Institute of Oceanology, Chinese Academy of Sciences
Article Information
- ESMAEILZADEH Marjan, KARBASSI Abdolreza, MOATTAR Faramarz
- Heavy metals in sediments and their bioaccumulation in Phragmites australis in the Anzali wetland of Iran
- Journal of Oceanology and Limnology, 34(4): 810-820
- http://dx.doi.org/10.1007/s00343-016-5128-8
Article History
- Received: Apr. 18, 2015
- Accepted: Jan. 26, 2016
2. Department of Environmental Engineering, Graduate Faculty of Environment, University of Tehran, Tehran, Iran
Metals enter into the aquatic environments from natural and anthropogenic sources(Suárez-Serrano et al., 2010; Abdallah and Mohamed, 2015). Industrial wastewaters, agricultural runoffs and weathering of rocks are processes that play important roles in release of heavy metals into the water sources(Nasehi et al., 2013; Xiao et al., 2013). Heavy metals in aquatic ecosystems are long-term contaminants because of their environmental stability, high toxicity and ability to transfer to food chain(Eid et al., 2012; Lü et al., 2015). Therefore, investigation of the accumulation and availability of metals to living organisms in aquatic ecosystems is of great importance. Wetland sediments act as "sources" of and "sinks" for these pollutants(Bai et al., 2014). In aquatic environments, most of the contaminants, particularly trace elements, are readily adsorbed onto the suspended solids and deposited in bed sediments. Metals stabilized in sediments can also enter water columns through chemical and biological processes(Devesa-Rey et al., 2010; Abdallah and Mohamed, 2015). Sediments, smooth out environmental variations in the overlying water, and thus are an appropriate tool for the monitoring pollution in aquatic environments (Karbassi et al., 2008; Xiao et al., 2013). However, the chemical properties of sediments alone fail to provide enough biological information on potential risks to the organisms(Piva et al., 2011). Bioavailability of contaminants, particularly heavy metals, depends on different physical(such as grain size and suspended solids)and chemical(such as solubility and pH)factors. However, measurement of bioaccumulation of metals in organisms is required to determine the bioavailability of metals(Calace et al., 2005; Champan, 2007). Biomonitoring has also proved to provide a suitable framework within which to measure heavy–metal accumulation and their bioavailability. Aquatic plants, as the first trophic level of the food chain, take up the heavy metals, and thus can be indicative of the relative increase in concentration of elements in water or sediments of an ecosystem(Bonanno, 2011; Ganjali et al., 2014). Therefore, sediments and aquatic plants, as two complementary factors, can show metal pollution in an aquatic environment. Various research has been conducted on metal pollution in sediment and aquatic plants in wetlands(Ganjali et al., 2014; Wang et al., 2014, 2015)Compared to other types of fl ora and fauna, wetland macrophytes show a higher capacity for accumulation of metals(Bonanno and Lo Giudice, 2010; Ganjali et al., 2014). Selection of the species to monitor depends on local conditions and availability of aquatic macrophytes(Bonanno and Lo Giudice, 2010). Phragmites australis, an emergent macrophyte, adsorbs heavy metals from sediments and is grown widely in constructed wetlands for the treatment of metal-containing wastewaters(Grisey et al., 2012; Eid and Shaltout, 2014). Furthermore, some characteristics, including immobility and being in continuous contact with contaminants, wide distribution across aquatic environments, and being a perennial plant(allowing integration of long-term contamination of the environment), make it a good candidate for biomonitoring contaminants in aquatic environments(Bonanno, 2011; Srivastava et al., 2014).
In the Anzali wetland, P . australis has been examined as a bioindicator of metals. The main aim of the present work has been to investigate the quality of aquatic environment in this wetland, that has been subject to serious sources of pollution. We also try to find out the ability of P . australis ’ different organs in metal absorption. For this purpose the concentrations of As, Cd, Co, Cu, Cr, Zn, V, Pb, Ni, Mn, Fe and Al in sediments, as well as in roots and shoots of P . australis in the Anzali wetland(Ramsar site)were investigated. It was also attempted to investigate the relationship between the concentrations of heavy metals in sediments and those in P . australis organs. Furthermore, the differences in accumulation of heavy metals in different parts of P . australis were investigated, and the most appropriate organ found has been is assessed for its suitability in monitoring heavy metals in sediments in the study area.
2 MATERIAL AND METHOD 2.1 Study areaAnzali wetland, a freshwater, eutrophic and shallow wetland, lies between latitudes 37°22′ and 37°32′N and longitudes 49°15′ and 49°36′E. It is located in the south-west of the Caspian Sea with an area of about 193 km 2 . Anzali has been registered as an international wetland since 1975 under the Ramsar Convention(Vesali Naseh et al., 2012; Zamani Hargalani et al., 2013). The catchment of the wetland covers about 3 610 km 2 and is limited by the Caspian Sea to the north, the Alborz mountain range to the south, the Talish Mountains to the west and the Sefid Rud delta to the east. Approximately 93 525 and 196 020 ha of the catchment are covered with farm lands(particularly rice farms)and forest lands, respectively. The wetland, with mean annual precipitation and evaporation rates of about 1 280 mm and 980 mm respectively, does not have a dry season (JICA, 2004). It is covered with reed beds and plays a key role in spawning and development of fish and also provides many water birds with a place for breeding as well for staging and wintering(Vesali Naseh et al., 2012; Zamani Hargalani et al., 2013). Anzali wetland’s environment has been put in jeopardy due by contaminants produced as a result of urbanization, population growth, and agriculture, industry and tourism(Jamshidi-Zhanjani and Saeedi, 2013).
2.2 Sampling and chemical analysisIn April 2013, the first set of samples was collected from surface sediments using a Peterson grab sampler, while the second set was collected from P . australis aquatic plants based on vegetation density at 7 stations in Anzali wetland. The sediment and plant samples were taken to the laboratory. To analyze metals in plant samples, roots, stems and leaves were segregated and washed with distilled water. The samples were first air dried for about 15 days and then placed in an oven for 24 h to be thoroughly dried at 65°C. The dried roots, stems and leaves were then powdered using an agate mortar and pestle (Bonanno and Lo Giudice, 2010; Hosseini Alhashemi et al., 2011). Since the tissues of P . australis contain large amounts of lignin and cellulose, they are very difficult to digest (Du Laing et al., 2009). Among the studied methods, microwave digestion has proved to be the best digestion method for the analysis of heavy metals in P . australis(LDu aing et al., 2009). Therefore, about 0.5 g of each plant was put into microwave vessels and digested with 7 mL of HNO 3(65% v/v) and 1 mL of H 2 O 2 (30% w/v) at high temperature and pressure. Subsequently, the samples were cooled and filtered at room temperature and then made up to volume with HNO 3 (4% v/v) in a 50 mL volumetric fl ask(Bonanno, 2011). For determination of the total metal content in sediment samples, air-dried samples were passed through a mesh size less than 63 μm and subsequently powdered by an agate mortar and pestle. About 0.5 g of powdered sample was treated with 5 mL aqua regia in a TFM beaker at about 125°C. This was followed by 3 mL HCLO 4 for digestion of organics. The samples were then cooled at room temperature, filtered and made up to volume by 1N HCl in a 50 mL volumetric fl ask(U. S. EPA3050, 1996; Chester and Hughes, 1967; Gibbs, 1973; Tessier et al., 1979). Finally, all the digested samples were analyzed for metals through inductively coupled plasma mass spectrometry(ICP-MS; Agilent 4500-MS).
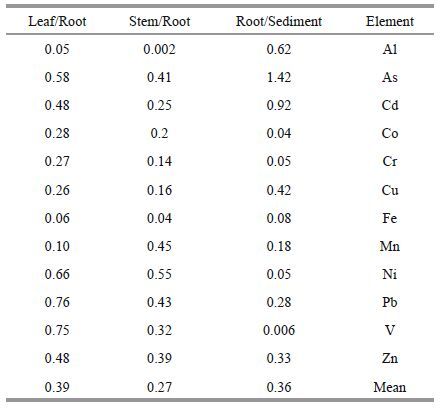
2.3 Statistical analysesThe correlation between the concentration of trace elements in sediments and plant organs was tested using the Pearson coefficient. One-way ANOVA, followed by Dunkan test using SPSS statistical software. Translocation factor (TF) or bioaccumulation factor (BAF) for the concentration of heavy metals in sediments and plant was shown using the ratios: [Trace element] Root / [Trace element] sediment and [Trace element] Leaves / [Trace element] Root(Wang et al., 2015; Xiao et al., 2015).
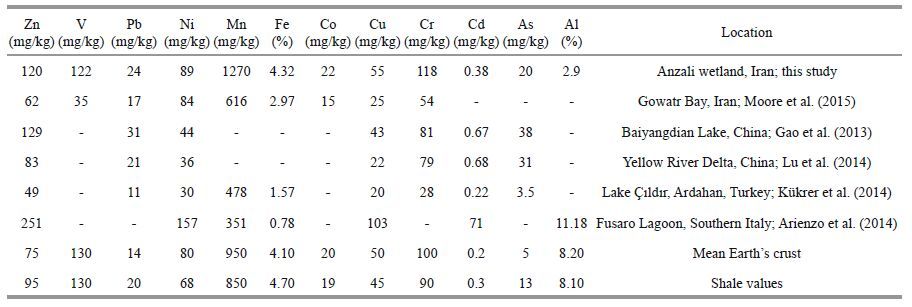
3 RESULT AND DISCUSSION 3.1 Metal concentration in sedimentTable 1 shows the concentration of elements that were found in the Anzali wetland sediments along with mean Earth’s crust and shale values. The mean concentrations of these elements in sediments followed the order: Fe>Al>Mn>V>Zn>Cr>Nitalic>Cu>P b>Co>As>Cd.
|
Generally, concentration of all the studied metals (except for Fe, V and Al) in the sediments was higher than the mean concentration in the Earth’s crust and shale. Concentrations of metals in the sediments were also compared by Ontario Ministry of the Environment and Energy Guideline and Guideline for Use at Contaminated Sites in Ontario (1998). The amounts found of Ni(88 mg/kg), Mn(1 270 mg/kg), Cu (54 mg/kg), Cr(117 mg/kg) and As (19.8 mg/kg) were higher than the standard limits, while the amounts of other metals were lower than the limits suggested in the Ontario Guideline. Standard limits of 6, 26, 16, 460, and 16 mg/kg have been specified for As, Cr, Cu, Mn, and Ni, respectively, in the Ontario Guideline.
The concentration of metals in the samples reveals that accumulation of metals in the sediments is higher in the eastern and central parts than in the western and southern parts of the wetland. The populated cities and industrial centers are mainly located at the eastern and central parts of the wetland and discharge domestic, industrial and agricultural wastewaters, which may be the main contributors of pollution in these area.
Metal concentrations in the sediments of Anzali wetland and in other aquatic ecosystems are compared in Table 1. In the sediments of Anzali wetland, concentrations of Cr, Mn and Fe were higher than, and concentrations of As, Cd, Pb and Zn similar to the sediments of other areas. Generally, it seems that metal pollution in the sediments of Anzali wetland is not much more serious than in other areas. (Areas shown in Table 1). Total organic matter(TOM) and grain size are among the factors affecting the spatial distributions of metals in sediments. Fine-grained sediments have higher ionic absorption power. Therefore, the fine-grained sediments tend to carry more organic materials and pollutants than coarsegrained sediments(Darvish Bastami et al., 2012). This may be one of the reasons for the variation in metal contents in sediments of the different areas.
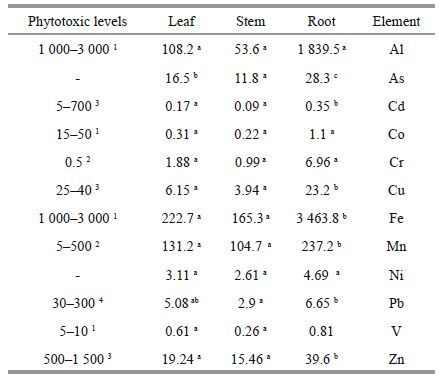
3.2 Metal concentration in plants 3.2.1 Accumulation of different metals in Phragmitesaustralis The mean concentrations of metals measured in roots, stems and leaves of P . australis in the Anzali wetland are compared with phytotoxic levels(Table 2). The mean concentration of trace elements in P . australis organs follows the order:
In roots:
Fe>Al>Mn>Zn>As>Cu>Cr>Pb>Nitalic>Co>V>Cd;
In stems:
Fe>Mn>Al>Zn>As>Cu>Pb>Nitalic>Cr>V>Co>Cd;
In leaves:
Fe>Mn>Al>Zn>As>Cu>Pb>Nitalic>Cr>V>Co>Cd.
Fe and Mn are known to be essential micronutrients for plants and they need to be present for enzyme activities and photosynthesis(Sasmaz et al., 2008). Iron toxicity in plants is related to the amount of Fe 2+ adsorbed by the roots. Availability of Fe to plants depends on different factors including pH and dissolved O 2in the soil(Goulet and Pick, 2001). High concentration of Fe in plants can induce formation of free radicals and consequently damage the cellular structure, membrane, protein molecules and DNA (Gill, 2014). At the studied stations, Table 1 shows that Fe concentrations in roots, stems and leaves were in the ranges 2 946–4 109 mg/kg, 105–196 mg/kg and 138–292 mg/kg, respectively.
Due to the abundance of Mn in lithosphere, it is usually found in most plant organs in high concentrations(Bonanno, 2012). Excessive increase of Mn in plants can disrupt the photosynthesis process by blocking the needed Fe(Gill, 2014). Manganese concentration in roots, stems and leaves was in the ranges 230–303 mg/kg, 81–133 mg/kg and 103– 195 mg/kg respectively.
Previous studies have shown that low Al concentrations can stimulate growth in plants (Kabata-Pendias and Mukherjee, 2007). However, high concentration of Al is toxic to plants and may cause damage to the structure of cytoskeleton, calcium homeostasis and phosphorus metabolism and can lead to oxidative stress in plants(Miyasaka et al., 2004). Al accumulations in roots, stems and leaves were respectively in the ranges of 1 400–2 489 mg/kg, 33.5–79 mg/kg and 54.5–142 mg/kg at different stations. Results obtained from the plant samples revealed that Mn concentration was at the phytotoxic level in roots, stems and leaves whereas Al and Fe were at the phytotoxic level only in roots.
Zn concentration in samples ranged from 22.1– 59 mg/kg in roots, 12.3–17 mg/kg in stems, and 19.7– 30.2 mg/kg in leaves. Zinc is an essential element for the growth of plants and plays a vital role in many metabolic and physiological processes within plants (Gill, 2014).
Zinc toxicity in plants can lead to poor or reduced root and shoot growth as well as chlorosis of leaves (Malik et al., 2011).
Copper is classified as a micronutrient element and plays a significant role in the vital activities of plants such as CO 2absorption and ATP synthesis. Higher concentrations of Cu can lead to oxidative stress and growth inhibition in plants(Gill, 2014). Copper was found to be in the ranges of 19.9–25.6 mg/kg in roots, 3.1–4.7 mg/kg in stems, and 5.4–7 mg/kg in leaves of P . australis at different stations in the Anzali wetland. The results also revealed that the mean concentrations of Zn and Cu in the plant samples were below the phytotoxic level.
Chromium is toxic to plants and its higher concentrations can affect the physiological processes of plants. Reduction of chlorophyll and photosynthesis as well as reduced plant growth are among the adverse effects of Cr (Chatterjee et al., 2015). Concentration of Cr in the roots, stems and leaves collected from the Anzali wetland ranged from 5–11.2 mg/kg, 0.52– 1.9 mg/kg and 0.6–3.32 mg/kg respectively. The mean concentrations of Cr were obtained to be far above the phytotoxic level of Cr(0.5 mg/kg) specified for plants.
Excess Pb in plants can reduce germination percentage and have adverse effects on metabolism. Moreover, lead toxicity inhibits root elongation (Kopyra and Gwóźdź, 2003). The contents of Pb in roots, stems and leaves of P . australis were respectively in the ranges 2.64–10.7 mg/kg, 1.19– 4.8 mg/kg and 0.6–3.32 mg/kg. These values are far below the phytotoxic level specified for Pb.
The most evident symptoms of nickel toxicity are chlorosis and necrosis. At high concentrations, Ni can form reactive oxygen species (ROS) in plants and induce membrane lipid peroxidase(Pandey and Sharma, 2002). Although Co has a positive effect on some plants, it is not regarded as an essential element for their growth(Sasmaz et al., 2008). Little is known about the toxicity of cobalt to plants; however, a few related studies have suggested that increase of cobalt in plants may infl uence their growth and biomass and cause problems in transfer of essential elements, including Zn, Cu, P, S and Mn, from roots to aboveground organs(Gill, 2014). Cobalt content in roots, stems and leaves of P . australis was in the ranges 0.77–1.67 mg/kg, 0.14–0.31 mg/kg and 0.14– 0.56 mg/kg respectively, in the plant samples of Anzali wetland, far below phytotoxic levels.
Cadmium is not an essential element for plants and its accumulation in plants would be toxic. It is easily absorbed and transferred to the different parts of plants through metabolism(Bonanno and Lo Giudice, 2010). It can also result in many morphological, physiological, biochemical, and structural changes in plants(Benavides et al., 2005). Compared to the studied metals, Cd had the lowest concentrations in the plant samples with the ranges 0.19–0.52 mg/kg in roots, 0.06–0.16 mg/kg in stems and 0.1–0.25 mg/kg in leaves. These values are considerably below the phytotoxic level.
The highest amount of V, ranging from 0.45 to 1.28 mg/kg, was found in the roots of P . australis . In the Anzali wetland, V content was about 0.001–0.49 mg/kg and 0.34–1.05 mg/kg in the stems and leaves respectively, below the phytotoxic level. Since the roots of P . australis can set up a protective mechanism to prevent uptake of vanadium(Bonanno, 2011), a lower amount of V can enter P . australis as compared to the sediments.
Arsenic toxicity depends on its chemical forms in the environment and the types of plant species. Because of having an affinity with phosphorous, arsenic can easily be absorbed by the plant system. It can lead to poor or reduced root and shoot growth, reduced germination, impaired photosynthesis and eventually plant death (Gupta and Khan, 2015). The amount of As in roots, stems and leaves was found to be in the ranges of 14.8–48.7 mg/kg, 8.8–16 mg/kg and 7.6–24.1 mg/kg, respectively. Accumulation of As is higher in roots than in above ground shoots because P . australis absorbs As from the environment through rhizofiltration process(Ghasemzadeh et al., 2008). The presence of elements in plants can be ascribed to the pollution sources in the wetland. Urban development, population growth, and industrial and agricultural activities are known to be the most important pollution sources which release the pollutants(such as metals) directly or through rivers into the wetland.
3.2.2 Distribution heavy metals in organs of Phragmites australisThere was a significant statistical difference between the rates of metals accumulation in the P . australis organs(Table 2). Bioaccumulation of the metals in organs of P . australis in various stations in the Anzali wetland, follows the order: root>leaf>stem. The highest bioaccumulation of metals was found in the roots of P . australis, and the lowest was observed in the stems. Roots of aquatic plants absorb heavy metals from sediments and store them in high concentrations(Baldantoni et al., 2004). This can be attributed to the fact that roots are the first organs which come into contact with metals. Therefore, metal ions are deposited in roots and prevented from being transferred to the above-ground organs. Therefore, roots play a significant role for immobilization of metals(Benavides et al., 2005). Since an important role of the stem is transport of nutrients and it has a little contact with pollutants, detoxification enzyme activity is very low in it (Pbugmacher et al., 1999). This can be the main reason for low accumulation of metals in stems. However, leaves are a place for photosynthesis and therefore, have a high rate of metabolic activity and a stronger defense system and detoxifying enzymes compared to the stems(Pbugmacher et al., 1999). Heavy metals become hazardous to the plant when they reach the cell cytosol. Cells store metals in leafcell vacuoles after changing them into a form tolerable for plants(Michalak, 2006; Bonanno, 2011). Thus, metal accumulation is higher in leaves than in stems.
|
The TF calculated for each element in the P . australis organs has been presented in Table 3. The mean TF indicates that metals mobility in P . australis is higher than metals translocation from the sediments to the plant. Translocation of metals within the plant tissues depends on the type of metal and the related organ(Bonanno, 2011).
Arsenic and Cd exhibited the highest rates of translocation from the sediments to the roots of P . australis . Phosphorous is one of the essential elements in plants; Arsenic is very similar to phosphorus and enters the plants through the same path for phosphorous transportation(Gupta and Khan, 2015). Such a similarity seems to be the main reason for the high uptake of As onto the plants. Cadmium has a high mobility in soil and thereby has a high availability and uptake rate in plants(Bonanno and Lo Giudice, 2010). Translocation Factor(TF) indicated that the elements accumulated in P . australis were stored in large amounts in roots. It should be pointed out that its value for all studied metals in stem/root was less than 1.
Metal mobility was also reduced from roots to the above-ground organs. The highest rate of translocation from root to stem was found in Ni and Mn. Vanadium and Pb possess the highest rate of translocation from root to leaf. In general, the rate of metal translocation was higher from root to leaf than from root to stem.
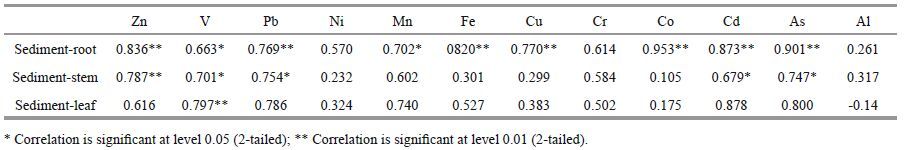
3.3 Relationship between heavy metalconcentration in sediment and plant Accumulation of trace elements in sediments and P . australis organs at different stations in Anzali wetland has been presented in Fig. 2.
|
| Figure 1 Location of the study area |
|
| Figure 2 Mean Concentration of heavy metals from sediment and organs of phragmites australis in Anzali Wetland |
A significant difference was observed between the accumulation of heavy metals in sediments and that in plant organs. Plants collected from various stations in the Anzali wetland were highly contaminated with metals. The highest concentration of Fe, Al and Mn, and the lowest concentration of Cd were observed in the sediment and in P . australis samples. Totally 12 metals were identified in the sediments and plant organs, and a significant positive, correlation (P <0.05) was observed between metal concentration(except for Al) in sediments and their accumulation in P . australis organs.(Table 4). As can be seen, metals in the Anzali wetland sediments bear the highest correlation with the metals in the P . australis roots. Moreover, among the metals in the sediments, As, Co, Cd and Zn had the highest correlation with the metals in roots. Most of the contaminants, particularly metals, can be absorbed by suspended particles in aquatic environments. Some of these particles are deposited and stabilized in sediments and, due to low solubility of heavy metals, they subsequently develop bonds with different organic and inorganic matters (Devesa-Rey et al., 2010). Sediment-rooted macrophytes, including P . australis, are mostly infl uenced by the metals existing in sediments rather than those existing in water. Therefore, a higher bioaccumulation will be found in P . australis if sediments are contaminated with heavy metals (Ganjali et al., 2014). Metal accumulation in aquatic plants depends on various factors including metal concentration in the environment, physical and chemical properties of water and sediments, contact time, condition of plant growth, type of absorption mechanism and time of sampling(Du Laing et al., 2009; Bonanno, 2011). However, high accumulation of metals in P . australis is indicative of their high bioavailability in the Anzali wetland sediments and their high absorption by P . australis in the study area.
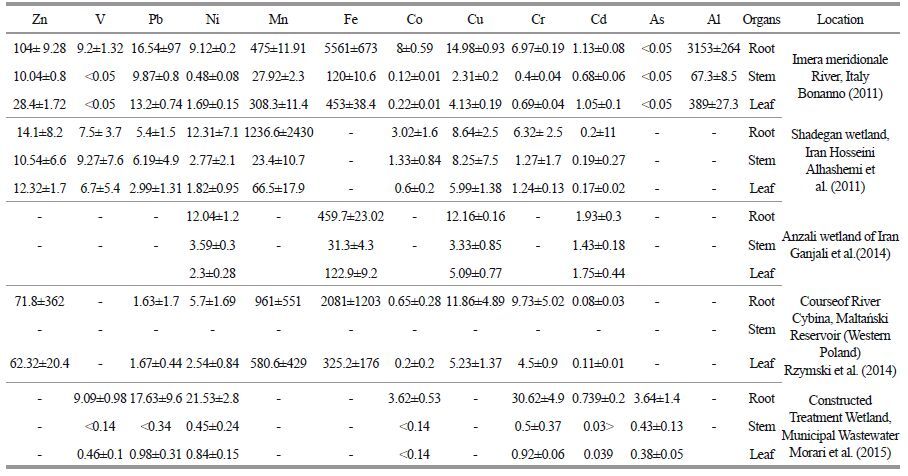
Table 5 shows metal concentrations in roots, stems and leaves of P . australis reported in previous studies. Ganjali et al. (2014)investigated the accumulation of Fe, Cu, Cd and Ni in sediments and P . australis in Anzali wetland. Comparison of the results obtained in the present study with the ones obtained by Ganjali et al.(2014) demonstrated the differences in amount of the studied metals in sediments and plant. Hosseini Alhashemi et al.(2011)studied heavy metal distribution in both sediments and P . australis in Shadegan wetland. Comparison of the results obtained in the present study with those obtained by Hosseini Alhashemi et al.(2011)shows identical TF values for Zn, Cu and Pb and totally different TF values for other metals. The differences between the results obtained in the present study and those reported in the previous ones can be attributed to the differences in contamination rates and physical and chemical characteristics of the sediments and water. Also, analytical methods applied to digest the P . australis material prior to analysis may have significantly affected the concentration of the observed metals(Du Laing et al., 2009)
The studies so far conducted support the results obtained in this study and imply that, in P . australis, the concentration of accumulated metals is higher in under-ground organs than in above-ground ones. In the other studies, metal accumulation follows the order: roots> leaves>stems(Bonanno, 2011; Hosseini Alhashemi et al., 2011; Ganjali et al., 2014; Rzymski et al., 2014; Morari et al., 2015).
In the studies conducted by Bonanno(2011), Ganjali et al.(2014)and Hosseini Alhashemi et al. (2011), a significant, linear correlation was observed between metal concentrations in sediments and their accumulation in P . australis organs. In full compliance with the results of the present study, they also suggested P . australis is useful for biomonitoring.
4 CONCLUSIONConcentrations of metals in the sediment and P . australis samples collected from Anzali wetland were measured. Results indicated that the concentration of most of metals in the sediment samples was higher than the mean concentrations of the Earth’s crust and shale. Moreover, the metals As, Cr, Cu, Ni and Mn had higher concentrations than the limits specified in the Ontario Guidelines, indicating that area of study is polluted. Investigation on the plant samples revealed that they are contaminated with metals. This also confirmed the high bioavailability of these metals in the wetland sediments. Statistical analysis demonstrated a high correlation between metal concentration in the sediments and in the P . australis organs, with the highest correlation observed between sediment and roots. According to the present study, Phragmites australis has many advantages, such as worldwide distribution, good growth and high toxic tolerance particularly to heavy metals and herbicides. Since it has good accumulation rate, therefore, it can be used as a biomonitor to refl ect metal pollution in sediments. Anzali international wetland is a unique and worthwhile ecosystem and, therefore, it seems necessary to develop an appropriate strategy to reduce the discharge of pollutants into it. Such strategies may include management of pesticide application and chemical fertilizers used over the wetland catchment area and finally treatment of the municipal and industrial wastewaters. The continuous monitoring of metal pollution would be essential. Selection of appropriate bioindicators can substantially contribute to monitoring program.
| Abdallah M A M, Mohamed A A, 2015. Assessment of heavy metals by sediment quality guideline in surficial sediments of Abu Qir Bay southeastern Mediterranean sea, Egypt. Environ. Earth Sci., 73 (7) : 3603 –3. Doi: 10.1007/s12665-014-3646-2 |
| Allen S E, 1989. Chemical Analysis of Ecological Material. Oxford: . |
| Arienzo M, Toscano F, Di Fraia M, Caputi L, Sordino P, Guida M, Aliberti F, Ferrara L, 2014. An assessment of contamination of the Fusaro Lagoon (Campania Province, southern Italy) by trace metals. Environ. Monit. Assess., 186 (9) : 5731 –5747. Doi: 10.1007/s10661-014-3816-4 |
| Bai J H, Xiao R, Zhao Q Q, Lu Q Q, Wang J J, Reddy K R, 2014. Seasonal dynamics of trace elements in tidal salt marsh soils as affected by the flow-sediment regulation regime. PLoS One, 9 (9) : e107738 . Doi: 10.1371/journal.pone.0107738 |
| Baldantoni D, Alfani A, Di Tommasi P, Bartoli G, Virzo De Santo A, 2004. Assessment of macro and microelement accumulation capability of two aquatic plants. Environ. Pollut., 130 (2) : 149 –156. Doi: 10.1016/j.envpol.2003.12.015 |
| Benavides M P, Gallego S M, Tomaro M L, 2005. Cadmium toxicity in plants. Braz. J. Plant Physiol., 17 (1) : 21 –34. |
| Bonanno G, Lo Giudice R, 2010. Heavy metal bioaccumulation by the organs of Phragmites australis (common Reed) and their potential use as contamination indicators. Ecol. Indic., 10 (3) : 639 –645. Doi: 10.1016/j.ecolind.2009.11.002 |
| Bonanno G, 2011. Trace element accumulation and distribution in the organs of Phragmites australis (common Reed) and biomonitoring applications. Ecotoxicol. Environ. Saf., 74 (4) : 1057 –1064. Doi: 10.1016/j.ecoenv.2011.01.018 |
| Bonanno G, 2012. Arundo donax as a potential biomonitor of trace element contamination in water and sediment. Ecotoxicol. Environ. Saf., 80 : 20 –27. Doi: 10.1016/j.ecoenv.2012.02.005 |
| Calace N, Ciardullo S, Maria Petronio B, Pietrantonio M, Abbondanzi F, Campisi T, Cardellicchio N, 2005. Influence of chemical parameters (heavy metals, organic matter, sulphur and nitrogen) on toxicity of sediments from the Mar Piccolo (Taranto, Ionian Sea, Italy). Microchem. J., 79 (1-2) : 243 –248. Doi: 10.1016/j.microc.2004.10.005 |
| Champan P M, 2007. Determining when contamination is pollution-weight of evidence determinations for sediments and effluents. Environ. Int., 33 (4) : 492 –501. Doi: 10.1016/j.envint.2006.09.001 |
| Chaney R L. 1989. Toxic element accumulation in soils and crops: protecting soil fertility and agricultural foodchains. In: Bar-Yosef B, Barrow N J, Goldshimd J eds. Inorganic Contaminants in the Vadose Zone. Springer-Verlag, Berlin. p. 140-158. |
| Chatterjee J, Kumar P, Sharma P N, Tewari R K. 2015. Chromium toxicity induces oxidative stress in turnip. Indian J. Plant Physiol., http://dx.doi.org/10.1007/s40502-015-0163-6. |
| Chester R, Hughes M J, 1967. A chemical technique for the separation of ferro-manganese minerals, carbonate minerals and adsorbed trace elements from pelagic sediments. Chem. Geol., 2 : 249 –262. Doi: 10.1016/0009-2541(67)90025-3 |
| Darvish Bastami K, Bagheri H, Haghparast S, Soltani F, Hamzehpoor A, Darvish Bastami M, 2012. Geochemical and geo-statistical assessment of selected heavy metals in the surface sediments of the Gorgan Bay, Iran. Mar. Pollut. Bull., 64 (12) : 2877 –2884. Doi: 10.1016/j.marpolbul.2012.08.015 |
| Devesa-Rey R, Díaz-Fierros F, Barral M T, 2010. Trace metals in river bed sediments: an assessment of their partitioning and bioavailability by using multivariate exploratory analysis. J. Environ. Manage., 91 (12) : 2471 –2477. Doi: 10.1016/j.jenvman.2010.06.024 |
| Du Laing G, van de Moortel A M K, Moors W, de Grauwe P, Meers E, Tack F M G, Verloo M G, 2009. Factors affecting metal concentrations in reed plants (Phragmites australis) of intertidal marshes in the Scheldt estuary. Ecol. Eng., 35 (2) : 310 –318. Doi: 10.1016/j.ecoleng.2008.01.002 |
| Eid E M, El-Sheikh M A, Alatar A A, 2012. Uptake of Ag, Co and Ni by the organs of Typha domingensis (Pers. ) Poir. ex Steud. in Lake Burullus and their potential use as contamination indicators. Open J. Mod. Hydr o l., 2 : 21 –27. |
| Eid E M, Shaltout K H, 2014. Monthly variations of trace elements accumulation and distribution in above-and below-ground biomass of Phragmites australis (Cav. ) Trin. ex Steudel in Lake Burullus (Egypt): a biomonitoring application. Ecol. Eng., 73 : 17 –25. |
| Ganjali S, Tayebi L, Atabati H, Mortazavi S, 2014. Phragmites australis as a heavy metal bioindicator in the Anzali wetland of Iran. Toxicol. Environ. Chem., 96 (9) : 1428 –1434. Doi: 10.1080/02772248.2014.942310 |
| Gao H F, Bai J H, Xiao R, Liu P P, Jiang W, Wang J J, 2013. Levels, sources and risk assessment of trace elements in wetland soils of a typical shallow freshwater lake, China. Stoch. Environ. Res. Risk Assess., 27 (1) : 275 –284. Doi: 10.1007/s00477-012-0587-8 |
| Ghasemzadeh F, Yosefzadeh H, Arab-Zavar M H, 2008. Removing arsenic and antimony by Phragmites australis: rhizofiltration technology. J. Appl. Sci., 8 (9) : 1668 –1675. Doi: 10.3923/jas.2008.1668.1675 |
| Gibbs R J, 1973. Mechanisms of trace metal transport in rivers. Science, 180 (4081) : 71 –73. Doi: 10.1126/science.180.4081.71 |
| Gill M, 2014. Heavy metal stress in plants: a review. Int. J. Adv. Res., 2 (6) : 1043 –1055. |
| Goulet R R, Pick F R, 2001. Diel changes in iron concentrations in surface-flow constructed wetlands. Water Sci. Technol., 44 (11-12) : 421 –426. |
| Grisey E, Laffray X, Contoz O, Cavalli E, Mudry J, Aleya L, 2012. The bioaccumulation performance of reeds and cattails in a constructed treatment wetland for removal of heavy metals in landfill leachate treatment (Etueffont, France). Water Air Soil Pollut., 223 (4) : 1723 –1741. Doi: 10.1007/s11270-011-0978-3 |
| Gupta M, Khan E. 2015. Mechanism of arsenic toxicity and tolerance in plants: role of silicon and signalling molecules. In: Tripathi B N, Müller M eds. Stress Responses in Plants. Springer International Publishing, Switzerland. p.143-157, http://dx.doi.org/10.1007/978-3-319-13368-3_6. |
| Hosseini Alhashemi A S, Karbassi A R, Hassanzadeh Kiabi B, Monavari S M, Nabavi S M B, Sekhavatjou M S, 2011. Bioaccumulation of trace elements in trophic levels of wetland plants and waterfowl birds. Biol. Trace Elem. Res., 142 (3) : 500 –516. Doi: 10.1007/s12011-010-8795-x |
| Jamshidi-Zanjani A, Saeedi M, 2013. Metal pollution assessment and multivariate analysis in sediment of Anzali international wetland. Environ. Earth Sci., 70 (4) : 1791 –1808. Doi: 10.1007/s12665-013-2267-5 |
| JICA. 2004. The study on integrated management for ecosystem conservation of the Anzali wetland. Nippon Koei Co, Ltd., Tokyo. |
| Kabata-Pendias A, Mukherjee A B. 2007. Trace Elements from Soil to Human. Springer, Berlin, Heidelberg. |
| Kabata-Pendias A, Pendias H, 2001. Trace Elements in Soils and Plants. Boca Raton, London: CRC Press. |
| Karbassi A R, Monavari S M, Nabi Bidhendi Gh R, Nouri J, Nematpour K, 2008. Metal pollution assessment of sediment and water in the Shur River. Environ. Monit. Assess., 147 (1-3) : 107 –116. Doi: 10.1007/s10661-007-0102-8 |
| Kopyra M, Gwóźdź E A, 2003. Nitric oxide stimulates seed germination and counteracts the inhibitory effect of heavy metals and salinity on root growth of Lupin u s luteus. Plant Physiol and Biochem., 41 (11-12) : 1011 –1017. Doi: 10.1016/j.plaphy.2003.09.003 |
| Kükrer S, Şeker S, Abacı Z T, Kutlu B, 2014. Ecological risk assessment of heavy metals in surface sediments of northern littoral zone of Lake Çıldır, Ardahan, Turkey. Environ. Monit. Assess., 186 (6) : 3847 –3857. Doi: 10.1007/s10661-014-3662-4 |
| Lü D W, Zheng B, Fang Y, Shen G, Liu H J, 2015. Distribution and pollution assessment of trace metals in seawater and sediment in Laizhou Bay. Chin. J. Oceanol. Limnol., 33 (4) : 1053 –1061. Doi: 10.1007/s00343-015-4226-3 |
| Lu Q Q, Bai J H, Gao Z Q, Zhao Q Q, Wang J J. 2014. Spatial and seasonal distribution and risk assessments for metals in a Tamarix Chinensis wetland, China. Wetlands., http://dx.doi.org/10.1007/s13157-014-0598-y. |
| Malik N J, Chamon A S, Mondal M D, Elahi S F, Faiz S M A, 2011. Effects of different levels of zinc on growth and yield of red amaranth (Amaranth us sp.) and rice (Oryza sativa, Variety-BR49). J. Bangladesh. Assoc. Young Res, 1 (1) : 79 –91. |
| Michalak A, 2006. Phenolic compounds and their Antioxidant activity in plants Growing under heavy metal stress. Polish J. Environ. Stud., 15 (4) : 523 –530. |
| Miyasaka S C, Hue N V, Dunn M A, 2007. CRC Press, Taylor and Francis Group, Boca Raton. . |
| Moore F, Nematollahi M J, Keshavarzi B, 2015. Heavy metals fractionation in surface sediments of Gowatr bay-Iran. Environ. Monit. Assess., 187 : 4117 . Doi: 10.1007/s10661-014-4117-7 |
| Morari F, Dal Ferro N, Cocco E, 2015. Municipal wastewater treatment with Phragmites australis L. and Typha latifolia L. for irrigation reuse. Boron and heavy metals. Water Air Soil Pollut., 226 : 56 . |
| Nasehi F, Hassani A H, Monavari M, Karbassi A R, Khorasani N, 2013. Evaluating the metallic pollution of riverine water and sediments: a case study of area river. Environ. Monit. Assess., 185 (1) : 197 –203. Doi: 10.1007/s10661-012-2543-y |
| Ontario Ministry of the Environment and Energy, Guideline for Use at Contaminated Sites in Ontario. 1998. Appendix 2, Table E, Sediment Quality Criteria Appendix. Revised September 1998, http://www.ene.gov.on.ca/envision/gp/3161e01_1.pdfH1997. |
| Pandey N, Sharma C P, 2009. Effect of heavy metals Co2+, Ni2+ and Cd2+ on growth and metabolism of cabbage. Plant Sci., 163 (4) : 753 –758. |
| Pbugmacher S, Geissler K, Steinberg C, 1999. Activity of phase I and phase II detoxication enzymes in different cormus parts of Phragmites australis. Ecotoxicol. Environ. Saf., 42 (1) : 62 –66. Doi: 10.1006/eesa.1998.1727 |
| Piva F, Ciaprini F, Onorati F, Benedetti M, Fattorini D, Ausili A, Regoli F, 2011. Assessing sediment hazard through a weight of evidence approach with bioindicator organisms: a practical model to elaborate data from sediment chemistry, bioavailability, biomarkers and ecotoxicological bioassays. Chemosphere, 83 (4) : 475 –485. Doi: 10.1016/j.chemosphere.2010.12.064 |
| Roos M S. 1994. Sources and forms of potentially toxic metals in soil-plant systems. In: Roos M S ed. Toxic Metals in Soil-Plant Systems. John Wiley, Chichester. p. 3-25. |
| Rzymski P, Niedzielski P, Klimaszyk P, Poniedziałek B, 2014. Bioaccumulation of selected metals in bivalves (Unionidae) and Phragmites australis inhabiting a municipal water reservoir. Environ. Monit. Assess., 186 (5) : 3199 –3212. Doi: 10.1007/s10661-013-3610-8 |
| Sasmaz A, Obek E, Hasar H, 2008. The accumulation of heavy metals in Typha latifolia L. grown in a stream carrying secondary effluent. Ecol. Eng., 33 (3-4) : 278 –284. |
| Srivastava J, Kalra S J S, Naraian R, 2014. Environmental perspectives of Phragmites australis (Cav. ) Trin. Ex. Steudel. Appl. Water Sci., 4 (3) : 193 –202. Doi: 10.1007/s13201-013-0142-x |
| Suárez-Serrano A, Alcaraz C, Ibáñez C, Trobajo R, Barata C, 2010. Procambarus cla r kii as a bioindicator of heavy metal pollution sources in the lower Ebro River and Delta. Ecotoxicol. Environ. Saf., 73 (3) : 280 –286. Doi: 10.1016/j.ecoenv.2009.11.001 |
| Tessier A, Campell P G C, Bisson M, 1979. Sequential extraction procedure for the Speciation of particulate trace metals. Anal. Chem., 51 (7) : 844 –851. Doi: 10.1021/ac50043a017 |
| U. S. EPA3050. 1996. Acid Digestion of Sediments, Sludges and Soils; Method 3050B. Environmental Protection Agency, USA. |
| Vesali Naseh M R, Karbassi A R, Ghazaban F, Baghvand A, 2012. Evaluation of heavy metal pollution in Anzali wetland, Guilan, Iran. Iran. J. Toxicol., 5 (15) : 565 –576. |
| Wang J J, Bai J H, Gao Z Q, Lu Q Q, Zhao Q Q. 2015. Soil as levels and bioaccumulation in Suaeda salsa and Phragmites australis wetlands of the Yellow River Estuary, China. BioMed Res. Int., 2015: 301898, http://dx.doi.org/10.1155/2015/301898. |
| Wang Z X, Yao L, Liu G H, Liu W Z, 2014. Heavy metals in water, sediments and submerged macrophytes in ponds around the Dianchi Lake, China. Ecotoxicol. Environ. Saf., 107 : 200 –206. Doi: 10.1016/j.ecoenv.2014.06.002 |
| Xiao R, Bai J H, Huang L B, Zhang H G, Cui B S, Liu X H, 2013. Distribution and pollution, toxicity and risk assessment of heavy metals in sediments from urban and rural rivers of the Pearl River delta in southern China. Ecotoxicology, 22 (10) : 1564 –1575. Doi: 10.1007/s10646-013-1142-1 |
| Xiao R, Bai J H, Lu Q Q, Zhao Q Q, Gao Z Q, Wen X J, Liu X H, 2015. Fractionation, transfer, and ecological risks of heavy metals in riparian and ditch wetlands across a 100-year chronosequence of reclamation in an estuary of China. Sci. Total Environ., 517 : 66 –75. Doi: 10.1016/j.scitotenv.2015.02.052 |
| Zamani Hargalani F, Karbassi A, Monavari S M, Abroomand Azar P, 2013. A novel pollution index based on the bioavailability of elements: a study on Anzali wetland bed sediments. Environ. Monit. Assess., 186 (4) : 2329 –2348. |
 2016, 34
2016, 34