Institute of Oceanology, Chinese Academy of Sciences
Article Information
- LI Wenjia(李文嘉), LU Xia(卢霞), LUAN Sheng(栾生), LUO Kun(罗坤), SUI Juan(隋娟), KONG Jie(孔杰)
- Heritability of body weight and resistance to ammonia in the Pacific white shrimp Litopenaeus vannamei juveniles
- Chinese Journal of Oceanology and Limnology, 34(5): 1025-1033
- http://dx.doi.org/10.1007/s00343-016-5034-0
Article History
- Received Jan. 27, 2015
- accepted in principle Jan. 6, 2015
- accepted for publication Jul. 18, 2015
The Pacific white shrimp, Litopenaeus vannamei, whose natural distribution is along the Pacific coast of the western American continent from Mexico to Peru, has been introduced into the Atlantic coast from USA to Brazil and into different Asian countries (Funge-Smith and Briggs, 2005). It is most widely cultured in many parts of the world, and its rapid growth, good survival in high-density culture and disease tolerance make it a good choice for intensive and/or biosecure closed grow-out strategies (Cuzon et al., 2004). However, these culture practices have resulted in the degradation of the culture water as well as a high frequency of diseases in recent years, which seriously affect output and quality of L. vannamei (Armstrong, 1979; Colt and Armstrong, 1981; Heath, 1995; Foss et al., 2003; Cuzon et al., 2004; Alagappan et al., 2010).
One of the limiting factors in culture systems is the toxicity of nitrogen waste products, such as ammonia and nitrites (Losordo, 1991) generated by the excretion and ammonification of unconsumed feed, organic wastes and sediments (Wright 1995; Zhao et al., 1997). The situation is exacerbated in closed and intensive shrimp farming operations (Chen et al., 1986, 1988; Chen and Kou, 1993): ammonia concentrations in the water increase, ammonia excretion by aquatic organisms diminishes, levels of ammonia in the blood and other tissues increase (Colt and Armstrong, 1981) and the metabolic pattern also changes and further affects growth, central nervous system function, immune response, ionic balance, energy metabolism, molting and survival (Heath, 1995; Wicks et al., 2002; Foss et al., 2003; Junkunlo et al., 2012). There are many published results concerning the toxic effect of ammonia in fish, mollusks and crustaceans (Johnson, 1985; Lewis and Morris, 1986; Chen et al., 1990a, b; Chen and Lin, 1992a, b; Noor-Hamid et al., 1994), and several studies have been carried out to determine the toxicity levels of ammonia in different life stages of penaeid shrimp, such as Penaeus monodon, Marsupenaeus japonicus, Fenneropenaeus chinensis, Litopenaeus setiferus and L. vannamei (Chin and Chen, 1987; Chen and Lin, 1991a,b; Lin et al., 1993; Alcaraz et al., 1999; Lin and Chen, 2001; Magallón-Barajas et al., 2006), but so far, not on genetic parameters.
Genetic improvement programs can increase the economic efficiency of farmed shrimp (Gjedrem, 2005; Cock et al., 2009). Several selective breeding programs are being developed in L. vannamei, and both ANOVA (analysis of variance) and restricted maximum likelihood (REML) methods have been used to estimate the heritability for growth-related, survival, reproduction, resistance to Taura syndrome virus (TSV), resistance to white spot syndrome virus (WSSV) and resistance to hypoxia traits in them (Goyard et al., 1999; Argue et al., 2002; Pérez-Rostro and Ibarra, 2003a, b; Gitterle et al., 2005a, b; Castillo-Juárez et al., 2007; Ibarra et al., 2007; Ibarra et al., 2009; Luan et al., 2012). Ammonia, toxic to aquaculture organisms, represents a potential problem in aquaculture systems; assessing the potential for the genetic improvement of resistance to ammonia in L. vannamei requires knowledge of the genetic parameters of this trait. Thus we present here an analysis of the genetic parameters for resistance to ammonia of L. vannamei juveniles. Our goal was to estimate the heritability of resistance to ammonia in the Pacific white shrimp (L. vannamei) and to reveal the correlation between resistance to ammonia and body weight in them.
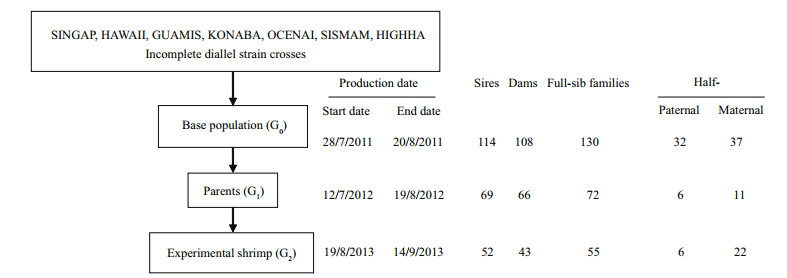
2 MATERIAL AND METHOD 2.1 Historical background and shrimp productionAll experimental procedures were conducted at the Mariculture Research Station of Yellow Sea Fisheries Research Institute, Chinese Academy of Fishery Sciences, located in the suburbs of Qingdao (latitude 36°20′32.22″N, longitude120°39′1.93″E, altitude 3.04 m), Shandong province, China. The base population (G0) was derived from seven commercial L. vannamei strains using an incomplete diallel cross experiment in 2011. The seven strains were separately introduced from Shrimp Improvement System Pte. Ltd (SINGAP), Shrimp Improvement System Hawaii LLC (HAWAII), University of Guam, CNAS (GUAMIS), Kona Bay Marine Resources, Waimea Aquatic Lab (KONABA), The Oceanic Institute (OCENAI), Shrimp Improvement System Florida (SISMAM) and High Health Aquaculture Inc (HIGHHA) in June 2011. Their origins were Singap, Oahu, Guam, Kauai, Oahu, Miami and Hawaii, respectively. The pedigrees of individuals in the seven strains were unknown. During subsequent generations (G1 to G2), the selection population was closed, and generations were discrete. G1 to G2 generations were produced using a full-sib/half-sib mating design. In 2012, 69 sires and 66 dams from the base population (G0) were used to produce parental shrimps (G1), which contained 72 full-sib families. A total of 55 full-sib families (consisted of 28 half-sib families) were produced as experimental shrimp (G2) from 52 sires and 43 dams of G1. There was an age difference of 24, 39 and 27 days for G0, G1 and G2 generation respectively, which was caused by the different mating and spawning time. The pedigree of all the individuals was known and was used to construct a relationship matrix. The number of sires, dams, fullsib families, half-sib families and the shrimp production scheme is shown in Fig. 1.
|
| Figure 1 Schematic presentation of production of experimental shrimp from seven commercial L. vannamei strains from Singapore and the United States Abbreviations: SINGAP: strain form Shrimp Improvement System Pte. Ltd; HAWAII: strain form Shrimp Improvement System Hawaii LLC; GUAMIS: strain form University of Guam, CNAS; KONABA: strain form Kona Bay Marine Resources, Waimea Aquatic Lab; OCENAI: strain form OCENAI The Oceanic Institute; SISMAM: strain form Shrimp Improvement System Florida; HIGHHA: strain form High Health Aquaculture Inc. |
Each breeding candidate was reared separately in a small net cage (0.25 m × 0.25 m × 0.25 m) labeled with a four digit identity code. Breeding candidates could then be individually identified using the cage ID. Female and male parents were carefully chosen to maximize mating success. Females with orange colored ovaries that occupied a large area of the cephalothorax were preferred. Males with a healthy appearance and filled spermatophore were obtained for mating with the sexually receptive females. Each full-sib group of fertilized eggs were hatched in separate tanks. After about one day, random samples of approximately 3 000 nauplii from each full-sib family were stocked in separate larvae-culture tanks. Hatched nauplius larvae passed through six nauplius stages, three zoea stages, three mysis stages and metamorphosis to post-larva in approximately ten days. Random samples of approximately 200 postlarvae from each family were transferred into larger tanks (3 m3) for separate rearing until the last family reached tagging size (with mean body weight of approximately 3 g, post-larval ages of 73–95 days). Then, the shrimps from each family were tagged using a visible implant elastomer, and each family received a unique tag code.
2.2 Challenge with high ammonia levelAfter a period of one week of temporary rearing to recover from tagging stress, 36 tagged individuals per family (totally 52 full-sib families) were assigned equally and randomly to four 3 m2 concrete tanks. Then, the shrimps were exposed to high ammonia concentration of 62.23 mg/L, that was the half lethal concentration for approximately 120 h according to our simple pre-experiment. An ammonia stock solution was prepared by adding NH4Cl (Aldrich, Milwaukee, WI, USA). The water temperature was kept at 24±1℃ during the experiment, and dissolved oxygen (DO), pH, and total ammonia were measured once a day, using a WTWR multi 340i meter and HACH kits. Dissolved oxygen was maintained no less than 5 mg/L; pH ranged between 7.9–8.3; ammonia ranged between 61.33–63.33 mg/L. Tanks were cleaned daily by suction to remove feces. Water that was removed during cleaning was replaced with clean water with the same concentration of NH4Cl. Tanks were observed once each hour for any shrimp mortality. Death was defined as the point at which shrimp lost balance, fell on their side, ceased body movements and lost their response to external stimulation. Throughout the experiment, dead shrimp were removed from the tanks at the beginning of each hour with a scoop net, and their tag, body weight (BW) and death time were recorded, which enabled us to quantify the survival time (ST) and survival status (1=alive, 0=dead) at the half lethal time (SS50) for each individual. Shrimp were fed feedstuff (Haid Dachuan #1, Guangdong Haid Group Co., Ltd.) four times a day according to their nutritional needs until the first death occurred.
2.3 Data analysisAn analysis of the descriptive statistics was conducted using the MEANS procedure (SAS Institute Inc., 2005). A complete pedigree in this breeding program from G0 was included in the below analysis. The common environment effect was omitted in the present analysis because of the short period of communal rearing.
The estimation of heritability for BW was performed using the ASReml package (Gilmour et al., 2009). The individual animal model was used to estimate the genetic parameters for BW of the shrimps challenged with high concentration ammonia. Age at the end of the experiment showed a linear relationship with body weight, and was fitted as a covariate in the model. All the fixed effects and the covariate were statistically significant (P < 0.01). The fitted model was:
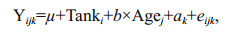 (Model 1)
(Model 1)where Yijk is the observed BW of the ak individual; μ is the overall mean; Tanki is the fixed effect of ith tank; b is the regression coefficient; Agej is the covariate of the jth individual age when the experiment ended; ak is the random additive genetic effect of the kth individual and eijk is the random residual effect associated with observation ijk. The phenotypic variance (σp2) was the sum of all variance components: σp2=σa2+σe2. The heritability was calculated as the ratio between animal genetic variance and the total phenotypic variance: (h2=σa2/σp2).
The resistance to ammonia was expressed as ST and SS50 in the present study. The individual animal model was used to estimate genetic parameters for ST of the shrimps challenged with high concentration ammonia. Body weight showed a linear relationship with ST (P < 0.01), and was fitted as a covariate in the model. The fixed effect of tank was also statistically significant (P < 0.01). The fitted model was:
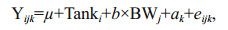 (Model 2)
(Model 2)where Yijk is the observed ST of the kth individual; μ is the overall mean; Tanki is the fixed effect of ith tank; BWj is the covariate of the jth body weight; b is the regression coefficient; ak is the random additive genetic effect of the kth individual and eijk is the random residual effect associated with observation ijk. The phenotypic variance (σp2) was the sum of all variance components: σp2=σa2+σe2. The heritability was calculated as the ratio between animal genetic variance and the total phenotypic variance (h2=σa2/σp2).
A standard threshold (probit) and a sire-dam model were used in this study to estimate the heritability for SS50. The model was written as in ASReml (Gilmour et al., 2009):
 (Model 3)
(Model 3)
where yijklm is the survival status (1=alive, 0=dead) of the mth shrimp; λijklm is the underlying liability of yijklm, assumed to be a cumulative standard normal distribution; μ is the overall mean; Tanki is the fixed effect of the ith test tank; BWj is the covariate of the jth body weight; b is the regression coefficient; Sirek and Dami are the additive genetic effects of the kth sire and the h2 dam, Sire or Dam~(0, Aσsd2) (σsd2=σs2=σd2), where A is the additive genetic relationship matrix among all shrimp and eijklm is the random residual error of the mth individual, with e (ô, Iσe2. The residual variance of λ was assumed to be 1. The phenotypic variance was the sum of 2σsd2 and σe2: σp2=σsd2+σe2. Heritability (h2) was computed as the ratio between 4σsd2 and σp2 (h2=4σsd2/σp2). The estimates then were adjusted according to Robertson and Lerner (1949), as they were overestimated in this model.
The phenotypic and genetic correlations between BW and ST were estimated using bivariate animal model with ASReml package (Gilmour et al., 2009). The bivariate animal model is the extension of Model 1 and Model 2, and the description for each term in the models was same as that for Model 1 and Model 2. The phenotypic and genetic correlations of BW/ SS50 and ST/ SS50 were calculated using bivariate analysis of ASReml package (Gilmour et al., 2009). The SS50 was set as the first variable, which has the same model as the univariate Model 3. BW and ST were set as the second variable, respectively, with the same fixed effects and covariate of Model 1 and Model 2. The description for each term of the models was same as that for Model 1, Model 2 and Mode1 3.
The Z-score was used for testing whether the heritability estimates were significantly different from each other and whether the heritability estimates and genetic correlations were significantly different from zero (Nguyen et al., 2007).
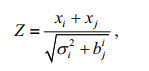
where xi and xj are the heritability estimates from different models or genetic correlations; σi2 and σj2 are their respective standard errors. Both xj and σj were set to be zero when testing whether an estimate was significantly different from zero. The resulting z-score was then tested against a large sample normal distribution.
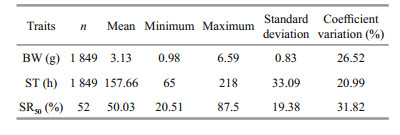
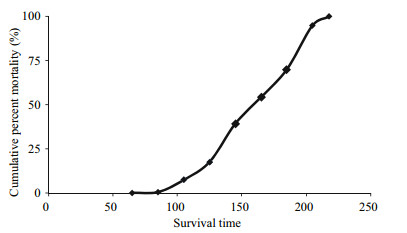
3 RESULT 3.1 Descriptive statisticsA total of 1 849 individuals’ records were obtained and analyzed in the present study, and 23 individuals were lost, which might be the result of cannibalism during the experiment process. The numbers of the samples, simple means, minimum and maximum, standard deviation and coefficient of variation for BW, ST and survival rate for each family at half lethal time (SR50) are given in Table 1. The means of BW, ST and SR50 were 3.13 g, 157.66 h and 50.03%, respectively. The SR50 of each family at half lethal time had the highest coefficient of variation, followed by the BW and lowest in the ST. During the high ammonia level challenge, mortality started gradually at 65 h, but increased dramatically at approximately 90 h and complete mortality occurred at 218 h (Fig. 2).
|
|
| Figure 2 Cumulative mortality of L. vannamei at different times during the high ammonia level challenge |
Estimates of variance components, heritability for BW, ST and SS50 and their standard errors are given in Table 2. The heritabilities of BW, ST and SS50 under high ammonia level challenge were 0.284 6±0.067 6, 0.154 4±0.044 6 and 0.147 5±0.040 0, respectively, and they were all significantly different from zero (P < 0.01). Moreover, the heritabilities of ST and SS50, which indicated the resistance to high ammonia, were not significantly different from each other.
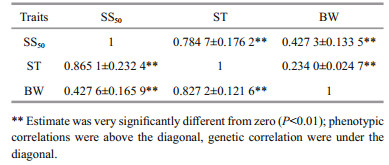
The phenotypic correlation and genetic correlation among BW, ST and SS50 are given in Table 3. There were significant positive phenotypic and genetic correlations between ST and SS50 (0.7847±0.1762 and 0.865 1±0.232 4 respectively, P < 0.01)). A significant positive phenotypic correlation was also shown between BW and resistance to ammonia (0.427 3±0.133 5 and 0.234 0±0.024 7 for BW/SS50 and BW/ST, respectively). The genetic correlations between them were even stronger (0.427 6±0.165 9 for BW/SS50 and 0.827 2±0.121 6 for BW/ST). These results indicated moderate to high positive correlations between the resistance to ammonia and BW.
|
Half lethal condition appeared at approximately 150 h under the ammonia concentrate of 62.23 mg/L in the present study. It showed a fairly higher resistance to ammonia than that of previous studies in L. vannamei and F. chinensis (Sun et al., 2002; He et al., 2008). This might be a result of the different temperature, salinity and post-larval age used in different experiments. It has been proven in many studies that a decrease in temperature and an increase in age and salinity improved the resistance of penaeids to the toxic effects of ammonia, and the effect also depended on different species (Young-Lai et al., 1991; Lin et al., 1993; Frías-Espericueta et al., 1999; Racotta and Hernández-Herrera, 2000; Kir et al., 2004).
Although the biochemical and metabolic response to ammonia toxicity has been studied for shrimp species, differences between individual and population capabilities to survive after toxic ammonia events have not been studied. Estimates of heritabilities for ammonia tolerance of L. vannamei using ST and SS50 were 0.1544±0.0446 and 0.1475±0.0400, respectively. Those are much lower than the values of TSV resistance in this species (0.28±0.14), tolerance to low-oxygen (0.23) and low-pH (0.23) in Larimichthys crocea (Argue et al., 2002; Wang et al., 2009), but is similar to those of cold tolerance (0.08±0.17) in Oreochromis niloticus, upper thermal tolerance (0.026±0.034) in Scophthalmus maximus and WSSV resistance (0.14±0.12) in Fenneropenaeus chinensis (Charo-Karisa et al., 2005; Liu et al., 2011; Kong et al., 2012).The common environment effect was omitted from the generalized linear mixed model in the present study, because the juveniles were with extremely short communal rearing period and lack of gene ties (only 25 half-sib families in 52 full-sib families), which may have contributed to the fact that the common environment variances could not be partitioned effectively (Gitterle et al., 2005a, b; Krishna et al., 2011; Luan et al., 2012). Thus, the heritability estimates might be overestimated with a confounding effect between the additive and nonadditive genetic and maternal effects included in the c2 effect (Pante et al., 2002; Rutten et al., 2005; Castillo-Juárez et al., 2007; Nguyen et al., 2007). Therefore, accurate estimates of heritability for ammonia might be lower than the results obtained in the present study and a more precise estimate should be obtained to enhance the accuracy of breeding.
Resistance to ammonia as a trait was described in two ways in the present study: ST and SS50. The estimates obtained using ST and SS50 were basically the same, and the Z-test also showed that they were not significantly different from each other. There were also high positive phenotypic and genetic correlations (nearly equal to 1) between them, indicating a strong relationship between the two descriptors of resistance to ammonia. All of these results suggested that both the ST and SS50 could be used as suitable descriptors of resistance to ammonia. More research is needed to confirm this conclusion, and once it is confirmed, SS50 may have widespread applications in select breeding programs of tolerance or resistance traits, as this would not only save resources and improve work efficiency but also keep the individuals with high tolerance or resistance alive so that they can be used as parent shrimps.
The effect of size on tolerance traits has been reported in this species. It showed a negative correlation between TSV resistance, WSSV resistance and the body weight of L. vannamei in early studies (Argue et al., 2002; Gitterle et al., 2005a, b). However, there showed a strong positive correlation between resistance to ammonia and body weight in our research, which is consistent with the result that resistance to ammonia increases with ontogenetic development (Barajas et al., 2006). This means that resistance to ammonia can be enhanced by the improvement of husbandry practices that increase body weight. The results from the present study suggest that the selection for higher body weight does not have any negative consequences for resistance to ammonia.
In addition to quantitative genetics, tools from molecular genetics, such as gene clone and RNAsequencing, which can help us to find the genes that play crucial roles in ammonia tolerance, can be applied to selective breeding programs to improve the efficiency of selection for traits with low heritability (Moore et al., 1999). To date, several genes might play crucial roles in stress tolerance, for example, heat shock proteins genes (HSPs) (Parsell and Lindquist, 1993), superoxide dismutase (SOD) (Lynch and Kuramitsu, 2000), metallothionein (MT) (Coyle et al., 2002) and cytochrome P450 (Anzenbacher and Anzenbacherová, 2001) and Aquaporin (AQP) (Peaydee et al., 2014) have been studied and some of them were proved can help shrimps to resist ammonia stress. With the development of RNA-sequencing, more and more ammonia resistant related genes and molecular markers will be identified and applied to shrimps selective breeding programs of improving the resistance to ammonia.
5 CONCLUSIONOverall, the present results indicate that the heritability estimate for ammonia resistance was low in L. vannamei juveniles, but it was significantly different from zero. Consequently, genetic gain for ammonia resistance still could be obtained in future by increasing the selection intensity in our selective breeding program. In addition, it has strongly positive correlation between ammonia resistance and body weight, suggesting that ammonia resistance and body weight could be improved simultaneously in our breeding program. Moreover, heritability estimates for ammonia resistance at different growth stages and genetic correlation with other economic traits should be investigated in the future studies.
| Alagappan K M, Deivasigamani B, Somasundaram S T, Kumaran S, 2010. Occurrence of Vibrio parahaemolyticus and its specific phages from shrimp ponds in east coast of India. Current Microbiology, 61 (4) : 235 –240. Doi: 10.1007/s00284-010-9599-0 |
| Alcaraz G, Chiappa-Carrara X, Espinoza V, Vanegas C, 1999. Acute toxicity of ammonia and nitrite to white shrimp Penaeus setiferus postlarvae. Journal of the World Aquaculture Society, 30 (1) : 90 –97. Doi: 10.1111/jwas.1999.30.issue-1 |
| Anzenbacher P, Anzenbacherová E, 2001. Cytochromes P450 and metabolism of xenobiotics. Cellular and Molecular Life Sciences, 58 (5-6) : 737 –747. |
| Argue B J, Arce S M, Lotz J M, Moss S M, 2002. Selective breeding of Pacific white shrimp (Litopenaeus vannamei)for growth and resistance to Taura syndrome Virus. Aquaculture, 204 (3-4) : 447 –460. Doi: 10.1016/S0044-8486(01)00830-4 |
| Armstrong D A. 1979. Nitrogen toxicity to crustacea and aspects of its dynamics in culture systems. In:Lewis D, Liang J eds. Proceedings of the 2nd Biennial Crustacean Health Workshop. 2nd edn. Texas A and M University Sea Grant Publication, Texas. p.329-360. |
| Barajas F J M, Villegas R S, Clark G P, Moreno B L, 2006. Litopenaeus vannamei (Boone) post-larval survival related to age, temperature, pH and ammonium concentration. Aquaculture Research, 37 (5) : 492 –499. Doi: 10.1111/are.2006.37.issue-5 |
| Castillo-Juárez H, Casares J C Q, Campos-Montes G, Villela C C, Ortega A M, Montaldo H H, 2007. Heritability for body weight at harvest size in the Pacific white shrimp, Penaeus (Litopenaeus) vannamei, from a multienvironment experiment using univariate and multivariate animal models. Aquaculture, 273 (1) : 42 –49. Doi: 10.1016/j.aquaculture.2007.09.023 |
| Charo-Karisa H, Rezk M A, Bovenhuis H, Komen H, 2005. Heritability of cold tolerance in Nile tilapia, Oreochromis niloticus, juveniles. Aquaculture, 249 (1-4) : 115 –123. Doi: 10.1016/j.aquaculture.2005.04.029 |
| Chen J C, Chin T C, Lee C K. 1986. Effects of ammonia and nitrite on larval development of the shrimp (Penaeus monodon). In:Maclean J L, Dizon L B, Hosillos L V eds.The First Asian Fisheries Forum. Asian Fisheries Society, Manila, Phillipines. p.657-662. |
| Chen J C, Kou Y Z, 1993. Accumulation of ammonia in the haemolymph of Penaeus monodon exposed to ambient ammonia. Aquaculture, 109 (2) : 177 –185. Doi: 10.1016/0044-8486(93)90214-J |
| Chen J C, Lin C Y, 1991a. Lethal effects of ammonia on Penaeus chinensis Osbeck juveniles at different salinity levels. Journal of Experimental Marine Biology and Ecology, 156 (1) : 138 –148. |
| Chen J C, Lin C Y, 1991b. Lethal doses of ammonia on Penaeus chinensis larvae. Bulletin of the Institute of Zoology, Academia Sinica, 30 (4) : 289 –298. |
| Chen J C, Lin C Y, 1992a. Effect of ammonia on growth of Penaeus penicillatus juveniles. Comparative Biochemistry and Physiology Part C:Comparative Pharmacology, 101 (3) : 443 –447. Doi: 10.1016/0742-8413(92)90067-H |
| Chen J C, Lin C Y, 1992b. Effects of ammonia on growth and molting of Penaeus monodon juveniles. Comparative Biochemistry and Physiology Part C:Comparative Pharmacology, 101 (3) : 449 –452. Doi: 10.1016/0742-8413(92)90068-I |
| Chen J C, Liu P C, Lei S C, 1990a. Toxicities of ammonia and nitrite to Penaeus monodon adolescents. Aquaculture, 89 (2) : 127 –137. Doi: 10.1016/0044-8486(90)90305-7 |
| Chen J C, Liu P C, Lin Y T, Lee C K, 1988. Super intensive culture of red-tailed shrimp Penaeus penicillatus. Journal of the World Aquaculture Society, 19 (3) : 127 –131. Doi: 10.1111/jwas.1988.19.issue-3 |
| Chen J C, Ting Y Y, Lin J N, Lin M N, 1990b. Lethal effects of ammonia and nitrite on Penaeus chinensis juveniles. Marine Biology, 107 (3) : 427 –431. Doi: 10.1007/BF01313424 |
| Chin T S, Chen J C, 1987. Acute toxicity of ammonia to larvae of the tiger prawn, Penaeus monodon. Aquaculture, 66 (3-4) : 247 –253. Doi: 10.1016/0044-8486(87)90110-4 |
| Cock J, Gitterle T, Salazar M, Rye M, 2009. Breeding for disease resistance of Penaeid shrimps. Aquaculture, 286 (1-2) : 1 –11. Doi: 10.1016/j.aquaculture.2008.09.011 |
| Colt J E, Armstrong D A. 1981. Nitrogen toxicity to crustaceans, fish and molluscs. In:Allen L J, Kinney E C eds.Proceedings of the Bio-Engineering Symposium for Fish Culture. Fish Culture Section, American Fisheries Society.Northeast Society of Conservation Engineers, Bethesda, MD. p.34-47. |
| Coyle P, Philcox J C, Carey L C, Rofe A M, 2002. Metallothionein:the multipurpose protein. Cellular and Molecular Life Sciences, 59 (4) : 627 –647. Doi: 10.1007/s00018-002-8454-2 |
| Cuzon G, Lawrence A, Gaxiola G, Rosas C, Guillaume J, 2004. Nutrition of Litopenaeus vannamei reared in tanks or in ponds. Aquaculture, 235 (1-4) : 513 –551. Doi: 10.1016/j.aquaculture.2003.12.022 |
| Foss A, Vollen T, Øiestad V, 2003. Growth and oxygen consumption in normal and O2 supersaturated water, and interactive effects of O2 saturation and ammonia on growth in spotted wolffish (Anarhichas minor Olafsen). Aquaculture, 224 (1-4) : 105 –116. Doi: 10.1016/S0044-8486(03)00209-6 |
| Frías-Espericueta M G, Harfush-Melendez M, Osuna-López J I, Páez-Osuna F, 1999. Acute toxicity of ammonia to juvenile Shrimp Penaeus vannamei Boone. Bulletin of Environmental Contamination and Toxicology, 62 (5) : 646 –652. Doi: 10.1007/s001289900923 |
| Funge-Smith S, Briggs M. 2005. The introduction of Penaeus vannamei and P. stylirostris into the Asia-Pacific region.In:Bartley D M, Bhujel R C, Funge-Smith S, Olin P G, Phillips M J eds. International Mechanisms for the Control and Responsible use of Alien Species in Aquatic Ecosystems, Report of an Ad Hoc Expert Consultation.Food and Agriculture Organization of the United Nations(FAO), Rome. p.149-167. |
| Gilmour A R, Gogel B J, Cullis B R, Thompson R. 2009.ASReml User Guide Release 3.0. VSN International Ltd, Hemel Hempstead. |
| Gitterle T, Rye M, Salte R, Cock J, Johansen H, Lozano C, Suárez J A, Gjerde B, 2005a. Genetic (co)variation in harvest body weight and survival in Penaeus (Litopenaeus)vannamei under standard commercial conditions. Aquaculture, 243 (1-4) : 83 –92. Doi: 10.1016/j.aquaculture.2004.10.015 |
| Gitterle T, Salte R, Gjerde B, Cock J, Johansen H, Salazar M, Lozano C, Rye M, 2005b. Genetic (co)variation in resistance to White Spot Syndrome Virus (WSSV) and harvest weight in Penaeus (Litopenaeus) vannamei. Aquaculture, 246 (1-4) : 139 –149. Doi: 10.1016/j.aquaculture.2005.02.011 |
| Gjedrem T. 2005. Selection and Breeding Programs in Aquaculture. Springer, The Netherlands. |
| Goyard E, Patrois J, Reignon J M, Vanaa V, Dufour R, Bédier E, 1999. IFREMER's shrimp genetics program. Global Aquaculture Advocate, 2 (6) : 26 –28. |
| He Y Y, Li J, Liu P, Huang F Y, Wang Q Y, 2008. Comparison of the resistance to pH value and ammonia in Chinese shrimp (Fenneropenaeus Chinensis) families. Periodical of Ocean University of China, 38 (5) : 761 –765. |
| Heath A G. 1995. Osmotic and ionic regulation. In:Heath A G.ed. Water Pollution and Fish Physiology. CRC Press, Boca Raton, FL, USA. p.141-170. |
| Ibarra A M, Famula T R, Arcos F G, 2009. Heritability of vitellogenin in hemolymph, a pre-spawning selectable trait in Penaeus (Litopenaeus) vannamei, has a large genetic correlation with ovary maturity measured as oocytes mean diameter. Aquaculture, 297 (1-4) : 64 –69. Doi: 10.1016/j.aquaculture.2009.09.015 |
| Ibarra A M, Pérez-Rostro C I, Ramírez J L, Ortega-Estrada E, 2007. Genetics of the resistance to hypoxia in postlarvae and juveniles of the Pacific white shrimp Penaeus(Litopenaeus) vannamei (Boone 1931). Aquaculture Research, 38 (8) : 838 –846. Doi: 10.1111/are.2007.38.issue-8 |
| Johnson E L. 1985. Ambient water quality for ammonia-1984.EPA 440/5-85-001. Environmental Protection Agency, Duluth, Minnesota, USA. |
| Junkunlo K, Prachumwat A, Tangprasittipap A, Senapin S, Borwornpinyo S, Flegel T W, Sritunyalucksana K, 2012. A novel lectin domain-containing protein (LvCTLD)associated with response of the whiteleg shrimp Penaeus(Litopenaeus) vannamei to yellow head virus (YHV). Developmental & Comparative Immunology, 37 (3-4) : 334 –341. |
| Kir M, Kumlu M, Eroldoğan O T, 2004. Effects of temperature on acute toxicity of ammonia to Penaeus semisulcatus juveniles. Aquaculture, 241 (1-4) : 479 –489. Doi: 10.1016/j.aquaculture.2004.05.003 |
| Kong J, Luo K, Luan S, Wang Q Y, Zhang Q W, Zhang T S, Meng X H, Wang W J, Ruan X H, 2012. The new variety of Fenneropenaeus chinensis "Huanghai No.2". Journal of Fisheries of China, 36 (12) : 1854 –1862. Doi: 10.3724/SP.J.1231.2012.28183 |
| Krishna G, Gopikrishna G, Gopal C, Jahageerdar S, Ravichandran P, Kannappan S, Pillai S M, Paulpandi S, Kiran R P, Saraswati R, Venugopal G, Kumar D, Gitterle T, Lozano C, Rye M, Hayes B, 2011. Genetic parameters for growth and survival in Penaeus monodon cultured in India. Aquaculture, 318 (1-2) : 74 –78. Doi: 10.1016/j.aquaculture.2011.04.028 |
| Lewis Jr W M, Morris D P, 1986. Toxicity of nitrite to fish:a review. Transactions of the American Fisheries Society, 115 (2) : 183 –195. Doi: 10.1577/1548-8659(1986)115<183:TONTF>2.0.CO;2 |
| Lin H P, Thuet P, Trilles J P, Mounet-Guillaume R, Charmantier G, 1993. Effects of ammonia on survival and osmoregulation of various development stages of the shrimp Penaeus japonicus. Marine Biology, 117 (4) : 591 –598. Doi: 10.1007/BF00349770 |
| Lin Y C, Chen J C, 2001. Acute toxicity of ammonia on Litopenaeus vannamei Boone juveniles at different salinity levels. Journal of Experimental Marine Biology and Ecology, 259 (1) : 109 –119. Doi: 10.1016/S0022-0981(01)00227-1 |
| Liu B S, Zhang T S, Kong J, Wang Q Y, Luan S, Cao B X, 2011. Estimation of genetic parameters for growth and upper thermal tolerance traits in turbot Scophthalmus maximus. Journal of Fisheries of China, 35 (11) : 1601 –1606. |
| Losordo T M. 1991. Engineering considerations in closed recirculating systems. In:Giovannini P ed. Aquaculture Systems Engineering:Proceedings of the World aquaculture Society and the American Society of Agricultural Engineers, WAS 22nd Annual Meeting American Society of Agricultural, SanJuan, PuertoRico.p.58-69. |
| Luan S, Yang G L, Wang J Y, Luo K, Zhang Y F, Gao Q, Hu H L, Kong J, 2012. Genetic parameters and response to selection for harvest body weight of the giant freshwater prawn Macrobrachium rosenbergii. Aquaculture, 362-363 : 88 –96. Doi: 10.1016/j.aquaculture.2012.05.011 |
| Lynch M, Kuramitsu H, 2000. Expression and role of superoxide dismutases (SOD) in pathogenic bacteria. Microbes and Infection, 2 (10) : 1245 –1255. Doi: 10.1016/S1286-4579(00)01278-8 |
| Magallón-Barajas F, Villegas S R, Clark G P, Mosqueda G J, Moreno L B, 2006. Daily variation in short-term static toxicity of unionized ammonia in Litopenaeus vannamei(Boone) postlarvae. Aquaculture Research, 37 (14) : 1406 –1412. Doi: 10.1111/are.2006.37.issue-14 |
| Moore S S, Whan V, Davis G P, Byrne K, Hetzel D J S, Preston N, 1999. The development and application of genetic markers for the Kuruma prawn Penaeus japonicus. Aquaculture, 173 (1-4) : 19 –32. Doi: 10.1016/S0044-8486(98)00461-X |
| Nguyen N H, Khaw H L, Ponzoni R W, Hamzah A, Kamaruzzaman N, 2007. Can sexual dimorphism and body shape be altered in Nile tilapia (Oreochromis niloticus) by genetic means? Aquaculture, 272(S1):S38-S46. Aquaculture, 272 (S1) : S38 –S46. |
| Noor-Hamid S, Fortes R D, Parado-Estepa F, 1994. Effect of pH and ammonia on survival and growth of the early larval stages of Penaeus monodon Fabricius. Aquaculture, 125 (1-2) : 67 –72. Doi: 10.1016/0044-8486(94)90283-6 |
| Pante M J R, Gjerde B, McMillan I, Misztal I, 2002. Estimation of additive and dominance genetic variances for body weight at harvest in rainbow trout, Oncorhynchus mykiss. Aquaculture, 204 (3-4) : 383 –392. Doi: 10.1016/S0044-8486(01)00825-0 |
| Parsell D A, Lindquist S, 1993. The function of heat-shock proteins in stress tolerance:degradation and reactivation of damaged proteins. Annual Review of Genetics, 27 : 437 –496. Doi: 10.1146/annurev.ge.27.120193.002253 |
| Peaydee P, Klinbunga S, Menasveta P, Jiravanichpaisal P, Puanglarp N, 2014. An involvement of aquaporin in heat acclimation and cross-tolerance against ammonia stress in black tiger shrimp, Penaeus monodon. Aquaculture International, 22 (4) : 1375 . |
| Pérez-Rostro C I, Ibarra A M, 2003a. Heritabilities and genetic correlations of size traits at harvest size in sexually dimorphic Pacific white shrimp (Litopenaeus vannamei)grown in two environments. Aquaculture Research, 34 (12) : 1079 –1085. Doi: 10.1046/j.1365-2109.2003.00913.x |
| Pérez-Rostro C I, Ibarra A M, 2003b. Quantitative genetic parameter estimates for size and growth rate traits in Pacific white shrimp, Penaeus vannamei (Boone 1931)when reared indoors. Aquaculture Research, 34 (7) : 543 –553. Doi: 10.1046/j.1365-2109.2003.00851.x |
| Racotta I S, Hernández-Herrera R, 2000. Metabolic responses of the white shrimp Penaeus vannamei, to ambient ammonia. Comparative Biochemistry and Physiology, Part A:Molecular & Integrative Physiology, 125 (4) : 437 –443. |
| Robertson A, Lerner I M, 1949. The heritability of all-or-none traits:viability of poultry. Genetics, 34 (4) : 395 –411. |
| Rutten M J M, Komen H, Bovenhuis H, 2005. Longitudinal genetic analysis of Nile tilapia (Oreochromis niloticus L.body weight using a random regression model). Aquaculture, 246 (1-4) : 101 –113. Doi: 10.1016/j.aquaculture.2004.12.020 |
| SAS Institute Inc. 2005. SAS/STAT Software:version 9.1.3(TS1M3) for Microsoft Windows. SAS Institute, Cary, NC, USA. |
| Sun G M, Tang J H, Zhong X M, 2002. Toxicity research of ammonia nitrogen and nitrite nitrogen to Penaeus vannamei. Journal of Aquaculture (1) : 22 –24. |
| Wang X Q, Wang Z Y, He X R, 2009. Heritability and tolerance of Larimichthys crocea to environmental factors. Oceanologia et Limnologia Sinica, 40 (6) : 781 –785. |
| Wicks B J, Joensen R, Tang Q, Randall D J, 2002. Swimming and ammonia toxicity in salmonids:the effect of sub lethal ammonia exposure on the swimming performance of Coho salmon and the acute toxicity of ammonia in swimming and resting rainbow trout. Aquatic Toxicity, 59 (1-2) : 55 –69. Doi: 10.1016/S0166-445X(01)00236-3 |
| Wright P A, 1995. Nitrogen excretion:three end products, many physiological roles. The Journal of Experimental Biology, 198 (Pt2) : 273 –281. |
| Young-Lai W W, Charmantier-Daures M, Charmantier G, 1991. Effect of ammonia on survival and osmo regulation in different life stages of the lobster Homarus americanus. Marine Biology, 110 (2) : 293 –300. Doi: 10.1007/BF01313716 |
| Zhao J H, Lam T J, Guo J Y, 1997. Acute toxicity of ammonia to the early stage-larvae and juveniles of Eriocheir sinensis H. Milne-Edwards, 1853 (Decapoda:grapsidae)reared in the laboratory. Aquaculture Research, 28 (7) : 517 –525. Doi: 10.1111/j.1365-2109.1997.tb01070.x |
 2016, Vol. 34
2016, Vol. 34



