Institute of Oceanology, Chinese Academy of Sciences
Article Information
- LI Mingzheng(黎明政), DUAN Zhonghua(段中华), GAO Xin(高欣), CAO Wenxuan(曹文宣), LIU Huanzhang(刘焕章)
- Impact of the Three Gorges Dam on reproduction of four major Chinese carps species in the middle reaches of the Changjiang River
- Chinese Journal of Oceanology and Limnology, 34(5): 885-893
- http://dx.doi.org/10.1007/s00343-016-4303-2
Article History
- Received Dec. 10, 2014
- accepted in principle Feb. 26, 2015
- accepted for publication Jul. 14, 2015
2 Key Laboratory of Aquatic Biodiversity and Conservation of Chinese Academy Of Sciences, Chinese Academy of Sciences, Wuhan 430072, China
Dams can alter fish populations and communities by changing recruitment, abundance and density, species richness, species composition, diversity (Głowacki and Penczak, 2012), and particularly, the reproductive activity of many native species (Normando et al., 2014). These effects have been reported on many rivers around the world, including the Parana River in Brazil (Sanches et al., 2006), the Sinnamary River in France (de Mérona et al., 2005), and the Colorado River Basin in the USA (Clarkson and Childs, 2000). These results have lead to proposed conservation measures, such as releasing more water to maintain minimal ecological flow (Agostinho et al., 2004) or creating artificial floods (King et al., 2010).
The Changjiang (Yangtze) River is the largest river in China and supports a highly diverse freshwater fish assemblage (Fu et al., 2003). The Three Gorges Dam (TGD) is on the mainstream of the upper Changjiang River and is the largest hydroelectric dam in the world. The Three Gorges Reservoir was initially impounded in 2003, and maximal water level reached 175 m above sea level in 2010. Many studies have investigated the effects of the change in hydrological conditions caused by the TGD on fish populations and communities (Gao et al., 2010; Yang et al., 2012) and revealed that many potamdromous fish species, which require long river reaches for migration and fast currents to transport eggs and larvae, have been impacted (Tang, 2010).
The four major Chinese carps are the silver carp, Hypophthalmichthys molitrix; the bighead carp, Aristichthysnobilis; the grass carp, Ctenopharyngodon idella; and the black carp, Mylopharyngodon piceus. They are the most important commercial freshwater fish in China. In 2012, aquaculture production of these four species accounted for 50.62% and 30.61% of farmed food fish production in China (Fishery Bureau of the Ministry of Agriculture of China, 2013) and worldwide (FAO, 2014), respectively. Production of these species often accounted for 60% of all catch in some Yangtze Basin lakes during the 20th century (Wu et al., 1992). These four carp species are also found in many Chinese rivers, but the Changjiang River has the largest populations. Adult fish migrate upstream to spawn during the flood season. Larvae are passively transported to the floodplains (nurseries) along the riverbanks, where they find favorable conditions for shelter and feeding (Anonymous, Fishes Study Lab, Institute of Hydrobiology of Hubei Province, 1976). The spawning grounds of the four major Chinese carps species are located mainly in the upper and middle reaches of the Changjiang River (Yi et al., 1988). Preliminary studies have shown that spawning activities and abundance of the four carp species in the middle Changjiang River have been affected downstream of the TGD, as spawning time has been delayed (Peng et al., 2012; Zhang et al., 2012; Wang et al., 2014), and larval abundance in the middle reaches of the Changjiang River has declined (Duan et al., 2009; Wang et al., 2014). However, longterm data is lacking, and no study has quantitatively evaluated the specific effects caused by the TGD.
In this study, the eggs and larvae of the four major Chinese carps, which are critical for recruitment, were investigated during 2005–2012 after construction of the TGD to evaluate the effects of the dam on reproduction. Specifically, the objectives of this study were (1) to investigate how changes in reproduction of the four major Chinese carps occurred; (2) to assess the influence of the TGD on reproduction of the four major Chinese carps in the middle reaches of the Changjiang River; and (3) provide suggestions for future conservation.
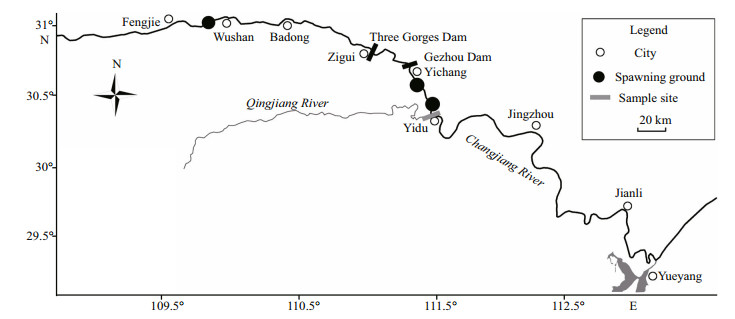
2 MATERIAL AND METHOD 2.1 Study sitesSamples were collected in the Yidu section (111°26′E, 30°25′N) of the Changjiang River (Fig. 1), which is about 80 km downstream of the TGD and 42 km downstream of the Gezhou Dam. The four major Chinese carps spawn successfully only in the main stream or large tributaries of the Changjiang River (Yi et al., 1988). No large tributary flows into the Changjiang River between the TGD and our sampling site, which means that the eggs and larvae sampled during this study originated only from the main stream.
|
| Figure 1 Locations of the sampling sites and spawning grounds of the four major Chinese carps in the Changjiang River Spawning ground 1 is from Yunchi to Yidu; spawning ground 2 is from Mojishan to Huyatan; and spawning ground 3 is in the Wushan section. |
The river is 1 600–2 000 m wide with corresponding discharge of 5 771–45 208 m3/s from May to July. The sampling site was situated 50–100 m away from the bank, at a depth of 6–8 m depending on water discharge. The river substrate consists of various combinations of silt, sand, gravel, and clay, and aquatic vegetation was abundant near shore.
2.2 Eggs and larvae samplingThe spawning season of the four major Chinese carps is May–July in the middle reaches of the Changjiang River (Yi and Liang, 1964; Yi et al., 1988); therefore, eggs and larvae were collected in the Yidu section during May–July each year from 2005 to 2012.
Routine sampling was conducted twice daily between 6:00 AM and 7:00 AM and between 17:00 PM and 18:00 PM. Each collection lasted 30–60 min (see also Li et al., 2013).
Routine sampling was conducted with a semiconical net (2 m long, 0.39 m2 semicircular mouth opening of 1 m diameter, and 500 μm mesh) (Yi et al, 1988; Cao et al., 2007) to collect eggs and larvae just below the water surface (water depth, 0.5 m). A collection box (40 cm×30 cm×30 cm), which was made of the same thin silk material as the semiconical net, was at the end of the semi-conical net. The upper part of the box was open to remove the sample. The box was buoyant, and the upper part remained above the water surface. The sampling net was fixed to a shipboard with ropes to maintain a position perpendicular to river flow. A mechanical flow meter was attached to the mouth of the net to calculate the water volume filtered during sampling. Routine sampling was conducted near the shore because of the high current velocity in the middle of the river (see also Li et al., 2013; Mu et al., 2014).
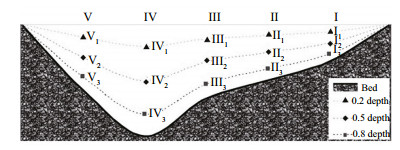
Sampling was conducted on a transect one or two times each year to estimate total numbers of eggs and larvae (Yi et al., 1988; Jiang et al., 2010). A conical net (2 m long, 0.5 m in diameter, with 500 μm in mesh size) was used to sample on the transect (Jiang et al., 2010). Five special sites (Ⅰ, Ⅱ, Ⅲ, Ⅳ, and Ⅴ) were set evenly along the river transect, each with three water depths (Fig. 2) (see also Mu et al., 2014). The routine sampling sites (sites I1 and V1) were among the transect sampling sites.
|
| Figure 2 Sampling sites on the transect Water temperature and velocity were measured at all sampling sites. Daily water discharge and water level data were obtained from the National Water and Rainfall Information Network from 2005 to 2012 (http://xxfb.hydroinfo.gov.cn/). |
Fish eggs and larvae were separated and counted immediately after sampling. The developmental stages and developmental times of each stage were defined according to Yi et al. (1988). Eggs were incubated in a tank (20 cm diameter and 18 cm high) for 6–10 days and identified to species level. Water temperature was maintained at 22–24℃ with air conditioning, each aquarium was stocked with 5–10 eggs at the same development stage, and the water was aerated (see also Li et al., 2013). The larvae were preserved in 5% sodium phosphate-buffered formalin (Kelso and Rutherford, 1996). Species were identified according to Yi et al. (1988) and Cao et al. (2007).
2.3 Data analysisSome studies have shown that density metrics data are insufficient to evaluate the numbers of eggs and larvae and result in incorrect estimates of egg and larval abundance because they do not account for large water volume differences (water discharge) among seasons or years (Martin and Paller, 2008). To avoid these limitations, the numbers of eggs and larvae transported through the sample transect were determined from estimates of daily mean egg and larval densities and station discharge. We assumed that all eggs and larvae in the water column were carried past the sampled river section and that no eggs and larvae remained in the vicinity of the sampled section (Martin and Paller, 2008). The numbers of eggs and larvae transported over time at each sample transect were calculated by averaging the numbers of eggs and larvae transported between each pair of consecutive sampling occasions, multiplying this mean by the elapsed time between sampling occasions, and summing the intervals (Yi et al., 1988; Martin and Paller, 2008; Mu et al., 2014). No effort was made to compensate for potential diel fluctuations in egg and larval abundance. This method has been used in many other studies (Yi et al., 1988; Robinson et al., 1998; Copp et al., 2005; Martin and Paller, 2008; Jiang et al., 2010; Mu et al., 2014).
The total numbers of eggs and larvae were calculated according to the following formulae (see also Mu et al., 2014),
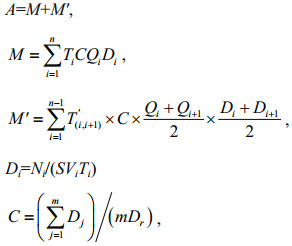
where A is the total number of eggs and larvae that drifted through the sampled river section during the entire sampling season; M is the total number of eggs and larvae counted during all routine samplings; M' is the total number of eggs and larvae collected during the time interval between each two adjacent routine sampling occasions; n is the number of routine samplings; i is one specific routine sampling; i+1 is the next sampling after the ith sampling; Qi is water discharge of the river reach during the ith sampling (m3/s); Di is drifting density of eggs and larvae during the specific ith sampling (number/m3); C is the distribution coefficient of fish eggs and larvae in the sampled section; T(i, i+1) is the time interval between the ith sampling and the (i+1)th sampling (second); Ni is the number of fish eggs and larvae collected during the ith sampling; S is net mouth area (m2); Vi is water velocity (m/s) at the net mouth during the ith sampling; Ti is duration of the specific ith sampling (second); Dj is density of eggs and larvae at each sampling site as determined by sampling on the transect (number/m3); j is the specific transect sampling site; m is the number of sampling sites when sampling on the transect; and Dr is the density of eggs and larvae at site I1 or V1 when sampling on the transect (number/m3).
The spawning grounds of the four major Chinese carps were determined using the formula (see also Mu et al., 2014),
K=V×H,
where K is the distance drifted from the spawning grounds to our sampling site (km); H is egg and larval development time (spawning grounds were only calculated based on eggs and larvae that had not reached the yolk exhaustion stage, which have no or minimal swimming ability) when captured (hour); and V is mean current velocity between the spawning grounds and sampling site (km/h). Current velocity below the TGD was measured at each sampling, and current velocity above the TGD was obtained from Li et al. (2008) and Lan (2005).
Data on the time of spawning onset of the four major Chinese carps and the date when water temperature reached 18℃ were available from Yi and Liang (1964), Yu et al. (1985), and Liu et al. (1986). The paired t-test was used to determine whether there were temporal trends in the timing of spawning onset of the four major Chinese carps and when water temperature reached 18℃ in the middle Changjiang River. April 1 was designated as day 0 for these analyses.
Larval abundance in the Jianli section (about 350 km below the TGD) during May and July from 1997 to 2012 was analyzed to determine the quantitative effects of the TGD on reproduction of the four major Chinese carps downstream. We obtained the larval abundance data from the Three Gorges Bulletin (1998–2013) (http://jcs.mep.gov.cn/hjzl/sxgb/), which is issued by the Ministry of Environmental Protection of the People’s Republic of China. A simple t-test was used to compare larval abundance before and after construction of the TGD. Analysis of covariance (ANCOVA) was used to control for the possibility that the difference was caused by a continuation of a previously decreasing trend. We predicted larval abundance postimpoundment based on a regression of larval abundance pre-impoundment vs. year, and a t-test was conducted between the predicted and observed values.
Analyses were performed with Excel 2010 (Microsoft Inc., Redmond, WA, USA) and SPSS 20.0 statistical software (SPSS Inc., Chicago, IL, USA).
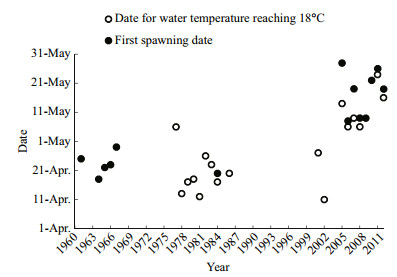
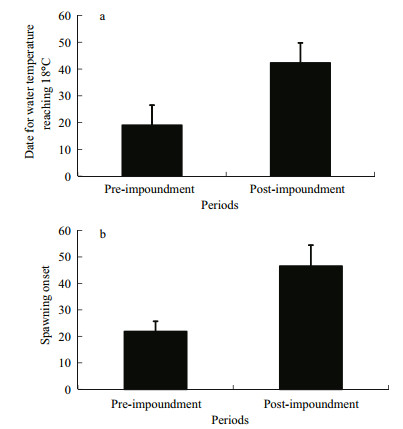
3 RESULT 3.1 Temporal variations in the onset of spawningFirst spawning of the four major Chinese carps was detected from May 8 to May 28 during 2005–2012 and usually started 0–3 d after water temperature was >18℃. Spawning onset was defined as the number of days from April 1 to the first spawning date, and the 18℃ date was defined as the number of days after April 1 until water temperature was >18℃. Spawning onset was 46.5±7.9 days (mean±standard error; range, 37–57 days) and the 18℃ date was 42.3±6.9 days (range, 35–53 days) during 2005–2012 (Fig. 3). A regression analysis showed a significant positive correlation between spawning onset and the 18℃ date (R2=0.6476, P < 0.05). Furthermore, the 18℃ date after construction of the TGD was significantly later (mean, 19±7.5 days; range, 10–35 days) than that before construction (t=-8.876, P < 0.001). Spawning onset after construction of the TGD was significantly later (mean, 21.8±3.9 days; range, 17–28 days) than that before construction (t=-6.118, P=0.002) (Fig. 4). Thus, spawning onset of the four major Chinese carps was delayed below the TGD in the middle reaches of the Changjiang River a mean of 25 days after construction of the TGD.
|
| Figure 3 First spawning dates of the four major Chinese carps and the 18℃ dates at the spawning grounds below the Three Gorges Dam The 1961 data were cited from Yi and Liang (1964); the 1964–1967 data were cited from Yu et al. (1985); the 1977–1984 were cited from Liu et al. (1986); the 1986 data were cited from Yi et al. (1988); and the 2001 and 2002 data are unpublished. |
|
| Figure 4 Comparisons of mean 18℃ dates (a) and spawning onset of the four major Chinese carps (b) between pre-and post-impoundment of the Three Gorges Reservoir (day 1=April 1) |
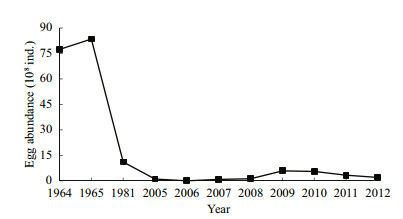
Egg abundance of the four major Chinese carps was 3–585 million (mean, 249±220 million eggs) from May to July during 2005–2012 (Fig. 5).
|
| Figure 5 Abundance of the four major Chinese carps eggs in the Yidu section of the Changjiang River below the Three Gorges Dam during 1964–2012 Abundance of the four major Chinese carps eggs in the Yidu section of the Changjiang River below the Three Gorges Dam during 1964–2012 |
A total of 1 681 eggs of the four major Chinese carps were collected, and 23 successive early development stages were identified. Among the samples, eggs at the optic vesicle closure (16.8%), blastopore (15.3%), and muscular contraction (10.5%) stages were dominant. Approximately 78.0% of the eggs were in the late gastrula and muscular contraction stages, which last 10–24 h after fertilization. Mean current velocity at the sampling site was 0.847 4±0.303 5 m/s. All eggs were released from spawning grounds downstream of the TGD based on the egg developmental stages collected and mean river velocity between the spawning grounds and the sampling site. Two main spawning grounds were identified between the TGD and Yidu section in the middle reaches of the Changjiang River from 2005 to 2012. One was about a 15 km stretch between Mojishan to Huyatan, and the other was a 10-km stretch from Yunchi to Yidu. The spawning grounds were very similar to those described in the 1980s (Liu et al., 1986; Yu, 1985).
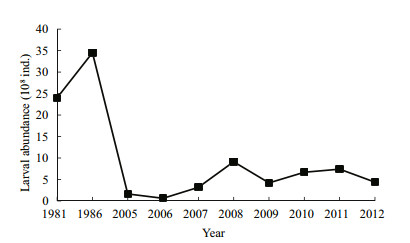
3.3 Abundance of fish larvae and upstream spawning groundsLarval abundance of the four major Chinese carps was 61–912 million (mean, 465±292 million larvae) from May to July during 2005–2012 (Fig. 6).
|
| Figure 6 Larval abundance of the four major Chinese carps in the Yidu section of the Changjiang River below the Three Gorges Dam during 1981–2012 Larval abundance of the four major Chinese carps in the Yidu section of the Changjiang River below the Three Gorges Dam during 1981–2012 |
A total of 2 667 larvae of the four major Chinese carps from the melanoid eye to the ventral fin formation stages were captured. Among the samples, larvae at the yolk exhaustion stage were the most abundant (64.1%), 10.36% were at the one chamber air bladder stage, and 1.16% were before the one chamber air bladder stage. Based on the larval development stage and mean river velocity between the spawning grounds and sampling sites, all larvae were from spawning grounds upstream of the TGD. The spawning grounds were distributed in the reaches upstream of Wushan city, which was about 110 km upstream of the TGD (Fig. 1).
3.4 Direct effects of construction of the TGD on reproduction of the four major Chinese carpsLarval abundance in the Jianli section of the river post-construction of the TGD was significantly lower than that pre-construction (t=10.185, P < 0.001). ANCOVA showed that construction of the TGD directly affected larval abundance in the Jianli section (F=16.553, P=0.002). A clear temporal trend of decreasing larval abundance was detected in the Jianli section before construction of the TGD (1997–2002) (R2=0.698, P < 0.05). However, no obvious temporal trend (P > 0.05) in larval abundance was observed in the years after construction of the TGD (2003–2012). At-test performed between the predicted and observed larval abundance values after construction of the TGD revealed a significant difference (P < 0.001) (Fig. 7). The observed value was 406 million in 2003, which was only 24.65% of the predicted value (1 646 million).

|
| Figure 7 Regression analysis for larval abundance of the four major Chinese carps in the Jianli section of the Changjiang River versus year from 1997–2002 (solid line) Dashed line represents the predicted value after impoundment of the TGR (2003–2012) based on the relationship between larval abundance and year (1997–2002). |
It has been proposed that downstream water temperature shifts due to hypolimnic release from reservoirs impair conditions favorable to native fish species (Cao and Yu, 1987). One study showed that spawning of the four carp species began in mid to late April downstream of the TGD before 2003 (Yi et al., 1988). In the present study, spawning of the four major Chinese carps downstream of the TGD began in early to late May, which was about 25 days later than before construction of the TGD (Fig. 4). These results support the prediction that spawning of the four major Chinese carps below the TGD has been delayed because of construction of the TGD. This delay shortens the growing season for juveniles and could decrease overwintering survival during the first year (Zhang et al., 2012).
4.2 Effect of the TGD on spawning grounds of the four major Chinese carps in the middle and upper reaches of the Changjiang RiverThe spawning grounds of the four major Chinese carps are distributed in reaches of the Changjiang River with specific habitat requirements (Yi and Liang, 1964). In the 1960s, 36 spawning grounds were found in the mainstream of the Changjiang River, located from Chongqing to Pengze within a range of 1 695 km. After construction of the Gezhou Dam, six spawning grounds were inundated by the reservoir; thus, 30 remained (Yi et al., 1988).
It was predicted that the 660 km long reservoir would submerge most of the spawning grounds upstream of the dam after construction of the TGD in 2003 (Cao and Yu, 1987) and that the fish would be forced to move more upstream to Chongqing City to search for new spawning grounds. These new spawning grounds were identified by previous studies and are mainly distributed in the river reaches from Chongqing to Luzhou (Jiang, 2009). In addition, we found that some spawning grounds remained within the area inundated by the Three Gorges Reservoir.
The spawning grounds between the TGD and the Yidu section were similar after construction of the TGD compared with the conditions in the 1980s (Liu et al., 1986; Yu, 1988), which agrees with the prediction of a previous study (Cao and Yu, 1987). Ten spawning grounds were distributed from Yichang to Chenglingji after construction of the TGD, which was similar to the 1980s (Duan et al., 2009).
4.3 Quantitative assessment of the effect of the TGD on reproduction of the four major Chinese carpsRegulation of a reservoir can alter the flow regime necessary for fish reproduction (Humphries et al., 2002). Considerable evidence indicates that damregulated flow affects reproductive activity of downstream fish populations. For example, a high percentage of native pelagophilic species that formerly occupied much of the Rio Grande Basin have been extirpated (Dudley and Platania, 2007). Fish spawning in sites downstream of dams is also restricted in the Uruguay River (Reynalte-Tataje et al., 2012). It was proposed that fluctuations in water flow and retention of eggs and larvae in the reservoir by the TGD would result in decreased larval abundance below the TGD (Yu, 1988). In the present study, larval abundance in the Jianli section (about 350 km below the TGD) of the river declined significantly after construction of the TGD (Fig. 7), which is direct evidence for the impact of the TGD, and the observed value in 2003 was just 24.65% of the predicted value. Therefore, construction of the TGD is directly responsible for the sharp declines in larval abundance of the four major Chinese carps in the middle reaches of the Changjiang River since 2003.
4.4 Larval and egg abundance in the Yidu section of the river after construction of the TGDEggs and larvae of the four major Chinese carps continue to drift through the Yidu section of the river after construction of the TGD and are important for recruitment of the fishery in the middle reaches of the Changjiang River. However, egg and larval abundance is clearly less than that before construction of the TGD. Egg abundance values of the four carp species downstream of the TGD were 7.74, 8.35, and 1.11 billion in 1964, 1965, and 1981 respectively (Survey Team of Spawning Grounds of Domestic Fishes in Chanjiang River et al., 1982; Yi et al., 1988) (Fig. 5), but mean abundance during 2005–2012 was only 0.25 billion. Larval abundance values were 2.42 and 3.45 billion in 1981 and 1986, respectively (Survey Team of Spawning Grounds of Domestic Fishes in Chanjiang River, 1982; Yi et al., 1988), but mean abundance was only 0.46 billion during 2005–2012.
4.5 Suggestions for conservationOur results indicate that the spawning grounds upstream of the TGD contribute to recruitment of the four major Chinese carps in the middle reaches of the Changjiang River. Thus, maintaining large free stretches upstream and a natural flood regime are very important for natural spawning and conservation of these four carp species. In addition, as the spawning grounds between the TGD and Yidu section of the river remain important, measures, such as creating artificial flood peaks to stimulate fish spawning, would facilitate more spawning at the present sites and contribute to recruitment.
Our finding of reduced larval abundance and delayed spawning of the four carp below the TGD strong supports the prediction that the TGD would affect their reproduction in the middle reaches of the Changjiang River. Additionally, one study indicated that larvae from spawning grounds nearer the TGD have a slower growth rate (Zhang et al., 2012). Thus, further research is needed to determine the influence of the TGD on early growth and mortality of the four major Chinese carps in the middle reaches of the Changjiang River.
5 CONCLUSIONThe Three Gorges Project has generated flood control, power, and navigation in the 10 years of operation since 2003. However, the ecological impacts of the TGD on the Changjiang River are also evident. Our data show that the first spawning date of four carp species has been delayed about 25 days since construction of the TGD, and larval abundance has decreased significantly downstream of the TGD. However, the present spawning grounds between the TGD and the Yidu section of the river are very similar to those reported in 1980s, and some spawning grounds remain upstream of the TGD, which contribute to recruitment of the four major Chinese carps in the middle reaches of the Changjiang River.
6 ACKNOWLEDGEMENTWe thank all of our colleagues for their help with the survey, particularly JIANG W, MU H X, and WANG H L. We also thank the editors and anonymous reviewers for their assistance.
| Agostinho A A, Gomes L C, Verissimo S, Okada E K, 2004. Flood regime, dam regulation and fish in the Upper Paraná River:effects on assemblage attributes, reproduction and recruitment. Rev. Fish Biol. Fisher., 14 (1) : 11 –19. Doi: 10.1007/s11160-004-3551-y |
| Anonymous, Fishes Study Lab, Institute of Hydrobiology of Hubei Province. 1976. The Fishes of the Yangtze River.Science Press, Beijing, China. p.93-98. (in Chinese) |
| Cao W X, Chang J B, Qiao Y, Duan Z H. 2007. Fish Resources of Early Life History Stages in Yangtze River. China Water Power Press, Beijing, China. p.69-183. (in Chinese) |
| Cao W X, Yu Z T. 1987. Preliminary assessment of impacts of the three gorges project on fish resources of the Changjiang river and approaches to the resource proliferation. In:Symposium on Impacts of the Three Gorges Project on Ecology and Environment and Possible Countermeasures.Science Press, Beijing, China. p.3-18. (in Chinese) |
| Clarkson R W, Childs M R, 2000. Temperature effects of hypolimnial-release dams on early life stages of Colorado River Basin Big-River Fishes. Copeia, 2000 (2) : 402 –412. Doi: 10.1643/0045-8511(2000)000[0402:TEOHRD]2.0.CO;2 |
| Copp G H, Spathari S, Turmel M, 2005. Consistency of diel behaviour and interactions of stream fishes and invertebrates during summer. River Res. Appl., 21 (1) : 75 –90. Doi: 10.1002/(ISSN)1535-1467 |
| de Mérona B, Vigouroux R, Tejerina-Garro F, 2005. Alteration of fish diversity downstream from Petit-Saut Dam in French Guiana. implication of ecological strategies of fish species. Hydrobiologia, 551 (1) : 33 –47. |
| Duan X B, Liu S P, Huang M G, Qiu S L, Li Z L, Wang K, Chen D Q, 2009. Changes in abundance of larvae of the four domestic Chinese carps in the middle reach of the Yangtze River, China, before and after closing of the Three Gorges Dam. Environ. Biol. Fish., 86 (1) : 13 –22. Doi: 10.1007/s10641-009-9498-z |
| Dudley R K, Platania S P, 2007. Flow regulation and fragmentation imperil pelagic-spawning riverine fishes. Ecol. Appl., 17 (7) : 2074 –2086. Doi: 10.1890/06-1252.1 |
| FAO. 2014. The State of World Fisheries and Aquaculture:Opportunities and Challenges. Food and Agriculture Organization of the United Nations (FAO), Roma, p.22. |
| Fu C Z, Wu J H, Chen J K, Wu Q H, Lei G C, 2003. Freshwater fish biodiversity in the Yangtze River basin of China:patterns, threats and conservation. Biodivers. Conserv., 12 (8) : 1649 –1685. Doi: 10.1023/A:1023697714517 |
| Gao X, Zeng Y, Wang J W, Liu H Z, 2010. Immediate impacts of the second impoundment on fish communities in the Three Gorges Reservoir. Environ. Biol. Fish., 87 (2) : 163 –173. Doi: 10.1007/s10641-009-9577-1 |
| Głowacki Ł, Penczak T, 2012. Large dam reservoirs are probably long-period oscillators of fish diversity. J. Fish Biol., 80 (6) : 2213 –2235. Doi: 10.1111/j.1095-8649.2012.03274.x |
| Humphries P, Serafini L G, King A J, 2002. River regulation and fish larvae:variation through space and time. Freshwater Biology, 47 (7) : 1307 –1331. Doi: 10.1046/j.1365-2427.2002.00871.x |
| Jiang W, Liu H Z, Duan Z H, Cao W X, 2010. Seasonal variation in drifting eggs and larvae in the upper Yangtze, China. Zool. Sci., 27 (5) : 402 –409. Doi: 10.2108/zsj.27.402 |
| Jiang W. 2009. Studies on Fish Early Resources in the Main Stream of State-Level Natural Protection Area for Rare and Endemic Fishes in the Upper Yangtze River. Institute of Hydrobiology, Chinese Academy of Sciences, Wuhan, China. p.38-40. (in Chinese) |
| Kelso W E, Rutherford D A. 1996. Collection, preservation, and identification of fish eggs and larvae. In:Murphy B R, Willis D W eds. Fisheries Techniques. 2nd edn. American Fisheries Society, Bethesda, Maryland. p.255-302. |
| King A J, Ward K A, O'Connor P, Green D, Tonkin Z, Mahoney J, 2010. Adaptive management of an environmental watering event to enhance native fish spawning and recruitment. Freshwater Biology, 55 (1) : 17 –31. Doi: 10.1111/fwb.2009.55.issue-1 |
| Lan K. 2005. Study on Flow Model of the Three Gorges Area in Chongqing Region. Chongqing University, Chongqing, China. p.86-97. (in Chinese) |
| Li C, Liao W G, Chen D Q, Xu T B, Liu S P, Liu J H, 2008. Hydrodynamic effect of different regulation scenarios for Three Gorges Reservoir on four major Chinese carps spawning. Sci. Technol. Rev., 26 (17) : 55 –61. |
| Li M Z, Gao X, Yang S R, Duan Z H, Cao W X, Liu H Z, 2013. Effects of environmental factors on natural reproduction of the four major Chinese carps in the Yangtze River, China. Zool. Sci., 30 (4) : 296 –303. Doi: 10.2108/zsj.30.296 |
| Liu L H, Wu G X, Cao W X, Wang Z L, 1986. Studies on the ecological effect on spawning of the black carp, the grass carp, the silver carp and the bighead carp in the Changjiang River after the constructions of the Gezhouba hydroelectric project. Acta Hydrobiol. Sinica, 10 (4) : 353 –364. |
| Martin F D, Paller M H, 2008. Ichthyoplankton transport in relation to floodplain width and inundation and tributary creek discharge in the lower Savannah River of Georgia and South Carolina. Hydrobiologia, 598 (1) : 139 –148. Doi: 10.1007/s10750-007-9146-6 |
| Mu H X, Li M Z, Liu H Z, Cao W X, 2014. Analysis of fish eggs and larvae flowing into the Three Gorges Reservoir on the Yangtze River, China. Fish. Sci., 80 (3) : 505 –515. Doi: 10.1007/s12562-014-0729-7 |
| Normando F T, Santiago K B, Gomes M V T, Rizzo E, Bazzoli N, 2014. Impact of the Três Marias dam on the reproduction of the forage fish Astyanax bimaculatus and A. fasciatus from the São Francisco River, downstream from the dam, southeastern Brazil. Environ. Biol. Fish., 97 (3) : 309 –319. |
| Peng Q D, Liao W G, Li C, Yu X Z, 2012. Impacts of four major Chinese carps' natural reproduction in the middle reaches of Changjiang River by Three Gorges Project Since the impoundment. Journal of Sichuan University(Engineering Science Edition), 44 (S2) : 228 –232. |
| Reynalte-Tataje D A, Agostinho A A, Bialetzki A, HermesSilva S, Fernandes R, Zaniboni-Filho E, 2012. Spatial and temporal variation of the ichthyoplankton in a subtropical river in Brazil. Environ. Biol. Fish., 94 (2) : 403 –419. Doi: 10.1007/s10641-011-9955-3 |
| Robinson A T, Clarkson R W, Forrest R E, 1998. Dispersal of larval fishes in a regulated river tributary. Trans. Am. Fish.Soc., 127 (5) : 772 –786. Doi: 10.1577/1548-8659(1998)127<0772:DOLFIA>2.0.CO;2 |
| Sanches P V, Nakatani K, Bialetzki A, Baumgartner G, Gomes L C, Luiz E A, 2006. Flow regulation by dams affecting ichthyoplankton:the case of the Porto Primavera Dam, Paraná River, Brazil. River Res. Applic., 22 (5) : 555 –565. Doi: 10.1002/(ISSN)1535-1467 |
| Survey Team of Spawning Grounds of Domestic Fishes in Chanjiang River, 1982. A survey on the spawning grounds of the "four famous Chinese carps" in the Changjiang River after dammed by the key water control project at Gezhouba. J. Fish. Sci. China, 6 (4) : 287 –305. |
| Tang X L. 2010. Studies on Early Fish Resources in Jiangjin Cross-Section in the Upstream of Yangtze River.Southwest University, Chongqing, China. p.15. (in Chinese) |
| The Fishery Bureau in Ministry of Agricultural of China. 2013.China Fishery Statistical Yearbook. China Agriculture Press, Beijing, China. p.31. (in Chinese) |
| Wang J N, Li C, Duan X B, Chen D Q, Feng S X, Luo H H, Peng Q D, Liao W G, 2014. Variation in the significant environmental factors affecting larval abundance of four major Chinese carps species:fish spawning response to the Three Gorges Dam. Freshwater Biology, 59 (7) : 1343 –1360. Doi: 10.1111/fwb.2014.59.issue-7 |
| Wu X W, Rao J Z, He B W. 1992. The history of the Chinese freshwater fisheries. In:Liu J K, He B W eds. Cultivation of the Chinese Freshwater Fishes. 3rd edn. Science Press, Beijing, China. p.5-29. (in Chinese) |
| Yang S R, Gao X, Li M Z, Ma B S, Liu H Z, 2012. Interannual variations of the fish assemblage in the transitional zone of the Three Gorges Reservoir:persistence and stability. Environ. Biol. Fish., 93 (2) : 295 –304. Doi: 10.1007/s10641-011-9936-6 |
| Yi B L, Liang Z S, 1964. Natural conditions of the spawning ground and polisheds of the "Domestic Fishes" in Yangtze River and essential external factor for spawning. Acta Hydrobiol. Sin., 5 (1) : 1 –15. |
| Yi B L, Yu Z T, Liang Z S, 1988. Gezhouba Water Control Project and Four Famous Fishes in Yangtze River. Hubei Science and Technology Press, Wuhan, China1-106. |
| Yu Z T, Zhou C S, Deng Z L, Xu Y G, 1985. On spawning grounds of four Chinese farm fishes in the River Changjiang after damming of Gezhouba area. Transaction of the Chinese Ichthyological Society, 4 : 1 –12. |
| Yu Z T, 1988. Preliminary assessment of impacts of the large hydraulic engineering on fish resources of the Yangtze River. Reservoir Fisheries, 2 : 38 –41. |
| Zhang G, Wu L, Li H T, Liu M, Cheng F, Murphy B R, Xie S G, 2012. Preliminary evidence of delayed spawning and suppressed larval growth and condition of the major carps in the Yangtze River below the Three Gorges Dam. Environ. Biol. Fish., 93 (3) : 439 –447. Doi: 10.1007/s10641-011-9934-8 |
 2016, Vol. 34
2016, Vol. 34


