Institute of Oceanology, Chinese Academy of Sciences
Article Information
- CHEN Dong(陈栋), WANG Wei(王蔚), RU Shaoguo(汝少国)
- Effects of dietary genistein on GH/IGF-I axis of Nile tilapia Oreochromis niloticus
- Chinese Journal of Oceanology and Limnology, 34(5): 1004-1012
- http://dx.doi.org/10.1007/s00343-016-4386-9
Article History
- Received Jan. 10, 2015
- accepted in principle Apr. 30, 2015
- accepted for publication Aug. 15, 2015
2 School of Environmental and Municipal Engineering, Qingdao Technological University, Qingdao 266033, China
Marine fishes require approximately 30%–50% protein in their feed, most of which is usually provided by fish meal (NRC, 2011). In recent years the price of fish meal has greatly increased because of the limited supplies of fishery resources, thus, soybean meal has gradually become an important alternative ingredient for fish meal because of its favorable amino acid profile, high level of crude protein, and its relatively low price (Refstie et al., 2006; Lilleeng et al., 2007). At present, soybean meal is tentatively substituted for fish meal in some fish feed production. However, as a result of the relatively simple processing technology, soybean meal is rich in isoflavone, also known as phenolic compounds, which may have potential biological effects on fish. In fact, Ishibashi et al. (2002) reported that the total isoflavone content in commercial carp and trout feed was 810 μg/g and 80 μg/g, respectively. The two most abundant and most biologically active isoflavones found in soybeans are genistein and daidzein, whose contents in different soybean origins are approximately 619.1–2 367.9 and 291.5–600.8 μg/g, respectively (Tepavčević et al., 2010). While Mambrini et al. (1999) reported that the maximum content of genistein and daidzein in soybean protein concentrates were~5 900 and 1 990 μg/g, respectively (Mambrini et al., 1999).
Thus, the effect of isoflavones on fish growth is a concerns for the application of the soybean protein in aquatic feed. Moreover, there is little research on the effect of single isoflavones on fish growth. Preliminary results have shown that low concentrations of genistein had no significant effect on fish growth, but that high concentrations inhibit growth. Our previous work demonstrated that 30 and 300 μg/g dietary genistein had no significant effect on growth performance in Nile tilapia (O. niloticus), but the higher levels of genistein (3 000 μg/g) significantly reduced the final body weight and specific growth rate (Chen et al., 2015). It has been suggested that genistein may reduce fish growth partly by inhibiting digestion because the activity of major digestive enzymes, including stomach and hepatopancreas protease and amylase in the liver and intestine, were significantly reduced by 3 000 μg/g dietary genistein (Chen et al., 2015). Refstie et al. (2006) also reported that diets in which 24% of total protein came from extracted soybean meal reduced the digestion of both amino acid and lipid metabolism in Atlantic cod (Gadus morhua). However, the endocrine system, especially hormone/insulin-like growth factor-I (GH/IGF-I) axis, plays a crucial role in the regulation of fish growth. It has been demonstrated that dietary daidzein decreases the production of GH in tilapia (Oreochromisco aureus) (Yu et al., 2006). At present, data on how isoflavone affects the GH/IGF-I system in fish is scarce. Therefore, this study was conducted to elucidate how genistein affects fish growth based on insights on the GH/IGF-I axis. Juvenile Nile tilapia (O. niloticus) were fed diet containing graded levels of genistein, we then investigated the plasma levels of GH and IGF-I, and determined the mRNA levels of gh, ghr, igf-I, igf-Ir, igfbp3, and other regulatory genes along the GH/IGF-I axis.
2 MATERIAL AND METHOD 2.1 Experimental dietsThe feed formulation and preparation process was according to that of Chen et al. (2015). In brief, fishmeal and fish oil were used as protein and lipid sources, respectively. Four isonitrogenous and isocaloric diets (diet 1, diet 2, diet 3, and diet 4) were formulated to contain graded levels of genistein (0, 30, 300, and 3 000 μg/g, respectively), which was purchased from Shenzhen Medherb Biotechnology Co. Ltd., Shenzhen, China. The ingredients were ground into a fine powder through a 320-μm mesh, and then thoroughly mixed with fish oil and water to produce stiff dough. The dough was pelletized with an experimental feed mill (F-26 (II), South China University of Technology, China) and dried for 24 h in a ventilated oven at 45℃. After drying, the diets were broken and sieved into proper pellet size (1.5 mm×3.0 mm), and stored at -20℃.
2.2 Experimental proceduresJuvenile tilapia (O. niloticus) were purchased from the National Tilapia Seed Farm of Qingdao. After acclimating in laboratory rearing conditions and fed with the control diet (diet 1) for 2 weeks, the fish were fasted for 24 h, anesthetized in 75 mg/L MS-222 (Sigma, MO, USA), then weighed. Fish of similar sizes (initial weight 10.47±1.24 g) were then randomly distributed into 12 tanks (50-L capacity filled to 30 L), and each tank was stocked 15 juveniles. Each diet was randomly assigned to triplicate tanks. Fish were handfed to apparent satiation twice daily (09:30 and 16:00) for 8 weeks. The consumption of food in each cage was recorded. During the experimental period, rearing water temperature ranged from 26.0 to 30.0℃, dissolved oxygen was maintained at approximately 7 mg/L.
Following feeding for 8 weeks, tilapia were fasted for 24 h and anesthetized in 75 mg/LMS-222 (Sigma), and mean body weight was recorded before harvest. For this study, tissue samples were taken from eight fish that were randomly chosen from the 15 in each of three replicate tanks. The remaining fish were used for experiments reported in another study (Chen et al., 2015). Blood was taken from the caudal vein with chilled heparinized syringes. After centrifugation (1 000×g, 10 min), plasma was frozen in liquid nitrogen for GH and IGF-I measurement by radioimmunoassay. The pituitary, hypothalamus and liver were dissected, then frozen in liquid nitrogen and stored at -80℃ for the quantification of gh, ghrelin, gnrhs, npy, npyrs, pacap, igf-I, ghrs, igf-Ir, and igfbp3 mRNA by real-time PCR.
2.3 RadioimmunoassayGH and IGF-I levels were detected using commercial radioimmunoassay (RIA) kits purchased from the Beijing North Institute of Biological Technology, China. Hormone levels were determined according to the manufacturer’s instructions. The RIA was based upon the competition of certain 125I labeled antigen and unlabeled antigen (standard or unknown) binding to the limited quantity of antibodies. The RIA kits for human GH and IGF-I were validated for use with tilapia samples by demonstrating parallelism between a series of diluted and spiked samples in relation to the standard curve attached to the assay kits. Standards and samples were added to the test tubes in duplicate. The assay detection ranges were 1–50 ng/mL for GH and 30–1 500 ng/mL for IGF-I.
2.4 Quantitative real-time PCRTotal RNA was isolated from the collected tissues using the phenolic reagent TRIzol (Invitrogen, CA, USA) according to the manufacturer’s protocol. The extracted RNA was measured by spectrometry at OD260/280 to check its quality. Traces of DNA were removed by treating with DNase I (Promega, WI, USA) prior to the process of reverse-transcriptase PCR. Equal amounts of RNA (1 μg) were reverse-transcribed into cDNA using a reverse transcriptase kit (Toyobo, Osaka, Japan) in a 20-μL reaction. The cDNA samples were diluted to 100 μL with dH2O and stored at -20℃.
Primers were designed for the specific amplification of target genes: gh, ghrelin, gnrhs, npy, npyrs, pacap, igf-I, ghrs, igf-Ir, igfbp3, housekeeping genes: β-actin and 18s rRNA, according to the Nile tilapia (O. niloticus) sequences published in GenBank (Table 1). Parallel PCR reactions were conducted to amplify the target and housekeeping gene cDNA. The amplifications were carried out in a Eppendorf Mastercycler® ep Realplex2 Real-time Quantitative PCR System (Eppendorf, Hamburg, Germany) using the SYBR Green Mix Kit (TaKaRa, Dalian, China). Quantitative real-time PCR was performed in a 20-μL volume system containing 10 μL SYBR® Premix Ex TaqTM II, 0.4 μL ROX Reference Dye, 0.8 μL forward primer and reverse primer (10 μmol/L) for each, 4 μL first-strand cDNA (template), and 4.8 μL dH2O. The thermal profile was 95℃ for 30 s followed by 40 cycles of 95℃ for 5 s and 60℃ for 30 s. The target gene mRNA abundance in each sample, relative to the abundance of β-actin and 18s rRNA mRNA, was analyzed according to the formula 2-ΔΔCt, and plotted on a logarithmic scale. Levels of β-actin and 18s rRNA mRNA expression were not changed under any of the experimental conditions in this study. Agarose gel electrophoresis of the PCR products was displayed to prove the presence of single amplicons of the correct predicted base-pair size (not shown).
All statistical analysis was carried out in SPSS 16.0, and all data are presented as the mean±standard deviation. Multiple comparisons were performed using one-way analysis of variance (ANOVA) followed by Tukey’s test. Statistical significance was considered to be P < 0.05.
3 RESULT 3.1 Effects of genistein on body weightAs we have reported previously (Chen et al., 2015), after 8 weeks of feeding, the average body weight of control tilapia fish increased from the 10.47±1.24 g to 43.75±1.64 g, and there were no significant differences in the final body weight among fish fed diet 2 (30 μg/g genistein; 43.30±2.10 g) and diet 3 (300 μg/g genistein; 45.20±2.39 g) compared with the control. However, the final body weight of fish fed with diet 4 (3 000 μg/g genistein) was 36.83±1.80 g, which was significantly lower than the control.
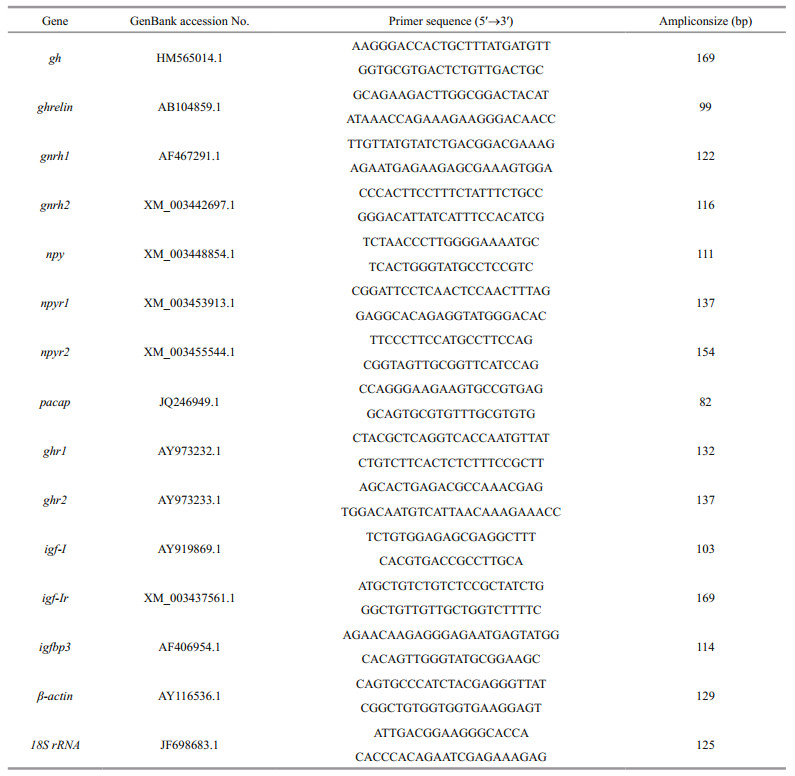
3.2 Effects of genistein on plasma GH and IGF-I levelsAs shown in Fig. 1a, the plasma GH concentration was 1.35±0.12 ng/mL in control fish, while it remained unaffected in fish fed diet 2 and diet 3, but significantly decreased to 0.88±0.04 ng/mL in fish fed diet 4. Similarly, IGF-I concentrations in the plasma of fish fed diet 4 was also significantly lower than those of control, from 118.29±12.76 ng/mL to 87.63±4.31 ng/mL (Fig. 1b).
|
| Figure 1 Quantification of plasma GH (a) and IGF-I (b) levels in Nile tilapia fed diets containing different concentrations of genistein Data are presented as the mean±standard deviation (n=24). Asterisks indicate statistically significant difference compared with the control (*P < 0.05). |
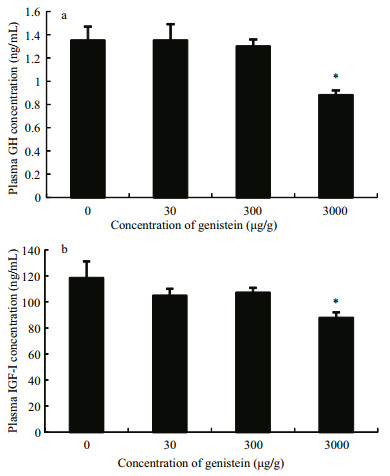
The relative mRNA levels of gh in the pituitary of tilapia fish fed diet 4 significantly decreased compared with that of the control, and there was no significant difference in the other two diet groups (Fig. 2a). Ghrelin and npyr1 mRNA expression were also significantly inhibited by feeding with diet 4 (Fig. 2b, f). In contrast, npy mRNA levels increased significantly after being fed diet 4 (Fig. 2e). However, gnrh1, gnrh2, npyr2, and pacap mRNA levels were not changed by any of the dietary treatments (P > 0.05) (Fig. 2c, d, g, h).
|
| Figure 2 Relative mRNA expression levels of gh in the pituitary and regulatory genes in the hypothalamus of Nile tilapia fed diets containing different concentrations of genistein diets containing different concentrations of genistein Fold change (Y-axis) represents the expression of mRNA relative to that of the control group (equals 1 by definition). Data are presented as the mean±standard deviation (n=24). Asterisks indicate statistically significant difference from the control group (*P < 0.05). |
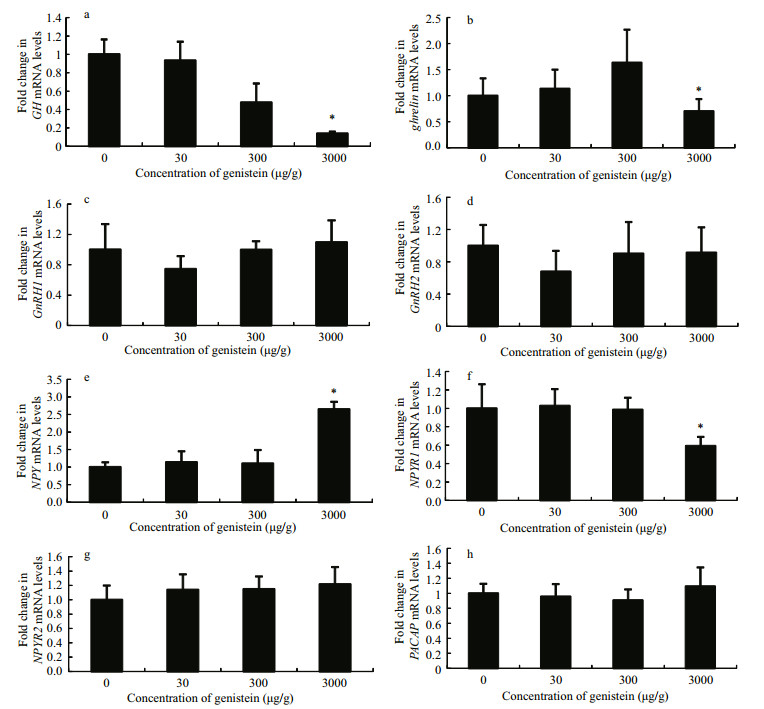
As shown in Fig. 3a, ghr1 mRNA levels in the livers of tilapia were not affected by any of the dietary treatments (P > 0.05). However, ghr2 mRNA levels in the livers of fish fed diet 4 decreased significantly compared with the control, and no significant difference was found in the other two dietary treatments (Fig. 3b).
|
| Figure 3 Relative mRNA expression levels of ghr1 and ghr2 in the liver of Nile tilapia fed diets containing different concentrations of genistein Fold change (Y-axis) represents the expression of ghr1 and ghr2 mRNA relative to that of the control group (equals 1 by definition). Data are presented as the mean±standard deviation (n=24). Asterisks indicate statistically significant difference from the control group (*P < 0.05). |
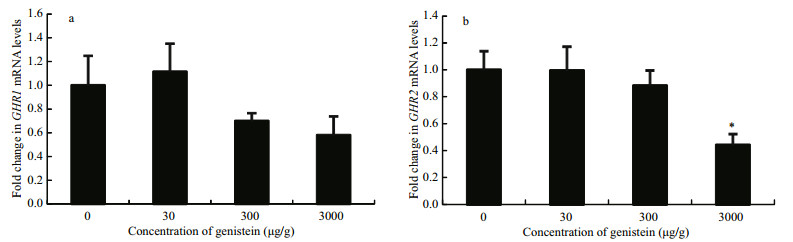
As shown in Fig. 4a, igf-I mRNA expression levels in the livers of tilapia fish fed diet 4 were significantly lower than those of control, and there was no significant difference in the other two groups. Igf-Ir mRNA expression were also significantly inhibited by diet 4, but were stimulated by diet 3 (Fig. 4b). In contrast, igfbp3 mRNA levels increased after feeding with diet 4 compared with the control, and no significant difference was found in the other two groups (Fig. 4c).
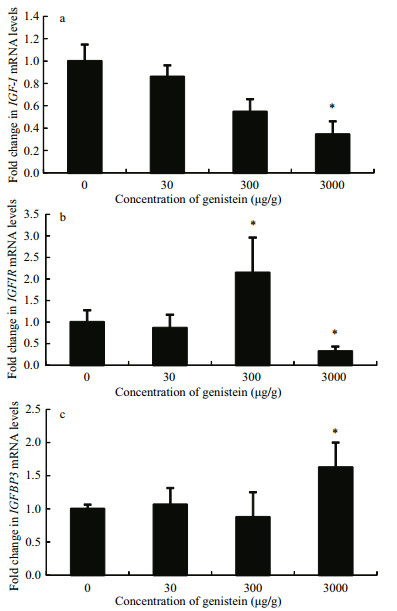
|
| Figure 4 Relative mRNA expression levels of igf-I, igf-Ir and igfbp3 in the liver of Nile tilapia fed diets containing different concentrations of genistein Fold change (Y-axis) represents the expression of igf-I, igf-Ir and igfbp3 mRNA relative to that of the control group (equals 1 by definition). Data are presented as the mean±standard deviation (n=24). Asterisks indicate statistically significant difference from the control group (*P < 0.05). |
Fish growth is influenced by genetic, environmental, nutritional, and endocrinal factors (Norbeck and Sheridan, 2011). In terms of nutrition, our previous study showed that high dietary levels of genistein (3 000 μg/g) significantly depressed the growth performance of Nile tilapia partly by reducing digestive enzyme activity (Chen et al., 2015). Other researchers have reported similar findings (Cai, 2006; Mao, 2009).
In terms of endocrine, the GH/IGF-I system plays a major role in the regulation of most important physiological processes in teleost fish, including somatic growth, metabolism, and reproduction (Reinecke et al., 2005; Nordgarden et al., 2006). It has been demonstrated that the two hormones act together (GH induces IGF-I secretion and increased IGF-I sensitivity of tissues) and independently in regulating the growth of vertebral and muscle tissue in fish (Nordgarden et al., 2006). Under normal circumstances, fish growth is positively correlated with GH levels (Yu et al., 2006; Ma et al., 2010). In the fish farming industry, GH is often used as a potential growth promoting agent (McLean et al., 1993; Ben-Atia et al., 2000). Additionally, soybean isoflavones may affect GH production. It has been reported that dietary daidzein significantly increases plasma GH levels and promotes somatic growth in tilapia (Yu et al., 2006). However, in this study, another soybean isoflavone, genistein, decreased plasma GH levels and pituitary gh mRNA expression and suppressed growth in Nile tilapia in high dietary concentrations (3 000 μg/g).
GH is synthesized by the somatotrophic cells of the anterior pituitary gland, and its secretion is regulated by hypothalamic neuroendocrine factors that either directly affect the somatotrophic cells or regulate the release or activity of other neuroendocrine factors (Holloway and Leatherland, 1998; Canosa et al., 2007). Growth hormone releasing hormone (GHRH), pituitary adenylate cyclase activating polypeptide (PACAP), gonadotropin releasing hormone (GnRH), and Neuropeptide Y (NPY) are stimulatory hypothalamic factors that stimulate the synthesis and release of pituitary GH (Canosa et al., 2007). Additionally, ghrelin is predominately synthesized in the fish gut and in a few other peripheral tissues and brain, and can also act directly on somatotrophic cells to stimulate GH secretion (Lin, 2000; Kaiya et al., 2003; Unniappan and Peter, 2005; Canosa et al., 2007). Isoflavones were found to influence these GH controlling factors in mammals. Some studies have suggested that dietary genistein inhibits the release of ghrelin in rats (Ryökkynen et al., 2006; Zhang et al., 2009), isoflavone depresses ghrelin and NPY levels in rats (Zhang et al., 2009), and the influence of isoflavone on GnRH is closely correlated with the developmental stage of experimental animals (Wang, 2002). The effects of isoflavones on hypothalamic neuroendocrine factors and ghrelin in fish are poorly understand at present.
In this study, the ghrelin, gnrhs, npy, npyrs and pacap mRNA expression levels in tilapia hypothalamus were examined. Ghrelin and npyr1 mRNA levels significantly decreased with high dietary genistein (3 000 μg/g) in Nile tilapia (O. niloticus), and npy mRNA levels increased significantly with high genistein levels, while npyr2, gnrh1, gnrh2, and pacap mRNA expression levels remained unaffected.
Ghrelin plays a role in the regulation of GH synthesis and release (Unniappan and Peter, 2005). Meyer et al. (2013) demonstrated that GH was a major downstream effector of the ghrelin receptor (Meyer et al., 2013). Peripheral injections of ghrelin stimulate circulating levels of GH in goldfish and rainbow trout (Unniappan and Peter, 2005) and channel catfish (Kaiya et al., 2005). Furthermore, Date et al. (2002) indicated that blocking the gastric vagal afferent of rats may eliminate ghrelin synthesis, and then impede ghrelin-induced feeding GH secretion and GHRH-producing neurons (Date et al., 2002). In our study, the reduction in ghrelin mRNA levels in tilapia by genistein may indicate that genistein affects pituitary GH synthesis by inhibiting ghrelin mRNA expression.
As for the effects of NPY on GH, NPY may improve GH secretion by either directly acting on somatotrophic cells or causing the release of GnRHs that in turn stimulate GH release (Peng et al., 1993). NPYR1 and NPYR2, the receptors of NPY, are crucial to achieving its physiological function (Yang et al., 2008). In this study, the npy mRNA levels increased significantly with the higher dietary genistein, while the gnrh1, gnrh2 and npyr2 mRNA levels remained unchanged, and npyr1 mRNA levels decreased significantly. Therefore, we suggest that NPYR1 may be crucial for NPY GH stimulating functions in Nile tilapia, and genistein likely inhibited npyr1 mRNA expression and, hence, suppressed the induction of NPY on GH.
Augmentation of fish growth by GH is primarily a result of regulation of IGF levels (Shamblott et al., 1995). IGF-I, as the central factor in GH/IGF-I axis, has a higher correlation with fish growth compared with GH. It has been demonstrated that liver igf-I mRNA levels in Nile tilapia are significantly positively correlated with body weight gain under different temperature and feeding conditions (Vera et al., 2006). Furthermore, Uchida et al. (2003) found that body weight and specific growth rate (SGR) decreased significantly during fasting in tilapia, along with the significant reduction of IGF-I levels. Injection of bovine GH into juvenile rainbow trout (Oncorhynchus mykiss) stimulated liver igf-I gene expression and increased plasma IGF-I levels (Shamblott et al., 1995). This stimulation of IGF-I is mediated by growth hormone receptor (GHR) on the surface of the target cells. Two types of GHRs have been reported in tilapia: GHR1 and GHR2, which had higher homology with vertebrates, and the affinity of GH to GHR2 was far higher than that of GHR1 (Pierce et al., 2007). The tissue specific expression revealed that GHR2 was the primary GH receptor with functions in growth and metabolism, while GHR1 was also a receptor for somatolactin, with functions in metabolic and chromatophore regulation (Pierce et al., 2007). Thus, the role of GH is generally considered to be mediated primarily by GHR2 (Zheng, 2012). In black seabream (Deng et al., 2004), catfish (Small et al., 2006), and salmon (Fukada et al., 2004), fasting improves pituitary gh mRNA expression and plasma GH levels, while decreasing ghr and igf-I mRNA levels in the liver, causing a decrease in fish growth. Lerner et al. (2012) found that E2 and 4-nonylphenol decreased GHR abundance and caused the decline of plasma IGF-I levels in Atlantic salmon. In this study, the plasma GH levels, relative gh and ghr mRNA expression levels were significantly inhibited by the higher dietary genistein, which was consistent with the changing trend of IGF-I levels, igf-I mRNA expression, and fish growth.
Nile tilapia possess six insulin-like growth factor binding proteins (IGFBPs): IGFBP1, IGFBP2, IGFBP3, IGFBP4, IGFBP5, and IGFBP6 (Xiong, 2012). Similar to mammals, over 99% of total circulating IGF-I are bound to IGFBPs in fish (Shimizu et al., 1999). Among all of the IGFBPs, IGFBP3 is the most crucial binding and carrier protein of IGF-I in blood (Jiao et al., 2009). Furthermore, Cheng et al. (2002) found that IGFBP3 accounted for the majority of IGFBPs that interact with IGF-I in circulation in tilapia (Oreochromis mossambicus) (Cheng et al., 2002).
The IGF-I of combined state has no biological activities, and only free IGF-I can combine with IGFIR, then promote the growth of fish (Bang et al., 2001). It was found that the final body weight of fish fed diet 3 was more weight than that of control, although there was no significant difference, which was consistent with the results of some investigations that isoflavone in a certain concentration range would probably promote the growth of fish, while it would depress the growth of fish beyond a certain concentration range. It may be the reason why igf-Ir mRNA expression levels were significantly inhibited by feeding with diet 4, but were stimulated greatly by feeding with diet 3. Hence the higher of IGFBP3 levels, the more of IGF-I of combined state, then the less of free IGF-I. In the present study, the igf-Ir mRNA levels reduced greatly with the higher dietary genistein, while the igfbp3 mRNA levels increased significantly, which may be one of the reasons that the growth of fish was greatly depressed by the higher dietary genistein.
5 CONCLUSIONThe highest dietary level of genistein (3 000 μg/g) depressed greatly the growth performance of Nile tilapia by reducing final body weight, while the lower level of genistein (0–300 μg/g) did not influence the growth performance. The higher dietary genistein could disturb the endocrine regulation of tilapia. On one hand, it inhibited the expression of ghrelin and npyr1, and thus reduced the synthesis and secretion of GH. On the other hand, it reduced the synthesis of IGF-I and free IGF-I by depressing the expression of liver ghr2, igf-Ir, and igfbp3, thereby restraining the action of GH mediated by IGF-I.
| Bang P, Ahlsén M, Berg U, Carlsson-Skwirut C, 2001. Free insulin-like growth factor-I:are we hunting a ghost?. Hormone Research, 55 (Suppl. 2) : 84 –93. |
| Ben-Atia I, Fine M, Tandler A, Funkenstein B, Maurice S, Cavari B, Gertler A, 1999. Preparation of recombinant gilthead seabream (Sparus aurata) growth hormone and its use for stimulation of larvae growth by oral administration. Gen. Comp. Endocrinol., 113 (1) : 155 –164. Doi: 10.1006/gcen.1998.7192 |
| Cai Y H. 2006. Eff ects of Antinutritional Factors in Soybean on the Growth Performance and Digestive Physiology of Japanese Flounder, Paralichthys olivaceus. MS thesis, Ocean University of China, Qingdao. 183p. (in Chinese) |
| Canosa L F, Chang J P, Peter R E, 2007. Neuroendocrine control of growth hormone in fi sh. Gen. Comp.Endocrinol., 151 (1) : 1 –26. Doi: 10.1016/j.ygcen.2006.12.010 |
| Chen D, Wang W, Ru S G, 2015. Eff ect of dietary genistein on growth performance, digestive enzyme activity, and body composition of Nile tilapia Oreochromis niloticus. Chinese Journal of Oceanology and Limnology, 33 (1) : 77 –83. Doi: 10.1007/s00343-015-4037-6 |
| Cheng R S, Chang K M, Wu J L, 2002. Diff erent temporal expressions of tilapia (Oreochromis mossambicus)Insulin-like growth factor-I and IGF binding protein-3 after growth hormone induction. Marine Biotechnology, 4 (3) : 218 –225. Doi: 10.1007/s10126-002-0014-0 |
| Date Y, Murakami N, Toshinai K, et al, 2002. The role of the gastric aff erent vagal nerve in ghrelin-induced feeding and growth hormone secretion in rats. Gastroenterology, 123 (4) : 1120 –1128. Doi: 10.1053/gast.2002.35954 |
| Deng L, Zhang W M, Lin H R, Cheng C H K, 2004. Eff ects of food deprivation on expression of growth hormone receptor and proximate composition in liver of black seabream Acanthopagrus schlegeli. Comp. Biochem.Physiol., 137 (4) : 421 –432. Doi: 10.1016/j.cbpc.2004.01.008 |
| Fukada H, Ozaki Y, Pierce A L, Adachi S, Yamauchi K, Hara A, Swanson P, Dickhoff W W, 2004. Salmon growth hormone receptor:molecular cloning, ligand specifi city, and response to fasting. Gen. Comp. Endocrinol., 139 (1) : 61 –71. Doi: 10.1016/j.ygcen.2004.07.001 |
| Holloway A C, Leatherland J F, 1998. Neuroendocrine regulation of growth hormone secretion in teleost fi shes with emphasis on the involvement of gonadal sex steroids. Reviews in Fish Biology and Fisheries, 8 (4) : 409 –429. Doi: 10.1023/A:1008824723747 |
| Ishibashi H, Kobayashi M, Koshiishi T, et al, 2002. Induction of plasma vitellogenin synthesis by the commercial fi sh diets in male goldfi sh (Carassius auratus) and dietary phytoestrogens. J. Health Sci., 48 (5) : 427 –434. Doi: 10.1248/jhs.48.427 |
| Jiao S, Lu L, Li Y, Duan C M, 2009. Role and mechanism of the insulin-like growth factor in signal transduction pathway in zebrafi sh. International Journal of Pathology and Clinical Medicine, 29 (3) : 235 –239. |
| Kaiya H, Kojima M, Hosoda H, Riley L G, Hirano T, Grau E G, Kangawa K, 2003. Identifi cation of tilapia ghrelin and its eff ects on growth hormone and prolactin release in the tilapia, Oreochromis mossambicus. Comp. Biochem.Physiol., 135 (3) : 421 –429. Doi: 10.1016/S1096-4959(03)00109-X |
| Lerner D T, Sheridan M A, McCormick S D, 2012. Estrogenic compounds decrease growth hormone receptor abundance and alter osmoregulation in Atlantic salmon. Gen. Comp.Endocrinol., 179 (2) : 196 –204. Doi: 10.1016/j.ygcen.2012.08.001 |
| Lilleeng E, Froystad M K, Vekterud K, Valen E C, Krogdahl A, 2007. Comparison of intestinal gene expression in Atlantic cod (Gadus morhua) fed standard fi sh meal or soybean meal by means of suppression subtractive hybridization and real-time PCR. Aquaculture, 267 (1-4) : 269 –283. Doi: 10.1016/j.aquaculture.2007.01.048 |
| Lin H R, 2000. The interactions of neuroendocrine regulation on reproduction and growth in fi sh. Zoological Research, 21 (1) : 12 –16. |
| Ma X L, Zhang Y, Liu X C, Zhou L B, Lin H R, 2010. Eff ects of csh on the growth and gene expression of the growth axis in Nile tilapia (Oreochromis niloticus). Oceanologia et Limnologia Sinica, 41 (2) : 240 –245. |
| Mambrini M, Roem A J, Cravèdi J P, Lallès J P, Kaushik S J, 1999. Effects of replacing fish meal with soy protein concentrate and of DL-methionine supplementation in high energy extruded diets on growth and nutrient utilization in rainbow trout, Oncorhynchus mykiss. J.Anim. Sci., 77 (11) : 2990 –2999. Doi: 10.2527/1999.77112990x |
| Mao S S. 2009. Effects of Genistein on Digestive Enzymes and Antioxidant Systems of Goldfish, Carassius auratus. MS thesis, Ocean University of China, Qingdao. 60p. (in Chinese) |
| Mclean E, Donaldson E M, Teskeredzic E, Souza L M, 1993. Growth enhancement following dietary delivery of recombinant porcine somatotropin to diploid and triploid coho salmon (Oncorhynchus kisutch). Fish Physiology and Biochemistry, 11 (1-6) : 363 –369. Doi: 10.1007/BF00004586 |
| Meyer R M, Burgos-Robles A, Liu E, Correia S S, Goosens K A, 2014. A ghrelin-growth hormone axis drives stressinduced vulnerability to enhanced fear. Molecular Psychiatry, 19 (12) : 1284 –1294. Doi: 10.1038/mp.2013.135 |
| National Research Council (NRC). 2011. Nutritional Requirements of Fish. National Academic Press, Washington, DC, USA. |
| Norbeck L A, Sheridan M A, 2011. An in vitro model for evaluating peripheral regulation of growth in fish:effects of 17 β-estradiol and testosterone on the expression of growth hormone receptors, insulin-like growth factors, and insulin-like growth factor type 1 receptors in rainbow trout (Oncorhynchus mykiss). Gen. Comp. Endocrinol., 173 (2) : 270 –280. Doi: 10.1016/j.ygcen.2011.06.009 |
| Nordgarden U, Fjelldal P G, Hansen T, Björnsson B T, Wargelius A, 2006. Growth hormone and insulin-like growth factor-I act together and independently when regulating growth in vertebral and muscle tissue of atlantic salmon postsmolts. Gen. Comp. Endocrinol., 149 (3) : 253 –260. Doi: 10.1016/j.ygcen.2006.06.001 |
| Peng C, Trudeau V L, Peter R E, 1993. Seasonal variation of neuropeptide Y actions on growth hormone and gonadotropin-Ⅱ secretion in the goldfish:effects of sex steroids. Journal of Neuroendocrinology, 5 (3) : 273 –280. Doi: 10.1111/jne.1993.5.issue-3 |
| Pierce A L, Fox B K, Davis L K, Visitacion N, Kitashashi T, Hirano T, Grau E G, 2007. Prolactin receptor, growth hormone receptor, and putative somatolactin receptor in Mozambique tilapia:tissue specific expression and differential regulation by salinity and fasting. Gen. Comp.Endocrinol., 154 (1-3) : 31 –40. Doi: 10.1016/j.ygcen.2007.06.023 |
| Refstie S, Førde S O, Rosenlund G, Rorvik K A, 2006. Feed intake, growth, and utilisation of macronutrients and amino acids by 1-and 2-year old Atlantic cod (Gadus morhua) fed standard or bioprocessed soybean meal. Aquaculture, 255 (1-4) : 279 –291. Doi: 10.1016/j.aquaculture.2006.01.002 |
| Reinecke M, Björnsson B T, Dickhoff W W, McCormick S D, Navarro I, Power D M, Gutiérrez J, 2005. Growth hormone and insulin-like growth factors in fish:where we are and where to go. Gen. Comp. Endocrinol., 142 (1-2) : 20 –24. Doi: 10.1016/j.ygcen.2005.01.016 |
| Ryökkynen A, Kukkonen J V K, Nieminen P, 2006. Effects of dietary genistein on mouse reproduction, postnatal development and weight-regulation. Animal Reproduction Science, 93 (3-4) : 337 –348. Doi: 10.1016/j.anireprosci.2005.08.007 |
| Shamblott M J, Cheng C M, Bolt D, Chen T T, 1995. Appearance of insulin-like growth factor mRNA in the liver and pyloric ceca of a teleost in response to exogenous growth hormone. Proc. Natl. Acad. Sci. USA, 92 (15) : 6943 –6946. Doi: 10.1073/pnas.92.15.6943 |
| Shimizu M, Swanson P, Dickho W W, 1999. Free and proteinbound insulin-like growth factor-I (IGF-I) and IGFbinding proteins in plasma of coho salmon, Oncorhynchus kisutch. Gen. Comp. Endocrinol., 115 (3) : 398 –405. Doi: 10.1006/gcen.1999.7328 |
| Small B C, Murdock C A, Waldbieser G C, Peterson B C, 2006. Reduction in channel catfish hepatic growth hormone receptor expression in response to food deprivation and exogenous cortisol. Domest. Anim.Endocrinol., 31 (4) : 340 –356. Doi: 10.1016/j.domaniend.2005.12.003 |
| Tepavčević V, Atanacković M, Miladinović J, et al, 2010. Isoflavone composition, total polyphenolic content, and antioxidant activity in soybeans of different origin. Journal of Medicinal Food, 13 (3) : 657 –664. Doi: 10.1089/jmf.2009.0050 |
| Uchida K, Kajimura S, Riley L G, Hirano T, Aida K, Grau E G, 2003. Effects of fasting on growth hormone/insulin-like growth factor I axis in the tilapia, Oreochromis mossambicus. Comp. Biochem. Physiol., 134 (2) : 429 –439. Doi: 10.1016/S1095-6433(02)00318-5 |
| Unniappan S, Peter R E, 2005. Structure, distribution and physiological functions of ghrelin in fish. Comp. Biochem.Physiol., 140 (4) : 396 –408. Doi: 10.1016/j.cbpb.2005.02.011 |
| Vera E M, Brown C L, Luckenbach J A, Picha M E, Bolivar R B, Borski R J, 2006. Insulin-like growth factor-I cDNA cloning, gene expression and potential use as a growth rate indicator in Nile tilapia, Oreochromis niloticus. Aquaculture, 251 (2-4) : 585 –595. Doi: 10.1016/j.aquaculture.2005.06.039 |
| Wang Y J. 2002. Effects of Maternal Daidzein on Growth and Expression of Growth-related Genes in Shaoxing Ducks.Nanjing Agricultural University, Nanjing. 183p. (in Chinese) |
| Xiong C Q. 2012. Overexpression of IGF3 in Gonad and Characterization of its Possible Binding Protein in Nile Tilapia. Southwest University, Chongqing. 71p. (in Chinese) |
| Yang K P, Guan H Y, Arany E, Hill D J, Cao X, 2008. Neuropeptide Y is produced in visceral adipose tissue and promotes proliferation of adipocyte precursor cells via the Y1 receptor. Federation of American Societies for Experimental Biology Journal, 22 (7) : 2452 –2464. Doi: 10.1096/fj.07-100735 |
| Yu Z G, Xia D Q, Wu T T, 2006. Effects of daidzein on growth, hormone and physio-biochemical parameters levels in Oreochromis aureus. Chin. J. Vet. Sci., 26 (2) : 183 –185. |
| Zhang Y B, Na X L, Zhang Y, Li L N, Zhao X Y, Cui H B, 2009. Isoflavone reduces body weight by decreasing food intake in ovariectomized rats. Annals of Nutrition and Metabolism, 54 (3) : 163 –170. Doi: 10.1159/000217812 |
| Zheng Y. 2012. Effects of Monocrotophos Pesticide on the Growth and GH/IGF-I Axis in Juvenile Nile Tilapia, Oreochromis niloticus. Ocean University of China, Qingdao. 73p. (in Chinese) |
 2016, Vol. 34
2016, Vol. 34