Institute of Oceanology, Chinese Academy of Sciences
Article Information
- LIU Haokun(刘昊昆), YAN Quangen(严全根), HAN Dong(韩冬), JIN Junyan(金俊琰), ZHU Xiaoming(朱晓鸣), YANG Yunxia(杨云霞), XIE Shouqi(解绶启)
- Effect of dietary cottonseed meal on growth performance, physiological response, and gossypol accumulation in preadult grass carp, Ctenopharyngodon idellus
- Chinese Journal of Oceanology and Limnology, 34(5): 992-1003
- http://dx.doi.org/10.1007/s00343-016-4115-4
Article History
- Received May. 18, 2015
- accepted in principle Aug. 2, 2015
- accepted for publication Aug. 15, 2015
2 Freshwater Aquaculture Collaborative Innovation Center of Hubei Province, Wuhan 430070, China
Grass carp (Ctenopharyngodon idellus) is a commonly farmed freshwater fish species in China. Total grass carp yield in 2012 was 4.78 million tons, which was > 20% of total freshwater fish yield (Fishery Bureau of Ministry of Agriculture of the People’s Republic of China, 2013).
Grass carp are typical herbivores, and cottonseed meal (CM) has been included in commercial feed for grass carp at up to 35% of total ingredients. CM is a co-product of extracting cottonseed oil. China produces the most cottonseed in the world, which was 13.2 million tons in 2011, accounting for 34% of world production (FAO, 2014). Depending on the technical level of dehulling and oil extraction, CM has been used in animal feeds since the early part of the 20th century (Cheng and Hardy, 2002). However, the presence of gossypol, high fiber content, protease inhibitors, and low availability of lysine, methionine, and cysteine in CM limits its use in formulated fish diets (Heuzé et al., 2013). Gossypol is a phenolic aldehyde and a major anti-nutritional factor in cottonseed. Gossypol causes anorexia, diarrhea, anemia, pathological changes in the pyloric ceca, kidney, and liver and abnormal gametogenesis (Lee Herman, 1970; Braham and Bressani, 1985; Yu, 1987; Randel et al., 1992). The cottonseed proteins derived from glandless varieties contain low levels of gossypol and can be substituted for much of the fish meal (FM) used in aquaculture diets (Siccardi III et al., 2012). More than 70% of the phosphorus in seeds is contained in phytic acid, which is indigestible by fish (Jackson et al., 1996). Phytates may form complexes with protein and chelate cationic minerals, such as Ca2+, Mg2+, Fe2+, and Zn2+, which reduces bioavailability of these nutrients to fish (Richardson et al., 1985; D’Mello et al., 1991).
Several studies have investigated the effects of dietary CM and free gossypol in fish. The recommended dietary CM level is 15%–50% based on feeding habit, life stage, and breeding mode. Channel catfish (Ictalurus punctatus), Nile tilapia (Oreochromis niloticus), and Siberian sturgeon (Acipenser baerii Brandt) can adapt to 40%–50% dietary CM content if they are supplemented with amino acids (El-Saidy and Gaber, 2004; Li and Robinson, 2006; Wu et al., 2010), whereas rainbow trout (Oncorhynchus mykiss), coho salmon (Oncorhynchus kisutch), and parrotfish (Oplegnathus fasciatus) can only tolerate 12%–22% dietary CM (Fowler, 1980; Lee et al., 2002; Lim and Lee, 2009).
The possibility of accumulating gossypol in muscle, liver, and other edible tissues of fish fed CM should be noted because monogastric animals including humans are highly susceptible to the toxic effects of gossypol. Thus, use of CM in fish feed may have consequences for fish health and food safety.
The objective of this study was to determine a suitable CM level to replace FM in the grass carp diet. The main response parameters were liver and muscle accumulation of gossypol and the physiological response of grass carp.
2 MATERIAL AND METHOD 2.1 Experimental dietsAll experimental procedures were authorized by the ethics committee of the Institute of Hydrobiology, Chinese Academy of Sciences. Seven isonitrogenous (crude protein, 32%–33% dry matter [DM]) and isoenergetic (gross energy, 15 kJ/g DM) diets containing CM replacing FM at 0, 20%, 40%, 60%, 80%, 90%, and 100% (C, R20, R40, R60, R80, R90, and R100) were prepared (Table 1). CM and FM were the only dietary protein sources. Corn starch was used to maintain a constant gross energy level in the diets; however, dietary starch level would decrease. The CM originated from Xinjiang Province, China and was produced by solvent extraction. White FM was made with Pacific hake (Merluccius productus) and Alaska pollock (Theragra chalcogramma) originating from the North Pacific Ocean. The experimental diets were produced by the Nanchang Guanglian Branch Co. (Qingshanhu, China) and Jiangxi Zhengbang S & T Co. Ltd. (Jiangxi, China). All ingredients were milled using a hammer mill to pass through a 40-mesh sieve. The mixed raw materials were pelleted through 3-mm dies using a single-screw pellet mill (35 Series Pellet Mill; Zhengchang, Jiangxi, China). The pellets were dried, sieved, packaged, and stored at 4℃ before use. The chemical and amino acid compositions are shown in Tables 1 and 2, respectively.
The experiment was carried out in a cage-in-pond outdoor system at Laohe Changjiang Protospecies Farm of Four Major Chinese Carp (29°49′48.8ʺN 112°29′01.3ʺE) located in Shishou, Hubei Province, China. The surface area of the pond was 1 960 m2 (70 m×28 m), and water depth was 1.8 m. Nylonnetted steel-pipe frame cages (2.0 m×2.0 m×2.0 m) were suspended 30 cm above the pond bottom, with a freeboard of 20 cm above the water surface. River water was added to the ponds every 3 days to replace one-third of the water and to compensate for evaporative loss. Total ammonia (NH4+ and NH3) and dissolved oxygen were monitored once weekly. Dissolved oxygen levels were > 5 mg/L, and total ammonia levels were < 0.6 mg/L, at a pH of approximately 7.2.
The experimental grass carp were purchased from Jingzhou, Hubei on September 2, 2011. The fish were acclimated to the net cages for 10 days and fed a mixture of the seven experimental diets to apparent satiation three times daily (9:00, 14:00, and 17:00). The fish were starved for 24 h before the experiment.
The feeding trial was conducted from September 14–October 13, 2011. Fish of similar size (mean body weight, 761 g) were selected, batch weighed, and added to 28 cages (18 fish/cage). Three cages were chosen randomly for each dietary treatment. Fish were feed to apparent satiation three times daily (9:00, 14:00, and 17:00) during the 42 d feeding trial.
2.3 SamplingAt the beginning of the trial, three samples (3 fish/ sample) were collected for the body composition analysis. At the end of the trial, all fish in each tank were fasted for 24 h and batch weighed. Three fish from each cage were selected randomly to determine their proximate composition. The fish were wiped individually with a towel, weighed, measured (total length), and stored at -20℃. Three randomly chosen individuals were taken from each cage, and their bodies, viscera, and livers were weighed to determine the viscerosomatic (VSI, %) and hepatosomatic (HSI, %) indices. The liver and dorsal muscle were collected and stored at -20℃ to determine chemical composition and gossypol contents.
Another three fish were selected randomly and anaesthetized with MS-222 (300 mg/L, Sigma, St. Louis, MO, USA). Blood was withdrawn from the caudal vein and collected into 1.5-mL centrifuge tubes. The blood was allowed to clot and was centrifuged at 1 300×g for 15 min (Eppendorf 5417c; Eppendorf, Hamburg, Germany). Serum samples were frozen in liquid nitrogen and stored at -80℃ until analysis.
The abdomen was opened, the hepatopancreas and mid-intestine (MI; from the first visible folds to the last folds) were separated, and visceral fat and connective tissue were removed. Approximately 5-mm×5-mm×5-mm tissue samples were taken from the hepatopancreas and MI and fixed in buffered formalin (4% formaldehyde; pH 7.4), and 2-mm× 2-mm×2-mm tissue samples were taken and fixed in glutaraldehyde solution for ultrastructural observations. The intestinal sections were frozen in liquid nitrogen and stored at -80℃ until the enzyme analysis.
2.4 Chemical and hematological analysesDM, crude protein, crude lipid, ash, moisture, and gross energy contents were determined for the diets and fish, following an AOAC (2002) protocol. Briefly, fish were homogenized, and the samples were dried in an oven. Moisture was measured by drying to constant weight (105℃). Crude protein content was determined by the Kjeldahl method (2300 Kjeltec Analyzer Unit, Foss Tecator, Höganäs, Sweden), and crude lipid content was determined using a Soxtec system (Soxtec System HT6; Tecator). Ash content was measured by incineration in a muffle furnace (550℃ for 12 h). Non-resistant and resistant starch contents were analyzed with a commercial kit based on enzymatic hydrolysis and spectrophotometric quantification of glucose (resistant starch kit, Megazyme International Ireland, Co., Wicklow, Ireland). Gross energy content was measured with a micro-bomb calorimeter (Parr 1425 semimicro calorimeter; Parr Instruments, Moline, IL, USA). After hydrolyzing the sample in vacuumed test tubes with 6 N HCl for 22 h at 110℃, the amino acids in the diets were analyzed with an amino acid analyzer (Hitachi L-8800; Hitachi, Tokyo, Japan).
Dorsal muscle and hepatopancreatic samples were freeze dried (Alpha 1-4 LD-plus; Christ, Osterode, Germany). Gossypol content (free and total gossypol) was determined by high performance liquid chromatography (DGU-20A5E; Shimadzu, Tokyo, Japan) in the Analysis Center of the Hubei Academy of Agricultural Sciences.
Serum was analyzed for alanine aminotransferase (ALT) and aspartate aminotransferase (AST) activities and for lipid fractions, including cholesterol (CHO), high-density lipoprotein cholesterol (HDL-C), lowdensity lipoprotein cholesterol (LDL-C), and triglycerides (TG) using an automated chemistry analyzer (Olympus AU400; Tokyo, Japan), as recommended by the International Federation of Clinical Chemistry.
2.5 Intestinal enzymatic activity analysesIntestinal sections were cut open longitudinally, rinsed, and the internal surface was scraped to collect the mucosa. The mucosa samples were weighed, four volumes of ice-cold saline was added, the tissues were homogenized with an electric homogenizer (S10 portable disperser, Xinzhi Co., Ningbo, China), and centrifuged (4℃, 12 000×g, 20 min; 5417c, Eppendorf AG). The supernatant was separated and stored at -80℃ for later assessment.
Protein concentration in each tissue homogenate was determined by the Bradford (1976) method, using bovine serum albumin as the standard, and expressed as μg protein/μL. Enzyme activities were expressed as units/mg protein.
Trypsin (TRY) and chymotrypsin (CT) activities were analyzed colorimetrically according to the methods described by Geiger (1988) and Geiger and Fritz (1988), using benzoyl-arginine-p-nitroanilide (Sigma B-4875) and N-succinyl-ala-ala-pro-phe p-nitroanilide (Sigma S-7388) as respective substrates. TRY and CT activities were calculated with the formulae: Activity (U/L)=10000ΔA×V/(ΔtvεL), where ΔA is the increase in absorbance at a wavelength of 405 nm, V is total volume of the reaction (1.32 mL), Δt is reaction time (10 min at 25℃), v is volume of the test sample (200 μL), ε is the extinction coefficient (TRYε=0.54 L/(mmol·cm), and CTε=10.2 L/(mmol·cm)), and L is optical path length (0.735 cm).
AMY activity was measured by the iodine-starch method (Gitlitz and Frings, 1976; α-amylase activity kit, Jiancheng Bioengineering Institute, Nanjing, China). One unit of α-amylase activity was defined as the amount of enzyme that hydrolyzes 10 mg starch per 30 min in 100 mL at 37℃.
γ-Glutamyl transpeptidase (γ-GT) activity was determined using l-γ-glutamyl-3-carboxy-4-nitroanilide as the substrate (γ-GT activity kit, Jiancheng Bioengineering Institute). One unit of γ-GT activity was defined as the amount of enzyme that produces 1 μmol/L nitroaniline/min at 37℃.
2.6 HistologyTissues were dehydrated in a graded ethanol series, equilibrated in xylene, and embedded in paraffin according to standard histological techniques. Transverse sections of 5-μm thickness were cut with a rotary microtome (RM2145; Leica, Nussloch, Germany), mounted on slides, and stained with hematoxylin and eosin. The tissues were observed and photographed using a microscope system (DM1000 microscope and EC3 Digital Color Camera; Leica Microsystems, Zena, Germany).
Tissues were post-fixed in 1% osmium tetroxide and embedded in epoxy resin (Spurr’s resin, ERL-4206; Sigma-Aldrich, Steinheim, Germany) for the ultrastructural observations. Ultrathin sections were observed under a transmission electron microscope (TECNAI G2; FEI-TEM, Roanoke, VA, USA).
2.7 Calculations and statistical analysisThe following parameters were calculated:
Feeding rate (FR, %BW/d)=100×feed intake/ [days×(initial body weight+final body weight)/2],
Specific growth rate (SGR, %/d)=100×[ln(final body weight)–ln(initial body weight)]/days,
Feed efficiency (FE, %)=100×wet weight gain/dry feed intake,
Protein retention efficiency (PRE, %)=100×(protein retained in fish body/protein intake),
Energy retention efficiency (ERE, %)=100×(energy retained in fish body/energy intake),
Condition factor (CF)=100×body weight/(body length)3,
Hepatosomatic index (HSI, %)=100×liver wet weight/body weight,
Viscerosomatic index (VSI, %)=100×visceral wet weight/body weight.
All data analyses were performed using IBM SPSS Statistics 19.0 software (IBM, Armonk, NY, USA). All data were tested for homogeneity of variance using Levene’s test, and one-way analysis of variance (ANOVA) was used to detect differences among groups. A P < 0.05 was considered significant. Duncan’s multiple-range test was used to rank significant differences. The relationship between dietary free gossypol (DFG) and hepatopancreatic total gossypol (HTG) was expressed by linear regression: HTG=a+b×DFG.
3 RESULT 3.1 Dietary compositionAs shown in Tables 1 and 2, when dietary CM level rose from 0 to 61%, dietary ash content decreased and DFG and HTG increased. The dietary amino acid profile also changed when CM increased; arginine concentration rose, whereas methionine, lysine, threonine, isoleucine, leucine, and valine concentrations decreased.
3.2 Growth and feed utilizationNo fish died during the feeding trial. Table 3 presents the FBW, FR, SGR, FE, PRE, and ERE of grass carp fed the experimental diets for 42 d. When the dietary CM replacement levels were 0–60%, these parameters were not different between the groups (P > 0.05). FBW, SGR, FE, PRE, and ERE of fish fed the diets containing dietary CM replacement levels of 80%–100% were significantly lower than those of fish fed the diet containing 0% CM (P < 0.05).
|
No differences were found between the CF, HSI, and VSI values among treatments (Table 3; P > 0.05).
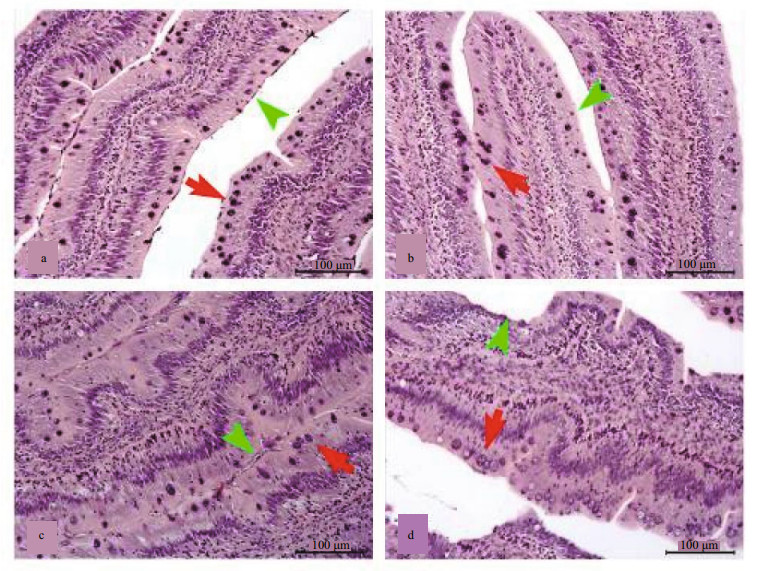
3.4 Intestinal and hepatopancreatic histologyThe MI sections of fish fed the FM, R20, R60, and R100 diets are shown in Fig. 1. The intestinal epithelium of fish in these groups was intact, and the goblet cells were regular (Fig. 1a–c). However, the mucosal folds of the intestinal epithelium were damaged, and the goblet cells were malformed in the R100 group (Fig. 1d).
|
| Figure 1 Mid-intestinal histology of grass carp fed the control, 20% cottonseed meal (R20), R60, and R100 diets (a, b, c, and d, respectively) Note the lymphocytes in the lamina of the epithelium (green arrowhead). Note hematoxylin and eosin-stained goblet cells (red arrow). |
The lamina propria broadened as dietary CM level increased. More lymphocytes were observed in the R100 group than that in the other groups.
TEM images of the MI microvilli of grass carp fed the FM, R20, R60, and R100 diets are displayed in Fig. 2. The changes in MI microvilli were similar to the changes detected in the intestinal epithelium presented in Fig. 1. Fish in the FM, R20, and R60 treatments had normal, regular, and organized microvilli (Fig. 2a–c), whereas microvilli in fish in the R100 group were dumpy and sparse (Fig. 2d).
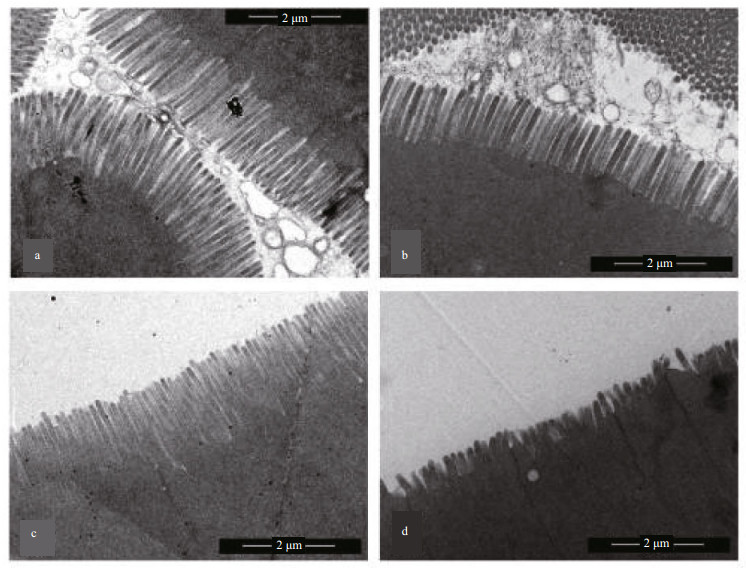
|
| Figure 2 Transmission electron micrographs of the mid-intestine of grass carp fed the control, 20% cottonseed meal (R20), R60, and R100 diets (a, b, c, and d, respectively) |
The activities of the MI enzymes are presented in Table 4. MI α-amylase activity decreased significantly in fish fed the diet with added starch (P < 0.05). γ-GT activity decreased in the MI of grass carp as dietary CM content increased (P < 0.05). TRY activity tended to increase in the CM-treated groups (P > 0.05). CT activity was not significantly affected by CM in the diet P > 0.05).
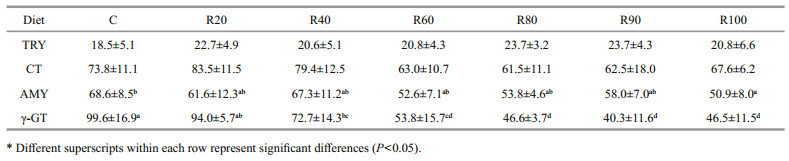
|
The serum enzyme activities are presented in Table 5. Serum ALT level increased significantly as dietary CM increased (P < 0.05), whereas AST level increased in the R20 and R40, groups and then tended to decrease (P > 0.05). Total CHO, HDL-C, and LDL-C levels decreased with increasing dietary CM (P < 0.05). No difference in serum TG level was observed among the groups (P > 0.05).
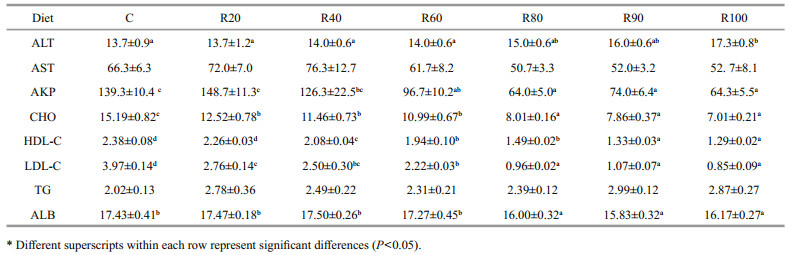
|
Table 6 shows the composition of the initial body samples, final body samples, and dorsal muscle samples of the grass carp. Only mean values are provided because these parameters were not different among the dietary treatments (P > 0.05).
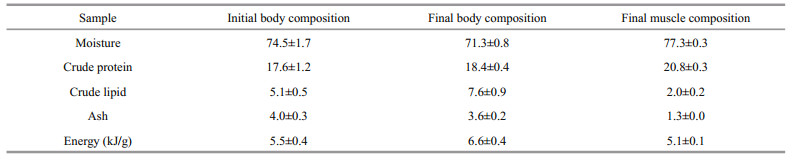
|
Table 7 shows the composition of the hepatopancreas in grass carp fed the experimental diets for 42 d. Moisture content of the hepatopancreas increased but energy level decreased with increasing dietary CM (P < 0.05). No differences were detected in moisture content or energy level between fish fed the R20, R40, and R60 diets and the C diet. The lipid level in the hepatopancreas increased initially and then decreased when dietary CM was increased (P < 0.05). The lipid level in the hepatopancreas of fish fed the R20 diet was not different from that of fish fed the R40 diet but it was higher than that of the other treatments.
|
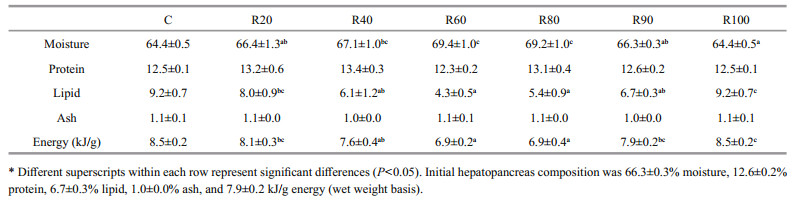
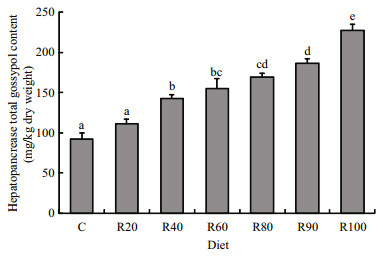
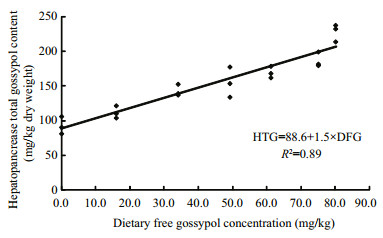
The accumulation of total gossypol in the hepatopancreas increased as dietary CM level increased (Fig. 3). The TG level in the hepatopancreas was significantly correlated with free gossypol contents in the diets (HTG=88.6+1.5 DFG, R2=0.89, P < 0.05; Fig. 4).
|
| Figure 3 Gossypol accumulation in the hepatopancreas of grass carp fed the control, 20% cottonseed meal (R20), R40, R60, R80, R90, and R100 diets for 42 d |
|
| Figure 4 Relationship between total gossypol accumulation in the hepatopancreas (mg/kg in dry matter) and free gossypol in the diet (mg/kg) |
The levels of CM included in the fish feed were higher than that in feeds for monogastric livestock and poultry, which can be as high as 30% (Gatlin et al., 2007). CM is a valuable substitute for expensive protein sources and has been tested in some fish species (Cai et al., 2011). However, it is important to evaluate the effect of dietary CM on growing fish. Our results show that up to 36.6% CM (replacement of 60% dietary FM) was tolerated by sub-adult grass carp. The same growth performance and feed utilization were observed under these conditions as fish fed a complete FM diet during outdoor culture. Large rainbow trout (1.5 year old adults and broodstock) fed CM-containing feed for more than 9 months do not show depressed growth (Dabrowski et al., 2000; Blom et al., 2001; Rinchard et al., 2003; Lee et al., 2006). In contrast, lower growth was observed in both sexes of mature tilapia (Oreochromis sp.) (Rinchard et al., 2002) and brood-sized male channel catfish (Robinson and Tiersch, 1995) fed a high-CM diet. These results suggest that the effect of increasing dietary CM level on growth performance is species-specific.
CM can be included at up to 24.1% in the juvenile grass carp diet (Yan, 2012), 24.5% for on-growing grass carp (100.0±0.3 g; Yan et al., 2014), and 36.6% for sub-adult grass carp (this study) without any negative effects on growth. Similar studies on parrotfish (Lim and Lee, 2009) used CM and soybean with added iron and phytase as substitutes for dietary FM to feed two sizes of parrotfish. The results showed that CM could replace up to 20% FM in the juvenile parrotfish diet and up to 30% in the on-growing parrotfish diet. Large fish had better plant protein utilization than that of juveniles.
The two major factors limiting the use of CM in aquafeeds are free gossypol and low lysine (El-Saidy and Gaber, 2004). The dietary lysine requirement of juvenile grass carp (initial body weight, ~3.5 g) is 1.94% at 38% dietary protein content, and the dietary methionine requirement is 1.1% at 37% dietary protein content (Wang, 2006). The dietary methionine requirement of mid-growth stage grass carp is 0.72% at 30% dietary protein content (Tang et al., 2012). The dietary lysine requirement is 1.68% and the dietary methionine requirement is 0.78%–0.98% at a 33% protein level. In the present study, 1.76% lysine was in the R40 diet, and 1.60% lysine was in the R60 diet. The R20 diet had 0.88% methionine, and 0.74% methionine was in the R40 diet. Both lysine and methionine were limiting amino acids in the grass carp CM diets.
Based on a factorial model, the lysine requirements of rainbow trout at different life stages (body weight: 0.2–20, 20–500, and 500–1 500 g) are 2.47%, 2.31%, and 1.92%, respectively (National Research Council, 2011). Adult aquatic animals have lower amino acid requirements and can adapt more efficiently to plant proteins than smaller animals (Ai and Xie, 2005). The reason that pre-adult grass carp could adapt to the R60 diet (1.60% lysine) is that they tolerated a lower essential amino acid level than that of juveniles.
Free gossypol ranged from 0 to 82.3 mg/kg in the experimental diets. The anti-nutritional effect of gossypol is not only determined by its toxicity but by its interaction with other nutrients. The ε-amino group of lysine combines with the reactive aldehyde group of gossypol to form a Schiff base, which lowers lysine bioavailability (Rinchard et al., 2003). Depressed growth was reported in juvenile and broodstock rainbow trout fed CM containing > 300 μg/g and 463 μg/g free gossypol, respectively (Lee Herman, 1970; Blom et al., 2001; Rinchard et al., 2002). In this study, the low free gossypol concentration in the CM diets was due to the high quality of the cottonseed originating from Xinjiang and the well-performed solvent extraction technology.
The toxic effects of gossypol, such as anemia and negative effects on reproduction, are of great concern to scholars and consumers. In the present study, gossypol content in the dorsal muscle of grass carp was below the detection limit, indicating that gossypol was not accumulating when the grass carp were fed a diet containing < 82.25 mg/kg gossypol. These data are consistent with studies in rainbow trout (Cheng and Hardy, 2002) and channel catfish (Robinson and Daniels, 1987; Robinson and Brent, 1989). After a 3-year rainbow trout feeding trial with 58.8% dietary CM, total muscle gossypol content was < 1 μg/g wet sample, which is far lower than the limit set by the FDA (450 mg/kg) (Lee et al., 2006). Based on these results, humans can safely consume fillets produced by fish fed CM-containing diets.
We did not collect fecal matter because of the outdoor cultivation. Digestive enzyme activities were tested to evaluate the changes in grass carp pancreatic function. CT and AMY activities decreased with increased dietary CSM. Dietary gossypol inhibits proteinase and AMY activities in Spodoptera littoralis larvae (Meisner et al., 1978) and in H. armigera cotton bollworm larvae (Wang and Qin, 1996; Abdurakhimov et al., 2009). In addition, glandless (without gossypol) cottonseed flour markedly inhibits CT activities in vitro (Bertrand et al., 1995). These in-vivo data indicate that the inhibition of digestive enzyme activities is a reason to reduce CM utilization in the grass carp diet.
The histological changes in villi and microvilli showed that the intestinal epithelium was injured in the grass carp. The decrease in γ-GT activity indicated that dietary CM affected the intestinal mucosa. One reason may be that gossypol interacted with membrane proteins and the lipid monolayer and bilayer membranes, thereby modifying the electrochemical properties of the membranes and inhibiting several membrane transport systems (Reyes et al., 1984; De Peyster et al., 1986; Cuéllar and Ramírez, 1993). Free gossypol can alter intestinal ion transport, resulting in impaired environmental adaptability of the animals (Trischitta and Faggio, 2008). Presumably, the breakpoint in growth performance after the R60 treatment occurred because of deteriorated absorption of nutrition’s and intestinal immunity.
Aminotransferases catabolize amino acids and divert α-amino acids into the tricarboxylic acid cycle as keto acids to augment energy production. ALT and AST activities in Nile tilapia increase with the addition of dietary CM (El-Saidy and Gaber, 2004; El-Kasheif et al., 2013). In this study, ALT activity increased significantly as dietary CM increased, which may have been caused by the low energy supplied from dietary starch.
Studies in rabbits and rats have shown that dietary CM lowers plasma CHO levels (Nwoha, 1995). In hamsters, CM may slightly elevate the HDL-C percentage of serum total CHO (Beynen and Liepa, 1987). In grass carp, dietary CM reduced serum CHO, HDL-C, and LDL-C concentrations, suggesting that serum lipid metabolism was altered. The high arginine-to-lysine ratio in CM has been held responsible for reducing total CHO and HDL-C (Park and Liepa, 1982). Gossypol can also markedly reduce total CO and LDL-C levels in adult rats (Obeidy et al., 1989). In monkeys (Macaca fascicularis), peroral gossypol decreases plasma total CHO, LDL-C, and very-low-LDL-C levels without reducing HDL-C (Shandilya and Clarkson, 1982). Thus, the hypolipidemic action of dietary CM is a combined consequence of the high arginine-to-lysine ratio and gossypol. These hematological data indicate that dietary CM affects hepatopancreatic metabolism.
5 CONCLUSIONCM is a palatable and nutritionally acceptable substitute for FM in the grass carp diet. Dietary CSM may affect nutrient digestion and absorption as well as hepatopancreatic metabolism. Replacing FM with 60% (36.59% in the diet formulation) CM in the diet of sub-adult grass carp had no negative effects on growth or feed utilization. Longer term studies are needed to closely monitor gossypol levels in fillets and ensure that gossypol levels remain in the safe range for human consumption.
| Abdurakhimov R S, Veshkurova O N, Uzbekov V V, Arzanova I A, Sultanova E M, Salikhov S I. 2009. Effect of cottonseed biocidal peptides and gossypol on resistance to biotic factors. Chem. Nat. Compd., 45(2):213-216, http://dx.doi.org/10.1007/s10600-009-9285-2. |
| Ai Q H, Xie X J. 2005. Advance in utilization of plant proteins by aquatic animals. Periodical of Ocean University of China, 35(6): 929-935, http://dx.doi.org/10.3969/j.issn.1672-5174.2005.06.010. (in Chinese with English abstract) |
| AOAC. 2002. Official Methods of Analysis of AOAC International. 17th edn. Association of Official Analytical Chemists, Washington, DC, USA. |
| Bertrand V, Prost J, Belleville J, 1995. In vitro rat pancreatic digestive enzyme activities and raw and heated glandless cottonseed and soybean flours. International Journal of Food Sciences and Nutrition, 46 (1) : 39 –45. Doi: 10.3109/09637489509003384 |
| Beynen A C, Liepa G U. 1987. Dietary cottonseed protein and cholesterol metabolism. Z. Ernährungswiss., 26(4): 219-225, http://dx.doi.org/10.1007/BF02023810. |
| Blom J H, Lee K J, Rinchard J, Dabrowski K, Ottobre J, 2001. Reproductive efficiency and maternal-offspring transfer of gossypol in rainbow trout (Oncorhynchus mykiss) fed diets containing cottonseed meal. J. Anim. Sci., 79 (6) : 1533 –1539. Doi: 10.2527/2001.7961533x |
| Bradford M M. 1976. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem., 72(1-2):248-254, http://dx.doi.org/10.1016/0003-2697(76)90527-3. |
| Braham J E, Bressani R. 1985. Biochemical and nutritional considerations of gossypol intake. In:Lobl T J, Hafez E S E eds. Male Fertility and Its Regulation. Springer, Netherlands. p.135-154. |
| Cai C, Li E, Ye Y, Krogdahl A, Jiang G, Wang Y, Chen L. 2011.Effect of dietary graded levels of cottonseed meal and gossypol on growth performance, body composition and health aspects of allogynogenetic silver crucian carp, Carassius auratus gibelio♀×Cyprinus carpio ♂.Aquaculture Nutrition, 17(4):353-360, http://dx.doi.org/10.1111/j.1365-2095.2010.00801.x. |
| Cheng Z J, Hardy R W. 2002. Apparent digestibility coefficients and nutritional value of cottonseed meal for rainbow trout(Oncorhynchus mykiss). Aquaculture, 212(1-4):361-372, http://dx.doi.org/10.1016/S0044-8486(02)00260-0. |
| Committee on the Nutrient Requirements of Fish and Shrimp, Board on Agriculture and Natural Resources, Division on Earth and Life Studies, National Research Council. 2011.Nutrient Requirements of Fish and Shrimp. National Academies Press, Washington, DC, USA. 63p. |
| Cuéllar A, Ramírez J. 1993. Further studies on the mechanism of action of gossypol on mitochondrial membrane.International Journal of Biochemistry, 25(8):1 149-1 155, http://dx.doi.org/10.1016/0020-711X(93)90593-4. |
| D'Mello J P F, Duffus C M, Duffus J H. 1991. Toxic Substances in Crop Plants. Woodhead Publishing, Cambridge, UK. 352p. |
| Dabrowski K, Rinchard J, Lee K J, Blom J H, Ciereszko A, Ottobre J. 2000. Effects of diets containing gossypol on reproductive capacity of rainbow trout (Oncorhynchus mykiss). Biology of Reproduction, 62(2):227-234, http://dx.doi.org/10.1095/biolreprod62.2.227. |
| De Peyster A, Hyslop P A, Kuhn C E, Sauerheber R D. 1986.Membrane structural/functional perturbations induced by gossypol:effects on membrane order, liposome permeability, and insulin-sensitive hexose transport.Biochemical Pharmacology, 35(19):3 293-3 300, http://dx.doi.org/10.1016/0006-2952(86)90426-0. |
| El-Kasheif M A, Gaber H S, Ibrahim S A. 2013. Histological and biochemical studies of Oreochromis niloticus fed on cottonseed meal. In:Proceedings of the International Conference of Environmental Sciences (ICES). Ain Shamas University, Cairo, Egypt. p.233-243. |
| El-Saidy D M S D, Gaber M M. 2004. Use of cottonseed meal supplemented with iron for detoxification of gossypol as a total replacement of fish meal in Nile tilapia, Oreochromis niloticus (L.) diets. Aquaculture Research, 35(9):859-865, http://dx.doi.org/10.1111/j.1365-2109.2004.01077.x. |
| FAO. 2014. FAOSTAT, commodity balances, crops primary equivalent. http://faostat3.fao.org/faostat-gateway/go/to/download/B/BC/E. Accessed on 2014-03-21. |
| Fishery Bureau of Ministry of Agriculture of the People's Republic of China. 2013. China Fishery Statistical Yearbook. China Agriculture Press, Beijing, China. p.32-35. (in Chinese) |
| Fowler L G. 1980. Substitution of soybean and cottonseed products for fish meal in diets fed to chinook and coho salmon. The Progressive Fish-Culturist, 42(2):87-91, http://dx.doi.org/10.1577/1548-8659(1980)42[87:Sosacp]2.0.Co;2. |
| Gatlin D M, Barrows F T, Brown P, Dabrowski K, Gaylord T G, Hardy R W, Herman E, Hu G S, Krogdahl Å, Nelson R, Overturf K, Rust M, Sealey W, Skonberg D, Souza E J, Stone D, Wilson R, Wurtele E. 2007. Expanding the utilization of sustainable plant products in aquafeeds:a review. Aquaculture Research, 38(6):551-579, http://dx.doi.org/10.1111/j.1365-2109.2007.01704.x. |
| Geiger R, Fritz H. 1988. Trypsin. In:Bergmeyer H U ed.Methods of Enzymatic Analysis:Enzymes 3:Peptidases, Proteinases and Their Inhibitors. 3rd edn. VCH Verlagsgesellschaft, Weinheim. p.119-129. |
| Geiger R. 1988. Chymotrypsin. In:Bergmeyer H U ed.Methods of Enzymatic Analysis:Enzymes 3:Peptidases, Proteinases and their Inhibitors. 3rd edn. VCH Verlagsgesellschaft, Weinheim. p.99-118. |
| Gitlitz P H, Frings C S, 1976. Interferences with the starchiodine assay for serum amylase activity, and effects of hyperlipemia. Clinical Chemistry, 22 (12) : 2006 –2009. |
| Heuzé V, Tran G, Bastianelli D, Hassoun P, Lebas F. 2013.Cottonseed meal. Feedipedia. org. http://www.feedipedia.org/node/550. |
| Jackson L S, Li M H, Robinson E H. 1996. Use of microbial phytase in channel catfish Ictalurus punctatus diets to improve utilization of phytate phosphorus. Journal of the World Aquaculture Society, 27(3):309-313, http://dx.doi.org/10.1111/j.1749-7345.1996.tb00613.x. |
| Lee Herman R. 1970. Effects of gossypol on rainbow trout Salmo guirdneri richardson. Journal of Fish Biology, 2(4):293-303, http://dx.doi.org/10.1111/j.1095-8649.1970.tb03288.x. |
| Lee K J, Dabrowski K, Blom J H, Bai S C, Stromberg P C. 2002. A mixture of cottonseed meal, soybean meal and animal byproduct mixture as a fish meal substitute:growth and tissue gossypol enantiomer in juvenile rainbow trout(Oncorhynchus mykiss). Journal of Animal Physiology and Animal Nutrition, 86(7-8):201-213, http://dx.doi.org/10.1046/j.1439-0396.2002.00375.x. |
| Lee K J, Rinchard J, Dabrowski K, Babiak I, Ottobre J S, Christensen J E. 2006. Long-term effects of dietary cottonseed meal on growth and reproductive performance of rainbow trout:three-year study. Animal Feed Science and Technology, 126(1-2):93-106, http://dx.doi.org/10.1016/j.anifeedsci.2005.06.007. |
| Li M H, Robinson E H. 2006. Use of cottonseed meal in aquatic animal diets:a review. North American Journal of Aquaculture, 68(1):14-22, http://dx.doi.org/10.1577/A05-028.1. |
| Lim S J, Lee K J. 2009. Partial replacement of fish meal by cottonseed meal and soybean meal with iron and phytase supplementation for parrot fish Oplegnathus fasciatus.Aquaculture, 290(3-4):283-289, http://dx.doi.org/10.1016/j.aquaculture.2009.02.018. |
| Meisner J, Ishaaya I, Asche K R S, Zur M, 1978. Gossypol inhibits protease and amylase activity of Spodoptera littoralis larvae. Annals of the Entomological Society of America, 71 (1) : 5 –8. Doi: 10.1093/aesa/71.1.5 |
| Nwoha P U. 1995. The blood constituents of gossypol-treated, protein-malnourished wistar rats. Contraception, 52(4):249-254, http://dx.doi.org/10.1016/0010-7824(95)00188-G. |
| Obeidy A, Fities I A, Sheriff D S. 1989. The effect of gossypol on serum lipoproteins of adult rats. Horm. Metab. Res., 21(12):649-651, http://dx.doi.org/10.1055/s-2007-1009311. |
| Park M S, Liepa G U, 1982. Effects of dietary protein and amino acids on the metabolism of cholesterol-carrying lipoproteins in rats. The Journal of Nutrition, 112 (10) : 1892 –1898. |
| Randel R D, Chase C C, Wyse S J, 1992. Effects of gossypol and cottonseed products on reproduction of mammals. Journal of Animal Science, 70 (5) : 1628 –1638. Doi: 10.2527/1992.7051628x |
| Reyes J, Allen J, Tanphaichitr N, Bellvé A R, Benos D J, 1984. Molecular mechanisms of gossypol action on lipid membranes. Journal of Biological Chemistry, 259 (15) : 9607 –9615. |
| Richardson N L, Higgs D A, Beames R M, McBride J R, 1985. Influence of dietary calcium, phosphorus, zinc and sodium phytate level on cataract incidence, growth and histopathology in juvenile chinook salmon (Oncorhynchus tshawytscha). The Journal of Nutrition, 115 (5) : 553 –567. |
| Rinchard J, Lee K J, Dabrowski K, Ciereszko A, Blom J H, Ottobre J S. 2003. Influence of gossypol from dietary cottonseed meal on haematology, reproductive steroids and tissue gossypol enantiomer concentrations in male rainbow trout (Oncorhynchus mykiss). Aquaculture Nutrition, 9(4):275-282, http://dx.doi.org/10.1046/j.1365-2095.2003.00253.x. |
| Rinchard J, Mbahinzireki G, Dabrowski K, Lee K J, GarciaAbiado M A, Ottobre J. 2002. Effects of dietary cottonseed meal protein level on growth, gonad development and plasma sex steroid hormones of tropical fish tilapia Oreochromis sp. Aquaculture International, 10(1):11-28, http://dx.doi.org/10.1023/A:1021379328778. |
| Robinson E H, Brent J R. 1989. Use of cottonseed meal in channel catfish feeds. Journal of the World Aquaculture Society, 20(4):250-255, http://dx.doi.org/10.1111/j.1749-7345.1989.tb01011.x. |
| Robinson E H, Daniels W H. 1987. Substitution of soybean meal with cottonseed meal in pond feeds for channel catfish reared at low densities. Journal of the World Aquaculture Society, 18(2):101-106, http://dx.doi.org/10.1111/j.1749-7345.1987.tb00424.x. |
| Robinson E H, Tiersch T R. 1995. Effects of long-term feeding of cottonseed meal on growth, testis development, and sperm motility of male channel catfish ictalurus punctatus broodfish. Journal of the World Aquaculture Society, 26(4):426-431, http://dx.doi.org/10.1111/j.1749-7345.1995.tb00838.x. |
| Shandilya L N, Clarkson T B. 1982. Hypolipidemic effects of gossypol in cynomolgus monkeys (Macaca fascicularis).Lipids, 17(4):285-290, http://dx.doi.org/10.1007/BF02534943. |
| Siccardi Ⅲ A J, Richardson C M, Samocha T M, Dowd M K, Wedegaertner T C. 2012. Glandless cottonseed meal replaces fishmeal in shrimp diet research. Global Aquaculture Advocate. p.66-67. |
| Tang B R, Feng L, Liu Y, Jiang J, Hu K, Jiang W D, Li S H, Zhou X Q. 2012. Methionine requirement of grass carp(Ctenopharyngodon idell) during middle growth stage. Chinese Journal of Animal Nutrition, 24(11):2 263-2 271, http://dx.doi.org/10.3969/j.issn.1006-267x.2012.11.027. |
| Trischitta F, Faggio C. 2008. Gossypol affects ion transport in the isolated intestine of the seawater adapted eel, Anguilla anguilla. Comparative Biochemistry and Physiology Part A:Molecular & Integrative Physiology, 151(1):139-143, http://dx.doi.org/10.1016/j.cbpa.2008.06.008. |
| Wang C Z, Qin J D, 1996. Effect of soybean trypsin inhibitor, gossypol and tannic acid on the midgut protease activities and growth of helicoverpa armigera larvae. Acta Entomologica Sinica, 39 (4) : 337 –341. |
| Wang S. 2006. Studies on Protein and Essential Amino Acid Requirements of Grass Carp, Ctenopharyngodon idella.Sun Yat-Sen University, Guangzhou, China. (in Chinese) |
| Wu X F, Xue M, Guo L Y, Wu L X, Zheng Y H. 2010. Effects of substitution of solvent-extracted cottonseed meal for part fish meal on growth, body composition and serum biochemical indices of juvenile Siberian Sturgeon(Acipenser baerii Brandt). Chinese Journal of Animal Nutrition, 22(1):117-124, http://dx.doi.org/10.3969/j.issn.1006-267x.2010.01.018. (in Chinese with English abstract) |
| Yan Q G, Zhu X M, Yang Y X, Han D, Jin J Y, Xie S W, Li Z J, 2014. Effect of replacement of fish meal with cottonseed meal on growth, hematological physiology, and body composition of grass carp. Acta Hydrobiologica Sinica, 38 (2) : 362 –369. |
| Yan Q G. 2012. Effects of Dietary Replacement of Fishmeal by Cottonseed meal on Grass Carp of Different Sizes.Graduate University of Chinese Academy of Sciences, Beijing, China. (in Chinese) |
| Yu Y W. 1987. Probing into the mechanism of action, metabolism and toxicity of gossypol by studying its (+)-and (-)-stereoisomers. Journal of Ethnopharmacology, 20(1):65-78, http://dx.doi.org/10.1016/0378-8741(87)90120-6. |
 2016, Vol. 34
2016, Vol. 34