Institute of Oceanology, Chinese Academy of Sciences
Article Information
- HU Zhongjun(胡忠军), JIA Xixi(贾茜茜), CHEN Xihua(陈细华), ZHANG Ying(张颖), LIU Qigen(刘其根)
- Spatial and seasonal pattern of macrozoobenthic assemblages and the congruence in water quality bioassessment using different taxa in artificial Mingzhu Lake in Shanghai
- Chinese Journal of Oceanology and Limnology, 34(5): 928-936
- http://dx.doi.org/10.1007/s00343-016-4244-9
Article History
- Received Oct. 10, 2014
- accepted in principle Jan. 3, 2015
- accepted for publication Jul. 14, 2015
2 Key Laboratory of Freshwater Biodiversity Conservation and Utilization, Certificated by Ministry of Agriculture, Yangtze River Fisheries Research Institute, Chinese Academy of Fishery Sciences, Wuhan 434000, China
Macrozoobenthos form a major link in energy flow and substance cycling between primary producers and consumers; thus it is an important component of lake ecosystems (Covich et al., 1999). Many works have long been conducted on spatial and seasonal distribution pattern of macrozoobenthic assemblages (Cowell and Vodopich, 1981; Muli, 2005; Ohtaka et al., 2010). For examples, Donouhe and Irvine (2004) and Hu et al. (2009) found that macrozoobenthic structural characteristics changed seasonally. However, their spatial variation pattern depended on the spatial size of the water bodies that studied.
Macrozoobenthic structure is ofen found heterogenous in a large (e.g., regional) spatial scale. In addition, species composition, abundance, and diversity display significant spatial distribution in a particular lake (Muli, 2005). Variations in water depth, water temperature, dissolved oxygen, organic matter, submerged macrophyte, sediment type, and interspecific interaction caused spatial heterogeneity in benthic fauna (Pan et al., 2012). However, spatial heterogeneity in community structure of macrozoobenthos has not been well studied at a fine scale. Particularly, the congruence of spatial or seasonal distribution of species composition, abundance, biomass, and diversities of macrozoobenthos at small scale has not been well-addressed.
Macrozoobenthos is sensitive to natural and anthropogenic disturbance because of their low mobility; thus it has been used widely as an indicator to aquatic environment quality (Slepukhina, 1984). Many indices based on macrozoobenthos have been designed and applied for water quality monitoring, and they fall into one of three categories: diversity indices, similarity indices, or biotic indices (Washington, 1984; Lydy et al., 2000). However, bioassessment results using different indices often disagree with each other. Among them, the similarity indices seems most promising in bioassessment among the three categories of indices (Lydy et al., 2000), but they can be used only when reference sites are deployed. Diversity indices perform better than biotic indices in some cases (Duran and Akyildiz, 2011), whereas in some other cases the opposite is true (Varnosfaderany et al., 2010). Therefore, application in combination of similarity, diversity, and biotic indices is recommended for better evaluation on water quality (Boyle et al., 1990; Pinto et al., 2009). Oligochaetes and chironomids are two widely distributed groups of freshwater macroinvertebrates. Chironomids are more mobile and depend less on sediment for food and reproduction than oligochaetes (Lang, 1999). When they are applied individually for bioassessment of a water body, the results may disagree with each other (Lang and Crozet, 1997; Lang, 1999).
Chongming Island (Fig. 1) in Shanghai at the Changjiang (Yangtze) River mouth is one of the world’s largest alluvial-build island and the country’s third largest island. It has been taken as an area of pioneer project for green city construction. Mingzhu Lake is the largest inland lake in the island, which was created by reclamation in the South Branch of the estuary in 1970s. The lake is a small water body in low spatial habitat heterogeneity but high seasonal variation. Recently, Chen et al.(2009, 2010, 2011) studied the protozoa, rotifer, and phytoplankton communities of the Lake. Hu et al. (2009) only used chironomid larvae to assess the water quality of the lake due to limited identification technique. Although water quality level of this lake using chironomid are consistent with those using planktons (Hu et al., 2009; Chen et al., 2009, 2010, 2011), deviations in water quality assessment using a single taxonomic category (chironomid) of benthic fauna carried out by Hu et al. (2009) might be produced (Lang and Crozet, 1997; Lang, 1999). In present study, we firstly identified the spatial and seasonal pattern of species composition, abundance, biomass and diversity of macrozoobenthic community and its relationship with environmental factors, and further explored the congruence among different bioassessment approaches respectively based on different taxonomic categories to find the best assessment method of the water quality for this lake.
2 STUDY AREAMingzhu Lake (31°43.7′–31°45.0′N, 121°14.8′– 121°15.5′E) is located in Lyuhua Town, southwest of Chongming Island. It is a semi-rectangle in shape, some 2.5 km north to south and 0.34–0.79 km east to west. The total surface area of the lake is about 1.33 km2 and water depth 1.0 to 4.5 m, mean at 2.8 m. The lake is in the subtropical oceanic monsoon zone; the annual mean water temperature is about 17.5°C and rainfall 1 123.7 mm. The lake had been suffered from considerable organic pollution as it served as a breeding base for economic aquatic animals before 2005 and artificial baits were used during the aquaculture.
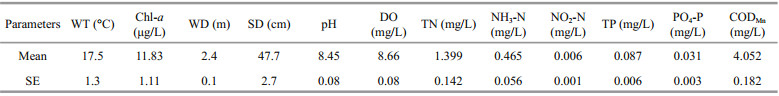
3 MATERIAL AND METHOD 3.1 Field investigationWater samples and two replicate sediment samples for macrozoobenthos were collected in January, April, July, and October in 5 stations (Stations 2 to 6 from Jul. 2006 to Jan. 2007) and 6 stations (Stations 1 to 6 from Apr. 2007 to Apr. 2008) in the profundal zone of the Lake (Fig. 1). Sampling for macrozoobenthos was performed with a modified Peterson grab whose opening area is 625 cm2. The samples were then wet sieved with a 0.45-mm mesh. The retained material was kept individually in polythene bottles and labeled. In laboratory, animals were sorted out from the retained material and preserved in 8% buffered formalin solution, all specimens were identified under dissecting and light micorscopy to species or genus level. Water temperature (WT), water depth (WD), Secchi depth (SD), pH, and dissolved oxygen (DO) were measured in situ. Total phosphorus (TP) was determined in colorimetry per Chinese national standard GB11893-1989, and total nitrogen (TN) was determined via alkaline potassium persulfate digestion and UV spectrophotometry per Chinese national standard GB11894-1989. Potassium permanganate index (CODMn) was determined using acidic potassium permanganate method per Chinese national standard GB/T11892-1989. Ammonia nitrogen (NH3-N), nitrite nitrogen (NO2-N) and orthophosphate (PO4-P) were determined using Nessler’s reagent method per GB 7479-87, spectrophotometry, and molybdenum blue technique per GB 11893-89, respectively. Chlorophyll a (Chl-a) was measured spectrophotometrically (Jin and Tu, 1990) (Table 1).

|
| Figure 1 Sampling station distribution of macrozoobenthos |
Diversity indices, including Margalef’s richness index (R), Shannon’s diversity index (H′), Simpson’s diversity index (1/D), Pielou’s evenness index (J) and Simpson’s evenness index (E), were calculated by the following equations: R=(S–1)/lnN, H'=-Σ(ni/N)ln(ni/N), 1/D=1/Σ(ni/N)2, J=H'/ln(S), E=(1/D)/S, where S, ni, and N are the species number, abundance or biomass of species i, and total abundance or biomass of all species in a station, respectively.
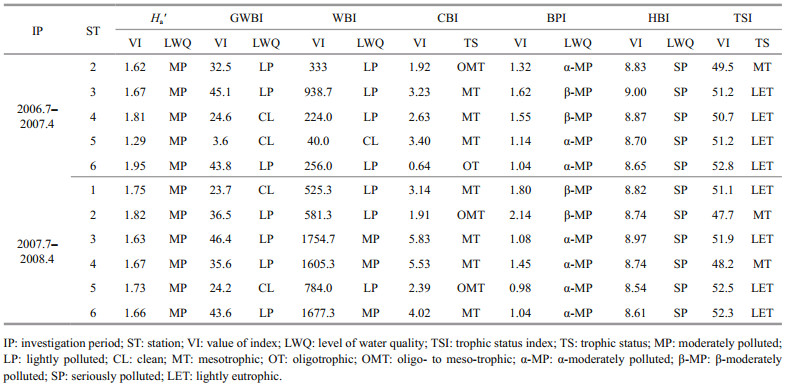
3.3 Assessment of water qualitySix biotic and diversity indices, including H', Goodnight-Whitley biotic index (GWBI), Wright biotic index (WBI), Carlander’s biotic index (CBI), Biological pollution biotic index (BPI) and Hilsenhoff biotic index (HBI), were used to assess water quality. The formulas for GWBI, WBI, CBI, BPI, and HBI and the water quality standard based on these parameters and H' followed three publications (Hu et al., 2009; Liu et al., 2011; Wu et al., 2011). Carson’s trophic state index (TSI) was calculated and used to indicate eutrophication degree according to a method and evaluation standards described by Jin and Tu (1990).
3.4 Statistical analysisData were square root transformed to normal distribution and homogeneity of variances. Two-way analysis of variance (ANOVA) was used to test for significant differences in the abundance, biomass and diversity of the whole macrozoobenthic community in different seasons and stations. If significant difference P < 0.05 by ANOVA, Duncan’s test was used to compare the mean values among groups.
Cluster analysis on the abundance was carried out in the “Pvclust” routine in the R environment (http://www.is.titech.ac.jp/~shimo/prog/pvclust/; Suzuki and Shimodaira, 2006a) to identify the spatial and seasonal differences in taxon composition of macrozoobenthic communities. Two types of probability values, the approximately unbiased (AU) P-value, and bootstrap probability (BP) were computed in parallel by the routine. The AU confidence that can correct the bias in the BP value caused by a constant sample size is more accurate and therefore was used to assess uncertainty of results caused by sampling error of data. The significance level of clusters was set to 95% (Suzuki and Shimodaira, 2006b). Similarity percentage (SIMPER) was analyzed using software PRIMER to identify the taxa responsible for observed differences. Data of taxa abundance and environmental variables except for pH were log (χ+1) transformed for normalization. Program CANOCO was used to conduct redundancy analysis (RDA) on the pretreated data. In the RDA, only the taxa on at least three occasions contributed≥1% of the total abundance included (Soininen et al., 2004). The RDA was run with forward selection of environmental variables. The significance of environmental variables was tested with 999 Monte Carlo permutations.
4 RESULT 4.1 Spatial and seasonal community compositionThirty-three taxa of macrozoobenthos collected were of classes Annelida, Arthropoda, Mollusca, and Nematomorpha. Among them, Arthropoda (15 taxa) and Annelida (12 taxa) were dominant for taking jointly 81.8% of the total in species number (Table 2).
Cluster analysis revealed two distinct groups: winter group (WG) and non-winter group (NWG) (Fig. 2). SIMPER showed that over 77.2% of the similarity in WG was due to Propsilocerus akamusi and about 89.7% of the similarity in NWG was contributed by Limnodrilus hoffmeisteri (51.0%), Microchironomus (31.0%) and Tanypus chinensis (7.7%). Propsilocerus akamusi (37.7%), L. hoffmeisteri (21.7%), Microchironomus (16.7%) and T. chinensis (6.1%) were the key members causing dissimilarity between WG and NWG.

|
| Figure 2 Dendrogram of cluster analysis based on abundance of macrozoobenthic communities The cluster elements are named as year+season+station number, e.g., A, B and C denote the year of 2006, 2007 and 2008, respectively; a, b, c, and d represent spring, summer, autumn, and winter; and the digits followed by the season are station number. |
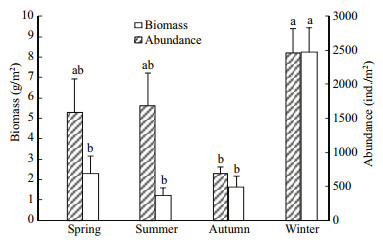
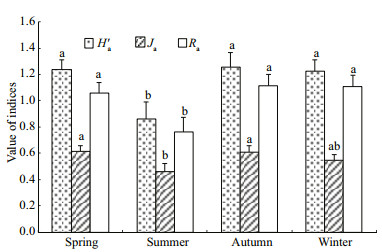
Two-way ANOVA of macrozoobenthic abundance, biomass, three indices of abundance-based diversity (Ra, Ha' and Ja, where subscripted a stands for abundance) and four indices of biomass-based diversity (Hb′, Jb, 1/Db and Eb, where subscripted b denotes biomass) indicated significant variation in season (all F > 3.14, P < 0.05), but in station (all F < 1.41, P > 0.05) and for station × season interactions (all F < 2.03, P > 0.05) (Figs. 3–5). However for 1/Da and Ea, neither significant variation in season (1/Da: F=2.159, P > 0.05; Ea: F=0.906, P > 0.05) and in station (1/Da: F=0.414, P > 0.05; Ea: F=0.329, P > 0.05) nor in station × season interaction (1/Da: F=1.971, P > 0.05; Ea : F=0.837, P > 0.05) were found.
|
| Figure 4 Seasonal variation in diversity indices based on macrozoobenthic abundance, referring to Fig.3 |

|
| Figure 5 Seasonal variation in diversity indices based on macrozoobenthic biomass, referring to Fig.3 |
The eigenvalues for RDA axis 1 (0.25) and axis 2 (0.13) explained 38.3% of the total variance of macrozoobenthic species. The species-environment correlations were 0.96 for the first axis and 0.78 for the second axis, and the cumulative percentage variance of species-environment relation was 75.8% for the first two axes, indicating a relatively strong correlation between 12 selected environmental variables and 17 taxa considered. Forward selection showed that 6/12 environmental variables contributed independently and significantly to the variance in the macrozoobenthic data. Water temperature was the key taking 24% of the total variance, followed by DO (10%), orthophosphate (5%), SD (4%), pH (3%), and water depth (3%). Axis 1 was correlated mainly to water temperature (R=-0.923) and pH (R=-0.451), whereas Axis 2 was mainly to DO (R=0.614), water depth (R=-0.445), pH (R=0.426), and SD (R=0.385) (Fig. 6).

|
| Figure 6 RDA biplot of macrozoobenthos and environmental variables (see abbreviations in Tables 1 & 2) |
Propsilocerus akamusi occurred mostly in cold water. L. hoffmeisteri preferred to habitats of warm and deep water, and low transparency and DO. Microchironomus sp. inhabited mainly in high pH and DO places.
4.4 Bioassessment to water qualityAssessment to water quality with six biological and diversity indices yielded somehow inconsistent results. The assessment results of the lake water quality were better with the GWBI, WBI, and CBI than with HBI, BPI and Ha'. The latter two indices indicated that the lake was moderately polluted, which is consistent with the results of TSI (Table 3).
The values of Ha' obtained based on both the chironomid and the whole community (WC) indicate moderate pollution in all stations, whereas those based on oligochaetes show serious pollution, reaching worsen than the results based on the other two methods (Tables 3, 4). The result by HBI assessment based on both oligochaete and WC indicates that all stations were seriously polluted. However, HBI based on the chironomid shows that all stations were polluted at a grade better than the results by the other two methods (Tables 3, 4). Moreover, Wilcoxon test shows that there were significant differences between HBI based on the two taxonomic groups and HBI based on the WC (oligochaetes versus the WC: Z=2.934, P=0.003; chironomids versus the WC: Z=2.936, P=0.003). The same results were obtained from Ha' (oligochaetes versus the WC: Z=2.934, P=0.003; chironomids versus the WC: Z=2.936, P=0.003).
 |
High spatial heterogeneity favors generation of abundant food and offers refuges for growth and reproduction of macrozoobenthos. The spatial variations of structural characteristics of macrozoobenthic community have been found usually being related to spatial heterogeneity of habitat (Shostell and Williams, 2007). Mingzhu Lake is a small water body with low spatial heterogeneity. It was expected that there were no significant spatial differences in structural properties of its macrozoobenthic community.
On the other hand, there were significant seasonal variations in community composition of macrozoobenthos in Mingzhu Lake, and water temperature was the most important ecological factor responsible for the seasonal and spatial distribution of macrozoobenthos. Liu and Liang (1997) reported that water temperature could affect reproduction, development, and overwintering of macrozoobenthos, resulting in obvious seasonal changes in macrozoobenthic structure. L. hoffmeisteri in Taihu Lake in South China breeds in March or April, July, and November (Li et al., 2012). This species may do so in Mingzhu Lake because it is near to Taihu Lake at a similar latitude and with a linear distance of approximately 86 km, causing probably the dominance of L. hoffmeisteri in spring, summer, and autumn in Mingzhu Lake. Propsilocerus akamusi is a cold water species and penetrates deep into sediment for diapause in warmer months (Yamagishi and Fukuhara, 1972). Its abundances in surface sediment of Lake Suwa in Japan are high from December to March of the following year, and it disappears from May to September (Yamagishi and Fukuhara, 1971). Similarly in Mingzhu Lake, seasonal population dynamics of this species was found by Hu et al. (2009), and its dominance in winter assemblage and negative relationship between its abundance and water temperature were determined in present study. In addition, two-way ANOVA indicated that the abundance, biomass, and diversity had significant seasonal variations, which may be related to the seasonal dynamics and individual weight of the dominant taxa and taxon composition of the whole community.
5.2 Bioassessment of water qualityProposiloceus akamusi and L. hoffmeisteri are pollution-tolerant and common dominant species in eutrophic lakes (Milbrink and Timm, 2002; Hirabayashi et al., 2004). Microchironomus is a representative group in highly productive lakes where TP>0.15 mg/L (Brodersen and Lindegaard, 1997). The macroinvertebrate community in Mingzhu Lake was characterized by the three above-mentioned taxa; therefore, this lake was in eutrophic state, which is in accordance with the results of other studies on plankton (Chen et al., 2011). This situation was probably related to aquaculture practice. Before 2005, Mingzhu Lake was an important breeding base in Shanghai for aquatic economic animals, and a large amount of artificial baits was used for aquaculture. Although the lake had been changed to an ecotourism area afterwards, endogenous pollution sources in the sediment resulted from previous artificial baits have not completely disappeared. Therefore, the lake was still in eutrophic state after its role change.
It is often difficult to reflect the real status of water quality of a lake by applying just a single biological or diversity index (Boyle et al., 1990; Pinto et al., 2009), which was true in this case. Washington (1984) disliked the biotic indices because of their specificity for a particular type of pollution typically an organic one. Boyle et al. (1990) particularly disliked diversity indices, except in conjunction with other indices. In addition, several other authors argued that it is inappropriate to use a single taxonomic group (e.g., chironomids or oligochaetes) of macrozoobenthos to assess water quality (Lang and Crozet, 1997; Lang, 1999; Nijboer et al., 2005). In this study, the results from chironomids and oligochaetes were not always consistent with those from the whole community when HBI or Ha' were applied to bioassessment of Mingzhu Lake, and biotic index BPI shows that the lake suffered from relatively heavy organic pollution during the investigation period. However, this finding shall be incorporated with those from diversity index (Shannon’ index) and TSI, by which water quality of Mingzhu Lake can be assessed more objectively and precisely.
6 CONCLUSIONWe did not find significant spatial variations in the main structural features, including species composition, abundance, biomass and all studied diversity indices, which can be ascribed to high spatial habitat homogeneity of the lake.
Both Shannon’s species diversity and biological pollution index (BPI) indicate that Mingzhu Lake was moderately polluted and eutrophicated by local aquaculture activities. However, Results of bioassessment on water quality of the lake using other four biotic indices (Goodnight-Whitley biotic index, Wright biotic index, Carlander’s biotic index, and Hilsenhoff biotic index) were inconsistent with those using trophic state index, indicating that diversity and biotic indices shall be used together for the bioassessment. Moreover, care should be taken when using single-taxonomic-group-based bioassessment because the results using chironomids and oligochaetes may differ from those using the whole community when abundance-based Shannon’s diversity index or Hilsenhoff biotic index were applied for the bioassessment.
| Boyle T P, Smillie G M, Anderson J C, Beeson D R, 1990. A sensitivity analysis of nine diversity and seven similarity indices. Research Journal of Water Pollution Control Federation, 62 : 749 –762. |
| Brodersen K P, Lindegaard C, 1997. Significance of subfossile Chironomid remains in classification of shallow lakes. Hydrobiologia, 324/343 : 125 –132. |
| Chen L J, Gu J, Hu Z J, Peng Z R, Liu Q G, 2010. The research of protozoa community structure in Mingzhu Lake of Chongming Island, Shanghai. Journal of Fisheries of China, 34 : 1404 –1413. |
| Chen L J, Gu J, Peng Z R, Hu Z J, Liu Q G, 2009. Community structure of rotifer in Mingzhu Lake of Chongming Island, Shanghai. Chinese Journal of Application Ecology, 20 : 3057 –3062. |
| Chen L J, Wu Z C, Hu Z J, Peng Z R, Liu Q G, 2011. Phytoplankton community structure in Mingzhu Lake of Chongming Island, Shanghai. Chinese Journal of Application Ecology, 22 : 1599 –1605. |
| Covich A P, Palmer M A, Crowl T A, 1999. The role of benthic invertebrate species in freshwater ecosystems. BioScience, 49 : 119 –127. Doi: 10.2307/1313537 |
| Cowell B C, Vodopich D S, 1981. Distribution and seasonal abundance of benthic macroinvertebrates in a subtropical Florida lake. Hydrobiologia, 78 : 97 –105. Doi: 10.1007/BF00007582 |
| Donouhe I, Irvine K, 2004. Seasonal patterns of sediment loading and benthic invertebrate community dynamics in lake Tanganyika, Africa. Freshwater Biology, 49 : 320 –331. Doi: 10.1111/fwb.2004.49.issue-3 |
| Duran M, Akyildiz G K, 2011. Evaluating benthic macroinvertebrate fauna and water quality of Suleymanli Lake (Buldan-Denizli) in Turkey. Acta Zoologica Bulgarica, 63 : 169 –178. |
| Hirabayashi K, Yoshizawa K, Yoshida N, Kazama F, 2004. Progress of eutrophication and change of Chironomid fauna in Lake Yamanakako, Japan. Limnology, 5 : 47 –53. Doi: 10.1007/s10201-003-0113-2 |
| Hu Z J, Liu Q G, Chen L J, Peng Z R, 2009. Structural characteristics of Chironomid community and their indicative significance in bioassessment of water quality in Mingzhu Lake of Chongming Island, Shanghai. Chinese Journal of Application Ecology, 20 : 929 –936. |
| Jin X C, Tu Q Y, 1990. Survey criteria of eutrophication for the lake. China Environmental Science Press, Beijing1-317. |
| Kalyoncu H, Zeybek M, 2011. An application of different biotic and diversity indices for assessing water quality:a case study in the Rivers Çukurca and Isparta (Turkey). African Journal of Agricultural Research, 6 : 19 –27. |
| Lang C, Lods-Crozet B, 1997. Oligochaetes versus chironomids as indicators of trophic state in two Swiss lakes recovering from eutrophication. Archiv fur Hydrobiologie, 139 : 187 –195. |
| Lang C, 1999. Contrasting responses of oligochaetes(Annelida) and chironomids (Diptera) to the abatement of eutrophication in Lake Neuchâtel. Aquatic Science, 61 : 206 –214. Doi: 10.1007/PL00001324 |
| Li Y, Cai Y J, Qin B Q, Gong Z J, 2012. Temporal and spatial patterns of Limnodrilus hoffmeisteri Claparède in Lake Taihu. Journal of Lake Sciences, 24 : 450 –459. Doi: 10.18307/2012.0318 |
| Liu B Y, Liang X M, 1997. Zoobenthos in Taipinghu Reservoir, Anhui Province. Journal of Lake Sciences, 9 : 237 –243. |
| Liu Q G, Zha Y T, Hu, Z J. 2011. Spatial distribution of macrozoobenthos in a large and deep impoundment:Xin'anjiang Reservoir, Zhejiang Province. In:Han B P, Liu Z W eds. Tropical and Sub-Tropical Reservoir Limnology in China. Springer Science+Business Media B.V. p.135-153. |
| Lydy M J, Crawford C G, Frey J W, 2000. A comparison of selected diversity, similarity, and biotic indices for detecting changes in benthic-invertebrate community structure and stream quality. Archives of Environment Contamination and Toxicology, 39 : 469 –479. Doi: 10.1007/s002440010129 |
| Milbrink G, Timm T, Lundberg S, 2002. Indicative profundal oligochaetes assemblages in selected small Swedish lakes. Hydrobiologia, 468 : 53 –61. Doi: 10.1023/A:1015274323026 |
| Muli J R, 2005. Spatial variation of benthic macroinvertebrates and the environmental factors influencing their distribution in Lake Victoria, Kenya. Aquatic Ecosystem Health and Management, 8 : 147 –157. Doi: 10.1080/14634980590953680 |
| Nijboer R C, Verdonschot P F M, van der Werf D C, 2005. The use of indicator taxa as representatives of communities in bioassessment. Freshwater Biology, 50 : 1427 –1440. Doi: 10.1111/fwb.2005.50.issue-8 |
| Ohtaka A, Watanabe R, Im S, Chhay R, Tsukawaki S, 2010. Spatial and seasonal changes of net plankton and zoobenthos in Lake Tonle Sap, Cambodia. Limnology, 11 : 85 –94. Doi: 10.1007/s10201-009-0283-7 |
| Pan B Z, Wang H J, Wang H Z, Wang Z Y, 2012. Macrozoobenthic assemblages in relation to environments of the Yangtze-isolated lakes. Frontiers of Environmental Science & Engineering, 6 : 246 –254. |
| Pinto R, Patrício J, Baeta A, Fath B D, Neto J M, Marques J C, 2009. Review and evaluation of estuarine biotic indices to assess benthic condition. Ecological Indicators, 9 : 1 –25. Doi: 10.1016/j.ecolind.2008.01.005 |
| Shostell J M, Williams B S, 2007. Habitat complexity as a determinate of benthic macroinvertebrate community structure in cypress tree reservoirs. Hydrobiologia, 575 : 389 –399. Doi: 10.1007/s10750-006-0385-8 |
| Slepukhina T D, 1984. Comparison of different methods of water quality evaluation by means of oligochaetes. Hydrobiologia, 115 : 183 –186. Doi: 10.1007/BF00027914 |
| Soininen J, Paavola R, Muotka T, 2004. Benthic diatom communities in boreal streams:Community structure in relation to environmental and spatial gradients. Ecography, 27 : 330 –342. Doi: 10.1111/eco.2004.27.issue-3 |
| Suzuki R, Shimodaira H. 2006a. Pvclust:an R package for hierarchical clustering with p-values. R package version 1.2-2. http://www.is.titech.ac.jp/~shimo/prog/pvclust/ |
| Suzuki R, Shimodaira H, 2006b. Pvclust:an R package for assessing the uncertainty in hierarchical clustering. Bioinformatics, 22 : 1540 –1542. Doi: 10.1093/bioinformatics/btl117 |
| Varnosfaderany M N, Ebrahimi E, Mirghaffary N, Safyanian A, 2010. Biological assessment of the Zayandeh Rud River, Iran, using benthic macroinvertebrates. Limnologica, 40 : 226 –232. Doi: 10.1016/j.limno.2009.10.002 |
| Washington H G, 1984. Diversity, biotic and similarity indices:A review with special relevance to aquatic ecosystems. Water Research, 18 : 653 –694. |
| Wu Z S, Cai Y J, Chen Y W, Shao X Y, Gao J F, 2011. Assemblage structure investigation of macrozoobenthos and water quality bioassessment of the main river systems in Taihu Basin. Journal of Lake Sciences, 23 : 686 –694. Doi: 10.18307/2011.0504 |
| Yamagishi H, Fukuhara H, 1971. Ecological studies on chironomids in Lake Suwa. I. Population dynamics of two large chironomids, Chironomus plumosus L. and Spaniotoma akamusi Tokunaga. Oecologia, 7 : 309 –327. |
| Yamagishi H, Fukuhara H, 1972. Vertical migration of Spaniotoma akamusi larvae (Diptera:Chironomidae)through the bottom deposits of Lake Suwa. Japanese Journal of Ecology, 22 : 226 –227. |
 2016, Vol. 34
2016, Vol. 34