Institute of Oceanology, Chinese Academy of Sciences
Article Information
- SONG Zhidong(宋志东), WANG Jiying(王际英), QIAO Hongjin(乔洪金), LI Peiyu(李培玉), ZHANG Limin(张利民), XIA Bin(夏斌)
- Ontogenetic changes in digestive enzyme activities and the amino acid profile of starry flounder Platichthys stellatus
- Chinese Journal of Oceanology and Limnology, 34(5): 1013-1024
- http://dx.doi.org/10.1007/s00343-016-5031-3
Article History
- Received May. 10, 2015
- accepted in principle Apr. 20, 2015
- accepted for publication Jul. 28, 2015
2 Shandong Shengsuo Aquatic Feed Research Center, Yantai 265601, China
Starry flounder (Platichthys stellatus) has been recently introduced to diversify aquaculture in North China. Similar to other flatfish, starry flounder larvae undergo several important morphological and physiological events, such as yolk depletion, firstfeeding, development of the digestive system, and metamorphosis before digestive ability improves (Fang and Wang, 2011). These events are related to nutritional changes, including ontogenic changes in the amino acid (AA) profile and digestive enzyme activities. Free amino acids (FAA) are produced by hydrolysis of yolk protein (Thorsen et al., 1996) and act as osmotic regulators and energy substrates for development as well as building-blocks for protein synthesis (Rønnestad and Fyhn, 1993; Finn, 1994; Finn et al., 1996). The FAA pool is consumed progressively after fertilization in most fish, and the precise sequence of use varies depending on the species (Finn et al., 1996; Conceição et al., 1997, 1998; Tulli and Tibaldi, 1997; Weltzien et al., 1999; Krautz et al., 2010; Dayal et al., 2003; Skjærven et al., 2003). The protein amino acid (PAA) profile also follows species-specific changes during larval development (Aragão et al., 2000). Pacu (Piaractus mesopotamicu) larvae utilize certain AAs and retain others for later developmental stages, which leads to a difference in the larval AA profile (Portella et al., 2013). Thus, changes in the AA profiles of fish larvae have important implications in terms of AA requirements (Conceição et al., 1997; Aragão et al., 2004).
The expression of digestive enzyme activities is a response of larval digestive organs to a food source. The activities of several digestive enzymes have been detected in fish larvae, including proteases (Applebaum et al., 2001; Bolasina et al., 2006; Kamaci et al., 2009), lipases (Murray et al., 2003; Sæle et al., 2010), and amylase (Lazo et al., 2000), suggesting that fish larvae have the potential to digest complex food. Starry flounder larviculture continues to rely on live food provided at different time based on larval size. Histological studies on the development of the digestive system in larval and juvenile starry flounder have been conducted (Liu et al., 2008; Fang and Wang, 2011). However, little information is available on digestive enzyme activities of starry flounder larvae. Analyzing changes in digestive enzyme activities will provide a better understanding of the digestive processes and the nutritional condition of larvae.
The main objective of this study was to elucidate the ontogenetic changes in digestive enzyme activities and the AA profiles during starry flounder larval development to assess larval digestion and dietary AA imbalances that could occur during larval production.
2 MATERIAL AND METHOD 2.1 Fish rearing and managementFertilized eggs were obtained from spontaneously spawning starry flounder broodstock cultured at the Rizhao Xinxiu fish farm (Rizhao, China). Eggs were incubated naturally in three closed concrete cement pools (6 m × 6 m) at 15±0.5℃ and stocking density of 30–80 eggs/L. After hatching, the temperature was increased gradually over 6 h to 18±0.5℃. Seawater (salinity, 31.0±1.0) was pre-filtered (5 μm) and UVtreated, and constant aeration was provided (25–30 L/(m3·h)). About 50% of the culture water was exchanged every second day and a 16:8 L/D photoperiod was maintained. The NH4-N level was never > 6 mmol/L, and pH was stable at 8.2 during the experimental period.
Larvae opened their mouths 3–4 days after hatching (DAH) and were fed Brachionus plicatilis rotifers (Chlorella enriched for 6 h) for 4 days, followed by mixed feed that included Artemia nauplii enriched with 50DE (Shengsuo Technologies, Yantai, China) until 12 DAH. A concentration of 4–7 rotifers m/L and 1 500–5 000 Artemia nauplii /L were maintained in the rearing water. Larvae received Artemia meta nauplii beginning 12 DAH until the end of experiment. The nutritional composition of the live food is presented in Table 1.
The sampling procedure followed those of Brown et al. (2005). In brief, samples were collected before feeding from each replicate tank at the egg stage and 0, 2, 4, 7, 12, 17, 24 DAH. Eggs (n=600–800) or larvae (n=1 500–1 800) were filtered on glass-fiber filters (Whatman GF/C 25 mm; Maidstone, UK) under a gentle vacuum. Forty larvae from each replicate tank were counted randomly under a stereomicroscope (SZX-ILLK200, Olympus Optical Co. Ltd., Tokyo, Japan). Absolute growth rate in length (LAGR, mm/d) for a given larval age was calculated by subtracting mean hatch length (1.91 mm) from mean length and dividing by the relevant number of days (Welch et al., 2013).
Similarly, rotifers and Artemia (200 mL suspension) were collected on glass-fiber filters, washed with 50 mL 0.5 mol/L ammonium formate, and approximately 2 mL of suspended larvae were transferred to cryovials. The cryovials were transferred to liquid nitrogen and freeze-dried into powder.
2.3 Digestive enzyme activity analysesPooled samples of 400, 200, 100, or 50 individuals (depending on age) were collected 0, 2, 4, 7, 12, 17, 24 DAH before feeding, homogenized in nine volumes (v/w) of cold 50-mol/L Tris-HCl buffer, pH 8.0, and centrifuged at 30 000×g for 30 min at 4℃. The supernatant was collected and stored at -80℃ for later digestive enzyme analyses. Pepsin activity was determined using 1% hemoglobin (Sigma, St. Louis, MO, USA) as the substrate. In brief, 0.5 mL 1% (w/v) hemoglobin, 0.4 mL glycine-HCl buffer (0.05 mol/L, pH 2.0), and 0.1 mL crude enzyme were mixed and incubated for 10 min in a 37℃ water bath. The reaction was stopped by adding 1.5 mL 5% trichloroacetic acid. After centrifugation at 2 500×g for 10 min, absorbance of the supernatant was recorded at 280 nm. One unit (U/mg prot) of pepsin was defined as the amount of enzyme in 1 mg of tissue homogenate that hydrolyzed protein to form 1-μg tyrosine equivalent/min at 37℃ and pH 2.0. Trypsin and chymotrypsin activities were determined using benzoyl-l-arginine ethyl ester hydrochloride (BAEE) (Amresco, Solan, OH, USA) and benzoyl-L-tyrosine ethyl ester (BTEE) (Amresco) as substrates, respectively. In brief, 1.5 mL of 2.0 mol/L substrate, 1.4 mL of 0.1 mol/L Tris-HCl (pH 8.0), and 0.1 mL crude enzyme were incubated at 37℃, and absorbance was recorded at 253 nm every 30 s. One unit (U/mg prot) of trypsin (or chymotrypsin) was defined as the amount of enzyme in 1 mg of tissue homogenate that hydrolyzed BAEE (or BTEE) per min at 37℃ and pH 8.0. Lipase activity was determined using 4-nitrophenyl palmitate (p-NPP) as the substrate. In brief, 4 mL of preheated p-NPP (0.09 mg/L) and 1 mL crude enzyme were mixed and incubated for 10 min in a 37℃ water bath. The reaction was stopped by adding 5 mL 0.5 mol/L trichloroacetic acid, and absorbance was recorded at 410 nm. One lipase unit (U/mg prot) was defined as the amount of enzyme in 1 mg of tissue homogenate that liberated 1 μmol p-nitrophenol/min at 37℃ and pH 7.5. Amylase activity was determined using 1% starch solution as the substrate. In brief, 1 mL of 1% starch solution, 3 mL phosphate buffer (pH 7.0), and 1 mL crude enzyme were mixed and incubated for 30 min at 37℃. The reaction was stopped by adding 1.5 mL 3, 5-dinitrosalicylic acid, and absorbance of the supernatant was recorded at 520 nm. One amylase unit (U/mg prot) was defined as the amount of enzyme in 1 mg of tissue homogenate that liberated 1.0 mg of maltose from 1% starch at 37℃ and pH 7.0.
Soluble protein content in the supernatant was determined by the Bradford assay using bovine serum albumin as the standard to normalize enzyme activity (Bradford, 1976). In brief, 0.1 mL of supernatant and 5 mL dye reagent containing 0.01% (w/v) Coomassie Brilliant Blue G250, 4.7% (v/v) ethanol, and 8.5% (v/v) H3PO4 were mixed. After standing at room temperature for 10 min, absorbance was recorded at 595 nm, and protein content was calculated according to the standard curve.
2.4 Amino acid analysisFreeze-dried eggs, larvae, rotifers, and Artemia were ground, and 4 mL of 80% methanol solution (v/v) was added to a 5-mL polyethylene tube to analyze FAAs. The tube was capped and sonicated for 40 min. The extract was centrifuged at 4 000×g/min for 10 min. One mL of the supernatant was freezedried, re-dissolved in 0.1 N HCl, and passed through a 0.22-μm filter into a 2-mL vial. The filtrate was loaded on a high-performance liquid chromatography (HPLC) system (LC1200, Agilent Technologies Inc., Palo Alto, CA, USA) equipped with an Agilent ZORBAX Eclipse Plus C18 column (150 μm × 5 μm). Signals of 16 amino acids were detected after derivatization with o-phthaldialdehyde. Asparagine, glutamine, proline, and tryptophan were not within the determination range. The HPLC conditions followed the protocol for the Agilent ZORBAX Eclipse Plus C18 column.
The residue was hydrolyzed with 6 mL of 6 N HCl at 110℃ for 22 h in an evacuated sealed tube to determine PAAs. The hydrolysates were dried under nitrogen gas to remove HCl, re-dissolved in 0.1 N HCl loading buffer, and filtered through a 0.22 μm polyethersulfone ultrafiltration membrane. The final hydrolysates were analyzed for PAAs.
2.5 Data analysis and statisticsThe statistical analysis was performed using SPSS 16.0 software (SPSS Inc., Chicago, IL, USA). After normality and homoscedasticity were tested, the larval AA profiles and digestive enzyme activities were compared by analysis of variance, and the means were compared using Duncan’s multiple comparison tests. A P-value < 0.05 was considered significant.
A regression analysis was performed using Office Excel 2007 (Microsoft Corp., Redmond, WA, USA). The essential amino acid (EAA) compositions of larvae and live food were compared, and the limiting EAAs were determined by: (EAA diet−EAA larva)×100×(EAA larvae)-1 according to the method of Aragão et al. (2004).
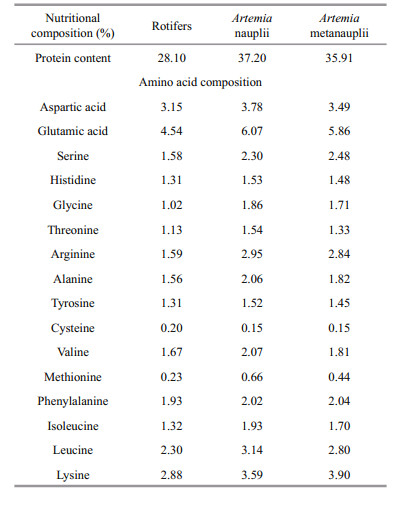
3 RESULT 3.1 Body length and LAGR of starry flounder larvaeThe mean length of starry flounder larvae at hatch was 1.91 mm, and mean body length at 24 DAH was 12.13 mm (Fig. 1). Size variations within the cohort increased over time. LAGR (mm/d) presented two phases of an increase (P < 0.05) from hatching to 7 DAH and then a decrease until the end of the experiment (Fig. 1).
|
| Figure 1 Starry founder larval body length (diamond) and absolute growth rate in length (triangle) during the experiment |
Starry flounder exhibited low digestive enzyme activities at hatching (Fig. 2). A similar variable pattern of trypsin and chymotrypsin activities was observed but changed slightly by 4 DAH (P > 0.05). Chymotrypsin activity increased significantly beginning 4 DAH, and trypsin activity increased 7 DAH (P < 0.05). Pepsin activity was negligible by 12 DAH but increased sharply at 17 DAH (P < 0.05). Lipase was detected at hatching. Lipase activity increased significantly at 4 DAH (P < 0.05) and increased progressively during larval growth. Amylase activity was also detected in newly hatched larvae. It increased at 4 and 7 DAH and then decreased gradually until the end of the experiment.

|
| Figure 2 Changes in digestive enzyme activity during larval development Dots are average activity±S.D. (n=3) of digestive enzymes. a. trypsin; b. pepsin; c. chymotrypsin; d. amylase; e. lipase. Dots with a common letter mean no significant difference (P >0.05). Dots are average activity ±S.D. (n=3) of digestive enzymes. a. trypsin; b. pepsin; c. chymotrypsin; d. amylase; e. lipase. Dots with a common letter mean no significant difference (P > 0.05). |
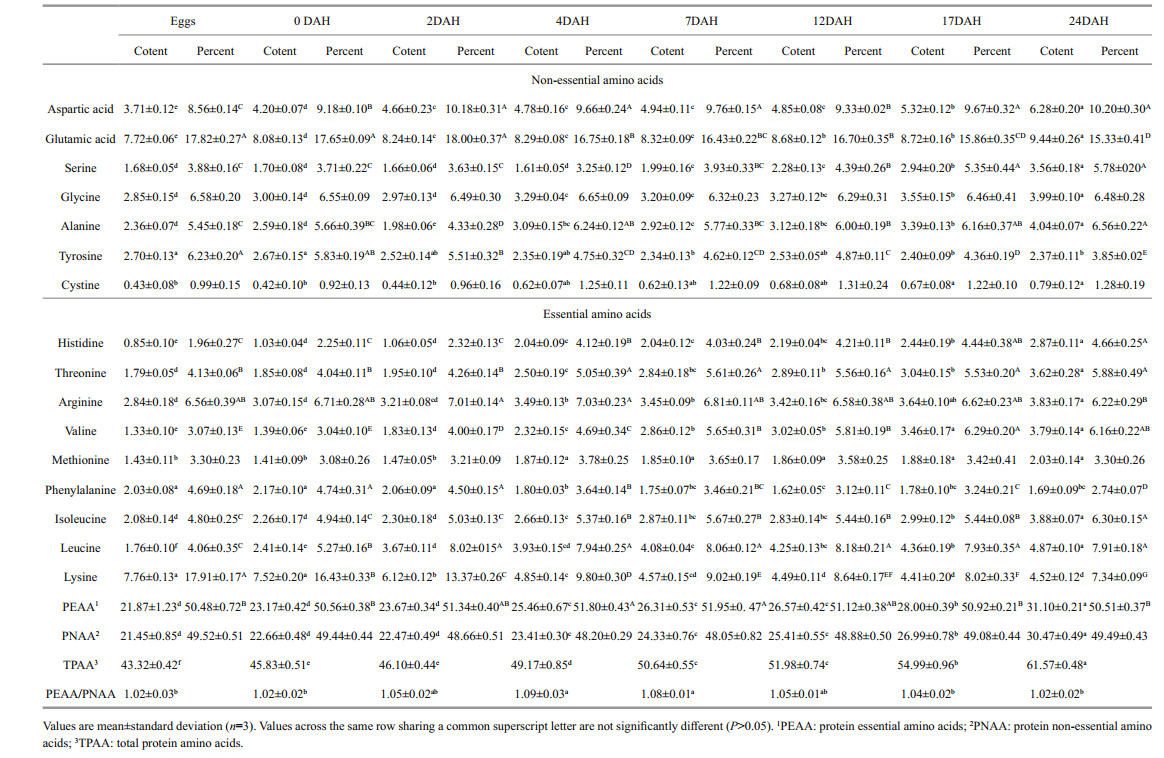
FAA contents in starry flounder larvae decreased by different degrees according to developmental stage (Table 2). The most dramatic change in the FAA pool occurred from the egg stage to 4 DAH (i.e., firstfeeding), and 60.90% of FAAs were depleted. Lysine and leucine were the two predominant free essential amino acids (FEAAs) (15.49 and 13.93 mg/g), accounting for 13.99% and 12.58% of FAAs in eggs, respectively. Leucine content dropped significantly to 1.80 mg/g in 4-DAH larvae and then decreased gradually to 0.42 mg/g in 24-DAH larvae. In contrast, lysine content decreased gradually to 8.22 mg/g in 4-DAH larvae and then decreased significantly to 4.84 mg/g in 7-DAH larvae, followed by a further gradual decrease to 0.75 mg/g in 24-DAH larvae. Valine, isoleucine, arginine, and threonine were medium-content FEAAs (10.87, 9.89, 8.12, and 7.93 mg/g, respectively), which constituted 9.82%, 8.93%, 7.33%, and 7.16% of total FAAs in eggs. Their contents decreased more than 50% in 4-DAH larvae. The EAAs histidine, phenylalanine, and methionine presented low contents (4.62, 3.57, and 2.74 mg/g) in eggs. Histidine and phenylalanine contents showed a similar decreasing trend and decreased > 50% by 4 DAH, and then decreased gradually until 24 DAH. Methionine content decreased gradually by 4 DAH and then fluctuated slightly thereafter. The FEAA and free non-essential amino acid (FNAA) ratio exhibited a continuously decreasing trend, suggesting that FEAAs were depleted faster than FNAAs.

|
The percentages of histidine, arginine, methionine, phenylalanine, and lysine increased significantly in 4-DAH larvae (P < 0.05) compared with those of newly hatched larvae, whereas threonine, valine, isoleucine, and leucine decreased. After first-feeding (4 DAH), the percentages of histidine and methionine increased gradually until the end of the trial, whereas the percentages of arginine, valine, phenylalanine, isoleucine, leucine, and lysine followed the opposite trend.
3.4 Changes in whole-fish protein amino acid contents during larval developmentChanges in whole-fish PAA composition were much milder than those observed for FAAs (Table 3). Histidine constituted only 0.85% of dry material (DM), which was the lowest among protein essential amino acids (PEAA) in eggs but increased to 2.87% in 24-DAH larvae. Lysine dominated the PEAAs in eggs (7.76% of DM) but decreased to 4.52% in 24-DAH larvae. Threonine, arginine, valine, isoleucine, and leucine contributed to 1.40%–2.98% of DM in larvae and increased to 3.62%–4.87% of DM in 24-DAH larvae. Changes in phenylalanine and methionine occurred in two phases: their contents were constant until 4 DAH. Then, methionine increased significantly and phenylalanine decreased. PEAAs, protein non-essential amino acids (PNAAs), and total PAAs increased gradually during larval development. However, the PEAA/PNAA ratio increased to a peak of 1.09 in 7-DAH larvae and then decreased gradually. The percentages of histidine, threonine, valine, isoleucine, and leucine increased during larval development, whereas those of phenylalanine and lysine decreased.
|
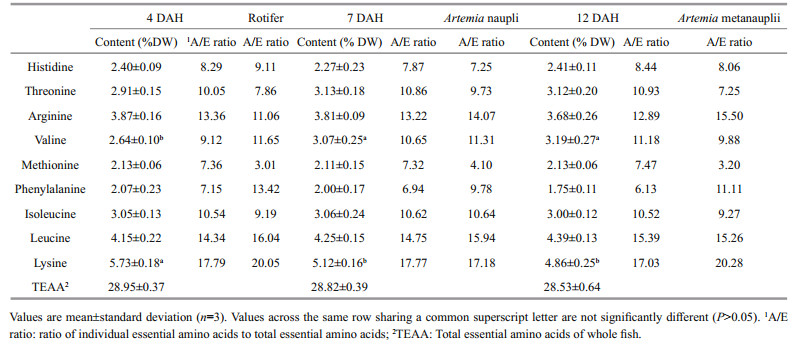
No significant differences in EAA composition or the A/E ratio were detected for histidine, threonine, isoleucine, leucine, arginine, methionine, or phenylalanine in 4-, 7-, or 12-DAH larvae (P > 0.05) (Table 4). Valine content increased significantly in 7-and 12-DAH larvae, whereas lysine content decreased (P < 0.05). The correlation between live food EAA content and larvae was low (Fig. 3), suggesting that dietary AA deficiencies occurred during the external feeding period. The comparison of the A/E ratio between live food and larvae indicated that rotifers were deficient in methionine, threonine, arginine, and isoleucine in 4-DAH larvae (Fig. 3a), suggesting that 59.14%, 21.83%, 17.28%, and 12.80% of AA intake was lost. Methionine and threonine deficiencies in Artemia nauplii caused estimated 53.60% and 27.02% AA losses in 7-DAH larvae (Fig. 3b). Methionine, threonine, isoleucine, and valine deficiencies were found in Artemia metanauplii when they were fed to 12-DAH larvae (Fig. 3c). As a result, the estimated maximum losses of ingested AAs were 57.12%, 24.53%, 11.84%, and 11.66%.
|
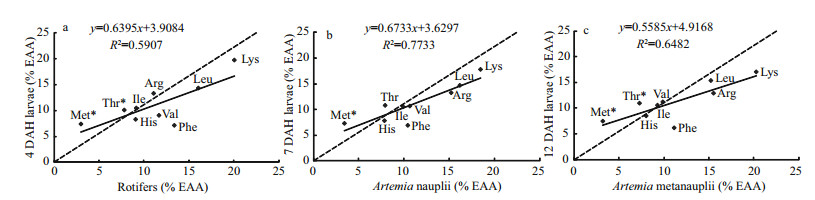
|
| Figure 3 Comparison of the ratio of individual essential amino acids to total essential amino acids (A/E ratio) in 4-, 7-, and 12-DAH larvae and their main live food, suggesting potential amino acid deficiencies (AA*) in live food Solid lines are linear regression curves. Points above the line with slope of 1.11 and intercept of 0 (dashed line: y=1.11x) suggest AA deficiencies in live food because the A/E ratios were at least 10% lower than those for the fish. |
Growth of starry flounder larvae was satisfactory and somewhat faster than that reported by Yamashita et al. (2014). They reported that starry flounder larvae had a total body length of 5.92±0.28 mm at 16–32 DAH and 6.43±0.25 mm at 23–32 DAH. However, we report values here of 9.23±0.66 mm at 17 DAH and 12.13±0.99 mm at 24 DAH, which agrees with Liu et al. (2008). The faster growth in these studies may have been caused by higher temperatures and a different larval feeding regime than those in the previous study. We observed a gradual decrease in larval LAGR beginning 7 DAH until the end of the experiment. The reduction in LAGR may have been caused by bending of the spine during the premetamorphic stage (Liu et al., 2008).
4.2 Changes in digestive enzyme activities during larval developmentPepsin activity was detected from hatching to 7 DAH and then became more apparent from 17 to 24 DAH. The timing of the appearance of pepsin activity may be associated with the development of the gastric glands (Fang and Wang, 2011), as observed in other fish species (Cara et al., 2003; Alvarez-González et al., 2008; Zamani et al., 2009).
Trypsin and chymotrypsin were detected before mouth opening in the present study, suggesting the presence of the pancreas. In our study, these enzymes increased sharply at the time of mouth opening (4 DAH), which would contribute to the establishment of a feeding strategy, particularly rotifers. The presence of these two enzymes and increased activities during early larval development may compensate for the absence of pepsin and facilitate protein digestion (Applebaum et al., 2001).
Lipase is produced mainly in the pancreas and catalyzes the breakdown of triacylglycerol to diacylglycerol and monoacylglycerol (Infante and Cahu, 1994). Several lipases have been found in fish larvae at different developmental stages, and a nonspecific lipase is predominant at hatching (Oozeki and Bailey, 1995; Murray et al., 2003). Bile saltactivated lipase, which develops later in fish larvae and is related to differentiation of the pyloric caeca, is responsible for digesting exogenous lipids (Sæle et al., 2010). In the present study, lipase was detected at hatching, and its activity increased significantly 4 DAH. The increased lipase activity may occur in response to the transition from yolk lipid to exogenous lipids, as rotifers were introduced at 4 DAH in this study. Bile salt-activated lipase may be responsible for the increased lipase activity detected at 4 DAH, which indicates the beginning of pyloric caeca differentiation.
Amylase activity initially increased and the decreased gradually in the present study, suggesting that amylase is synthesized during early larval stages, even in the absence of food, and that carbohydrates are actively catalyzed during organogenesis. This change suggests a natural predisposition of young larvae to use carbohydrate (Krogdahl and Sunby, 1999). Several authors have reported that amylase expression is regulated at the transcriptional level and that the low mRNA level found in older larvae results in a decline in amylase activity (Douglas et al., 2000; Ma et al., 2001). The change in nutrient source from yolk (internal glycogen) to an external diet (protein) may be responsible for this pattern.
4.3 Changes in the larval amino acid profileOur results show that the FAA pool and FEAA/ FNAA ratio decreased considerably in young larvae, and 60.90% of the initial FAAs were depleted at first feeding. Then, a continual decrease was detected after exogenous feeding commenced. At the same time, the PAA pool increased continuously during morphogenesis, and larval EAA composition remained almost unchanged. The decrease in FAAs and increase in PAAs indicates that morphogenesis largely converts soluble matter into insoluble matter, but that body composition does not change greatly despite the morphological development after feeding. Several marine fish larvae favor using NAAs rather than EAAs as energy substrates, indicating that they have the ability to control AA metabolism (Conceição et al., 2001, 2003; Rønnestad2001 et al., 2001; Portella et al., 2013). However, the continuous decrease in the FEAA/FNAA ratio in our study indicates that more FEAAs were depleted than FNAAs. We speculate that the larger depletion of FEAAs in starry flounder larvae was associated with their roles as fuel and selective utilization for organogenesis. This speculation is based on the decreasing trend in FEAA contents accompanied by increasing PEAA contents. In addition, PEAAs increased more (16.42%) than PNAAs (9.14%) in 4-DAH larvae compared with the eggs, indicating that young larvae prefer retaining EAAs rather than NAAs. However, the larvae retained fewer EAAs relatively to NAAs after 7 DAH, which lead to a gradual decrease in the EAA/NAA ratio. These combined FAA and PAA data suggest that starry flounder larvae have a high EAA requirement, and that they regulate retention of EAAs according to developmental stage.
Free leucine, isoleucine, valine, and threonine contents decreased sharply before first feeding, which may indicate a special requirement of starry flounder larvae. Yolk-sac larvae prioritized use of threonine, valine, isoleucine, and leucine, resulting in a gradual reduction in the percentage of FAAs and a corresponding increase in the PAA pool. This result suggests that yolk-sac larvae converted most of the threonine, valine, isoleucine, and leucine from FAAs to PAAs. High conversion rates of these four AAs were also found in post-larvae and was reflected as a percent increase in the PAA pool. Selective retention of some proteins as an energy source may change the whole-fish PAA profile, indicating high bioavailability. High relative bioavailabilities of isoleucine, leucine, and valine are also found in Diplodus puntazzo larvae, as these AAs are retained more efficiently than others (Saavedra et al., 2007). However, Conceição et al. (2003) concluded that threonine has low relative bioavailability and is retained less efficiently by seabream larvae compared with other AAs, whereas most lysine is retained. Thus, these findings suggest that specific individual AA requirements vary depending on the species.
4.4 Estimated possible dietary AA imbalance based on differences in the AA profiles between live food and larvaeThe whole-body EAA profile better reflects fish AA requirements compared with the EAA profiles of different tissues (Mambrini and Kaushik, 1995). A good correlation between the AA requirement and whole-fish body A/E ratio was reported by Wilson and Poe(1985). We found a poor association between the A/E ratios of live food and whole fish, suggesting that a dietary AA imbalance occurred during starry flounder development under culture conditions. Rotifers and Artemia contain methionine at trace or low levels, regardless of enrichment with phytoplankton, yeast, or DHA products (James et al., 1987; Naz, 2008; Jeeja et al., 2011). Therefore, methionine is regarded as the first limiting essential AA for most fish larvae (Helland et al., 2003; Aragão et al., 2004). Our results support this conclusion and indicate that methionine may also be the first limiting AA for starry flounder larvae fed rotifers and Artemia. Previous studies have demonstrated that a dietary AA imbalance may be species dependent and change during larval development (Conceição et al., 1997; Aragão et al., 2004; Saavedra et al., 2006). In the present study, the whole-fish A/E ratios of histidine, threonine, isoleucine, leucine, and valine increased gradually, suggesting high post-larval requirements for these AAs. As a result, threonine, isoleucine, and valine deficiencies were detected in larvae after 12 DAH.
Starry flounder larvae selectively utilized nutrients at different stages based on changes in growth rate, AA profiles, and digestive enzyme activities. Abundant FAAs played an important role in yolk-sac larvae and may be responsible for their rapid growth. Exogenous FAAs were lacking in live food and may have been insufficient to compensate for the FAAs consumed for energy and protein synthesis, resulting in a reduction in the FAA pool. However, the increased digestive enzyme activities illustrated the increasing importance of exogenous nutrients, particularly carbohydrates and lipids. This result suggests that post-larvae used carbohydrates and lipids as metabolic fuel to spare protein or FAAs.
The changing patterns of FAAs and PAAs in larvae also reflected their AA requirements and provide a strategy for enriching live food and artificial feed. Starry flounder larvae must be supplied with a natural diet rich in FAAs with a suitable EAA and DAA ratio that mimics a balanced AA profile to support the high larval growth rate. This may be important to ensure a successful transition from endogenous to exogenous feeding by flatfish larvae. Many efforts have been directed at manipulating dietary AA profiles with some success. Tonheim et al. (2000) enriched Artemia nauplii directly with free methionine using a liposome technique. Supplementing potentially limiting AAs (leucine and phenylalanine) has improved AA retention, growth, and nitrogen utilization of Senegalese sole, Solea senegalensis (Aragão et al., 2004). In addition, starry flounder developed a progressively enhanced digestive system after firstfeeding, indicating the feasibility of introducing an artificial compound feed with appropriate protein, carbohydrate, and lipid contents.
| Alvarez-González C A, Moyano-López F J, Civera-Cerecedo R, Carrasco-Chávez V, Ortiz-Galindo J L, Dumas S, 2008. Development of digestive enzyme activity in larvae of spotted sand bass Paralabrax maculatofasciatus1.Biochemical analysis. Fish Physiol. Biochem., 34 (4) : 373 –384. Doi: 10.1007/s10695-007-9197-7 |
| Applebaum S L, Perez R, Lazo J P, Holt G J, 2001. Characterization of chymotrypsin activity during early ontogeny of larval red drum (Sciaenops ocellatus). Fish Physiol. Biochem., 25 (4) : 291 –300. Doi: 10.1023/A:1023202219919 |
| Aragão C, Conceição L E C, Martins D, et al, 2004. A balanced dietary amino acid profi le improves amino acid retention in post-larval Senegalese sole (Solea senegalensis). Aquaculture, 233 (1-4) : 293 –304. Doi: 10.1016/j.aquaculture.2003.08.007 |
| Aragão C, Conceição L, Fyhn H J, Dinis M T, 2000. Whole body amino acid profi le of Solea senegalensis larvae changes during ontogeny. Comparative Biochemistry and Physiology-Part A:Molecular & Integrative Physiology, 126 (S1) : 6 . |
| Bolasina S, Pérez A, Yamashita Y, 2006. Digestive enzymes activity during ontogenetic development and eff ect of starvation in Japanese flounder, Paralichthys olivaceus. Aquaculture, 252 (2-4) : 503 –515. Doi: 10.1016/j.aquaculture.2005.07.015 |
| Bradford M M, 1976. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal yt. Biochem., 72 (1-2) : 248 –254. Doi: 10.1016/0003-2697(76)90527-3 |
| Brown M R, Battaglene S C, Morehead D T, et al, 2005. Ontogenetic changes in amino acid and vitamins during early larval stages of striped trumpeter (Latris lineata). Aquaculture, 248 : 263 –274. Doi: 10.1016/j.aquaculture.2005.04.018 |
| Cara J B, Moyano F J, Cárdenas S, Fernández-Díaz C, Yúfera M, 2003. Assessment of digestive enzyme activities during larval development of white bream. J. Fish Biol., 63 (1) : 48 –58. Doi: 10.1046/j.1095-8649.2003.00120.x |
| Conceição L E C, Grasdalen H, Rønnestad I, 2003. Amino acid requirements of fi sh larvae and post-larvae:new tools and recent fi ndings. Aquaculture, 227 (1-4) : 221 –232. Doi: 10.1016/S0044-8486(03)00505-2 |
| Conceição L E C, Ozório R O A, Suurd E A, et al, 1998. Amino acid profi les and amino acid utilization in larval African catfi sh (Clarias gariepinus):eff ects of ontogeny and temperature. Fish Physiol. Biochem., 19 (1) : 43 –58. Doi: 10.1023/A:1007738228055 |
| Conceição L E C, Rønnestad I, Tonheim S K, 2001. Metabolic budgets for lysine and glutamate in unfed herring (Clupea harengus) larvae. Aquaculture, 206 (3-4) : 305 –312. |
| Conceição L E C, van der Meeren T, Verreth J A J, et al, 1997. Amino acid metabolism and protein turnover in larval turbot (Scophthalmus maximus) fed natural zooplankton or Artemia. Mar. Biol., 129 (2) : 255 –265. Doi: 10.1007/s002270050166 |
| Dayal J S, Ali S A, Thirunavukkarasu A R, et al, 2003. Nutrient and amino acid profi les of egg and larvae of Asian seabass, Lates calcarifer (Bloch). Fish Physiol. Biochem., 29 (2) : 141 –147. Doi: 10.1023/B:FISH.0000035936.93304.c2 |
| Douglas S E, Mandla S, Gallant J W, 2000. Molecular analysis of the amylase gene and its expression during the development in the winter flounder, Pleuronectes americanus. Aquaculture, 190 (3-4) : 247 –260. Doi: 10.1016/S0044-8486(00)00398-7 |
| Fang H H, Wang B, 2011. Histological studies on the development of digestive system in larval and juvenile starry flounder. Chinese Agricultural Science Bulletin, 27 (14) : 50 –54. |
| Finn R N, Fyhn H J, Henderson R J, et al, 1996. The sequence of catabolic substrate oxidation and enthalpy balance of developing embryos and yolk-sac larvae of turbot(Scophthalmus maximus L). Comp. Biochem. Physiol., 115 (2) : 133 –151. Doi: 10.1016/0300-9629(96)00026-6 |
| Finn R N. 1994. Physiological energetics of developing marine fi sh embryos and larvae. University of Bergen, Bergen, Norway. |
| Helland S, Terjesen B F, Berg L, 2003. Free amino acid and protein content in the planktonic copepod Temora longicornis compared to Artemia franciscana. Aquaculture, 215 (1-4) : 213 –228. Doi: 10.1016/S0044-8486(02)00043-1 |
| Infante J L Z, Cahu C, 1994. Development and response to a diet change of some digestive enzymes in sea bass(Dicentrarchus labrax) larvae. Fish Physiol. Biochem., 12 (5) : 399 –408. Doi: 10.1007/BF00004304 |
| James C, Dias P, Salman A E, 1987. The use of marine yeast(Candida sp) and bakers' yeast (Saccharomyces cerevisiae) in combination with Chlorella sp. for mass culture of the rotifer Brachionus pliatilis. Hydrobiologia, 147 : 263 –268. Doi: 10.1007/BF00025752 |
| Jeeja P K, Joseph I, Raj R P, 2011. Nutritional composition of rotifer (Brachionus plicatilis Müller) cultured using selected natural diets. Indian J. Fish., 58 (2) : 59 –65. |
| Kamaci H O, Suzer C, Coban D, Firat K, Saka S, 2009. Organogenesis and enzymatic functionality of exocrine pancreas in cultured Gilthead Sea Bream (Sparus aurata)Larvae. J. Anim. Vet. Adv., 8 (12) : 2 –2484. |
| Krautz M C, Vásquez S, Castro L R, et al, 2010. Changes in metabolic substrates during early development in anchoveta Engraulis ringens (Jenyns 1842) in the Humboldt Current. Mar. Biol., 157 (5) : 1137 –1149. Doi: 10.1007/s00227-010-1395-7 |
| Krogdahl Å, Sundby A. 1999. Characteristics of pancreatic function in fi sh. In:Pierzynowski S G, Zabielski R eds.Biology of the pancreas in growing animals. Elsevier Science, Amsterdam. p.437-458. |
| Lazo J P, Holt G J, Arnold C R, 2000. Ontogeny of pancreatic enzymes in larval red drum Sciaenops ocellatus. Aquac.Nutr., 6 (3) : 183 –192. Doi: 10.1046/j.1365-2095.2000.00124.x |
| Liu Z H, Wang B, Yao Z G, Sun P X, Liu P, Wang Z L, 2008. Morphological development and growth of larval and juvenile fi sh of starry fl ounder, Platichthys stellatus. Advances in Marine Science, 26 (1) : 90 –97. |
| Ma P, Sivaloganathan B, Reddy P K, Chan W K, Lam T J, 2001. Ontogeny of α-amylase gene expression in sea bass larvae (Lates calcarifer). Mar. Biotechnol., 3 (5) : 463 –469. Doi: 10.1007/s10126-001-0018-1 |
| Mambrini M, Kaushik S J, 1995. Indispensable amino acid requirements of fi sh:correspondence between quantitative data and amino acid profi les of tissue proteins. J. Appl.Ichthyol., 11 (3-4) : 240 –247. Doi: 10.1111/jai.1995.11.issue-3-4 |
| Murray H M, Gallant J W, Perez-Casanova J C, Johnson S C, Douglas S E, 2003. Ontogeny of lipase expression in winter fl ounder. J. Fish Bio l., 62 (4) : 816 –833. Doi: 10.1046/j.1095-8649.2003.00067.x |
| Naz M, 2008. The changes in the biochemical compositions and enzymatic activities of rotifer (Brachionus plicatilis, Müller) and Artemia during the enrichment and starvation periods. Fish Physiol. Biochem., 34 (4) : 391 –404. Doi: 10.1007/s10695-007-9199-5 |
| Oozeki Y, Bailey K M, 1995. Ontogenetic development of digestive enzyme activities in larval walleye pollock, Theragra chalcogramma. Mar. Biol., 122 (2) : 177 –186. |
| Portella M C, Takata R, Leitão N J, et al, 2013. Free amino acids in Pacu, Piaractus mesopotamicus, eggs and larvae. J. World Aquacult. Soc., 44 (3) : 425 –434. Doi: 10.1111/jwas.2013.44.issue-3 |
| Rønnestad I, Fyhn H J, 1993. Metabolic aspects of free amino acids in developing marine fi sh eggs and larvae. Rev.Fish. Sci., 1 (3) : 239 –259. Doi: 10.1080/10641269309388544 |
| Rønnestad I, Rojas-Garcıa C R, Tonheim S K, et al, 2001. In vivo studies of digestion and nutrient assimilation in marine fi sh larvae. Aquaculture, 201 (1-2) : 161 –175. Doi: 10.1016/S0044-8486(01)00595-6 |
| Saavedra M, Beltran M, Pousão-Ferreira P, Dinis M T, Blasco J, Conceição L E C, 2007. Evaluation of bioavailability of individual amino acids in Diplodus puntazzo larvae:towards the ideal dietary amino acid profi le. Aquaculture, 263 (1-4) : 192 –198. Doi: 10.1016/j.aquaculture.2006.10.027 |
| Saavedra M, Conceição L E C, Pousão-Ferreira P, Dinis M T, 2006. Amino acid profi les of Diplodus sargus (L., 1758)larvae:implications for feed formulation. Aquaculture, 1758 (2) : 587 –593. |
| Sæle Ø, Nordgreen A, Olsvik P A, Hamre K, 2010. Characterization and expression of digestive neutral lipases during ontogeny of Atlantic cod (Gadus morhua). Comp. Biochem. Physiol. A. Mol. Integr. Physiol., 157 (3) : 252 –259. Doi: 10.1016/j.cbpa.2010.07.003 |
| Skjærven K H, Finn R N, Kryvi H et al. 2003. Yolk resorption in developing plaice (Pleuronectes platessa). In:Browman H I, Skiftesvik A B eds. The Big Fish Bang. Proceedings of the 26th Annual Larval Fish Conference. Institute of Marine Research, Bergen, Norway. ISBN 82-7461-059-8. |
| Thorsen A, Kjesbu O S, Fyhndr H J, et al, 1996. Physiological mechanisms of buoyancy in eggs from brackish water cod. J. Fish Biol., 48 (3) : 457 –477. Doi: 10.1111/jfb.1996.48.issue-3 |
| Tonheim S K, Koven W, Rønnestad I, 2000. Enrichment of Artemia with free methionine. Aquaculture, 190 (3-4) : 223 –235. Doi: 10.1016/S0044-8486(00)00402-6 |
| Tulli F, Tibaldi E, 1997. Changes in amino acids and essential fatty acids during early larval rearing of dentex. Aquacult.Int., 5 (3) : 229 –236. Doi: 10.1023/A:1018339401963 |
| Welch A, Hoenig R, Stieglitz J, et al, 2013. Growth rates of larval and juvenile bigeye scad Selar crumenophthalmus in captivity. Springer Plus, 2 : 634 . Doi: 10.1186/2193-1801-2-634 |
| Weltzien F A, Planas M, Cunha I, et al, 1999. Free amino acid and protein contents of start-feeding larvae of turbot(Scophthalmus maximus) at three temperatures. Mar.Biol., 133 (2) : 327 –336. Doi: 10.1007/s002270050471 |
| Wilson R P, Poe W E, 1985. Relationship of whole body and egg essential amino acid patterns to amino acid requirement patterns in channel catfish, Ictalurus punctatus. Comp. Biochem. Physiol. B. Comp. Biochem., 80 (2) : 385 –388. |
| Yamashita Y T, Aritaki M, Kurita Y, Tanaka M, 2014. Early growth and development of reciprocal hybrids of the starry fl ounder Platichthys stellatus and stone flounder Kareius bicoloratus. J. Fish Biol., 84 (5) : 1503 –1518. Doi: 10.1111/jfb.2014.84.issue-5 |
| Zamani A, Hajimoradloo A, Madani R, Farhangi M, 2009. Assessment of digestive enzymes activity during the fry development of the endangered Caspian brown trout Salmo caspius. J. Fish Biol., 75 (4) : 932 –937. Doi: 10.1111/jfb.2009.75.issue-4 |
 2016, Vol. 34
2016, Vol. 34