
| Reed L A, Siewicki T C, Shah J C, 2004. Pharmacokinetics of oxytetracycline in the white shrimp, Litopenaeus setiferus. Aquaculture, 232 (1-4) : 11 –28. Doi: 10.1016/S0044-8486(03)00451-4 |
| Samuelsen O B, Ervik A, Wennevik V, 1995. Absorption, tissue distribution, metabolism and excretion of ormetoprim and sulphadimethoxine in Atlantic salmon (Salmo salar) after intravenous and oral administration of Romet30. Xenobiotica, 25 (11) : 1169 –1180. Doi: 10.3109/00498259509046674 |
| Samuelsen O B, Lunestad B T, Jelmert A, 1997. Pharmacokinetic and efficacy studies on bath-administering potentiated sulphonamides in Atlantic halibut, Hippoglossus hippoglossus L. Journal of Fish Diseases, 20 (4) : 287 –296. Doi: 10.1046/j.1365-2761.1997.00294.x |
| Shepard T A, Reuning R H, Aarons L J, 1985. Estimation of area under the curve for drugs subject to enterohepatic cycling. Journal of Pharmacokinetics and Biopharmaceutics, 13 (6) : 589 –608. Doi: 10.1007/BF01058903 |
| Sumano L H, Gutiérrez O L, Zamora M A, 2001. Bioequivalence of four preparations of enrofloxacin in poultry. Journal of Veterinary Pharmacology and Therapeutics, 24 (5) : 309 –313. Doi: 10.1046/j.1365-2885.2001.00355.x |
| Sun Y Z, Liu H H, Qin H W, Zhang S J, Xing H Y, Xu Y J, Gao J Q, 2009. Pharmacokinetics of sulfamethoxazole in turbot after oral administration. Progress in Fishery Sciences, 30 (6) : 42 –47. |
| Toutain P L, 2002. Pharmacokinetic/pharmacodynamic integration in drug development and dosage-regimen optimization for veterinary medicine. AAPS PharmSci, 4 (4) : 160 –188. Doi: 10.1208/ps040438 |
| Toutain P L, Lees P, 2004. Integration and modelling of pharmacokinetic and pharmacodynamic data to optimize dosage regimens in veterinary medicine. Journal of Veterinary Pharmacology and Therapeutics, 27 (6) : 467 –477. Doi: 10.1111/jvp.2004.27.issue-6 |
| Uno K, Aoki T, Ueno R, 1993. Pharmacokinetics of sulphamonomethoxine and sulphadimethoxine following oral administration to cultured rainbow trout (Oncorhynchus mykiss). Aquaculture, 115 (3-4) : 209 –219. Doi: 10.1016/0044-8486(93)90137-N |
| Uno K, Aoki T, Ueno R, Maeda I, 1997. Pharmacokinetics and metabolism of sulphamonomethoxine in rainbow trout (Oncorhynchus mykiss) and yellowtail (Seriola quinqueradiata) following bolus intravascular administration. Aquaculture, 153 (1-2) : 1 –8. Doi: 10.1016/S0044-8486(97)00012-4 |
| Vree T B, Hekster Y A, Nouws J F M, Baakman M, 1986. Pharmacokinetics, metabolism, and renal excretion of sulfadimidine and its N4-acetyl and hydroxy metabolites in humans. Therapeutic Drug Monitoring, 8 (4) : 434 –439. Doi: 10.1097/00007691-198612000-00010 |
| Vree T B, Reekers-Ketting J J, Hekster C A, Nouws J F, 1983. Acetylation and deacetylation of sulphonamides in dogs. Journal of Veterinary Pharmacology and Therapeutics, 6 (2) : 153 –155. Doi: 10.1111/j.1365-2885.1983.tb00393.x |
| Wang Q, Sun X T, Liu D Y, Liu Q, Li J, 2001. Pharmacokinetics study of sulfamethazine in perch. Marine Sciences, 25 (2) : 35 –38. |
| Yang F, Li Z L, Shan Q, Zeng Z L, 2014. Pharmacokinetics of doxycycline in tilapia (Oreochromis aureus × Oreochromis niloticus) after intravenous and oral administration. Journal of Veterinary Pharmacology and Therapeutics, 37 (4) : 388 –393. Doi: 10.1111/jvp.2014.37.issue-4 |
| Yuan Z H, 2001. Comparative metabolic study on sulfonamides. Chinese Journal of Veterinary Drug, 35 (5) : 46 –51. |
| Yuan Z H, Fung K F, 1990. Pharmacokinetics of sulfadimidine and its N4-acetyl metabolite in healthy and diseased rabbits infected with Pasteurella multocida. Journal of Veterinary Pharmacology and Therapeutics, 13 (2) : 192 –197. Doi: 10.1111/j.1365-2885.1990.tb00768.x |
| Zheng Q, Zhang R J, Wang Y H, Pan X H, Tang J H, Zhang G, 2012. Occurrence and distribution of antibiotics in the Beibu Gulf, China: impacts of river discharge and aquaculture activities. Marine Environmental Research, 78 : 26 –33. Doi: 10.1016/j.marenvres.2012.03.007 |
 2016, 34
2016, 34


