Institute of Oceanology, Chinese Academy of Sciences
Article Information
- PENG Limin(彭丽敏), ZHENG Yuan(郑媛), YOU Feng(尤锋), WU Zhihao(吴志昊), ZOU Yuxia(邹玉霞), ZHANG Peijun(张培军)
- Establishment and characterization of a testicular Sertoli cell line from olive flounder Paralichthys olivaceus
- Chinese Journal of Oceanology and Limnology, 34(5): 1054-1063
- http://dx.doi.org/10.1007/s00343-016-5091-4
Article History
- Received Mar. 24, 2015
- accepted in principle Mar. 25, 2015
- accepted for publication Jul. 10, 2015
2 University of Chinese Academy of Sciences, Beijing 100049, China
In vitro cell culture has grown substantially as an essential biological method to study specific cellular physiology and molecular mechanisms without individual variability. Unlike primary cultures, permanent cell lines are easy to manipulate and can produce highly reproducible research (Lakra et al., 2011). More than 280 cell lines from freshwater and marine fish species have been established (Lakra et al., 2011; Zhang and Chen, 2011; Higaki et al., 2012; Qi, 2014). These cell lines are derived mainly from fish tissues such as fin, heart, brain, kidney, liver, spleen, and embryos (Tong et al., 1997a; Chen et al., 2005; Zhou et al., 2007; Ku et al., 2009; Wang et al., 2010a). However, there is a limited number of cell lines (about 20 gonadal cell lines) originating from testicular and ovarian tissues in fish (Lakra et al., 2011; Zhang et al., 2011; Higaki et al., 2012; Qi, 2014), among them, only about four testicular cell lines.
Testicular cells primarily contain Sertoli cells and germ cells. Sertoli cells create a microenvironment to control the self-renewal of germ cells in the process of spermatogenesis and orchestrate the structural organization and differentiation of testis particularly through formation of the blood-testis barrier (BTB) (Cheng and Mruk, 2012; Smith and Braun, 2012; Sekido and Lovell-Badge, 2013; Su et al., 2014). However, compared with mammals, there are fewer studies on Sertoli cells from lower vertebrates especially fish. To date, research has mainly focused on the establishment of spermatogenesis in Japanese eel (Anguilla japonica) (Miura et al., 1991), primary culture of spermatocytes (Saiki et al., 1997) and a stable stem cell line SG3 (Hong et al., 2004) in medaka (Oryzias latipes), and functional sperm obtention in co-culture with Sertoli-like cells in zebrafish (Danio rerio) (Sakai, 2002). There are four in vitro somatic cell lines from the testis of fish including CCT from color carp (Cyprinus carpio) (Ku and Chen, 1992), RMT1 from Honmoroko (Gnathopogon caerulescens) (Higaki et al., 2012), SCT1 from Southern catfish (Silurus meridionalis Chen) (Qi, 2014), and CSGC from the half smooth tongue sole (Cynoglossus semilaevis) (Zhang et al., 2011). Among them, only RMT1 was verified as a Sertoli cell.
Olive flounder Paralichthys olivaceus, which is mainly found in coastal waters of Japan, Korea, and China, is one of the most important mariculture fish species. Because the females grow faster than males when cultured under the same environmental conditions, its developmental and reproductive biology, especially sex determination and differentiation have been extensively studied. However, little is known about the mechanism of sex determination and differentiation, in particular at the cellular level owing to the lack of appropriate cell lines. Although four P. olivaceus cell lines from gill (FG-9307, Tong et al., 1997b), spleen (FSP), fin (FFN) (Kang et al., 2003) and embryo (FEC; Chen et al., 2004) have been established, there was no cell line from flounder gonads including ovary and testis. To reveal the related molecular and cellular mechanism, an in vitro cell line from the testis of P. olivaceus would be indispensable. In this study, a Sertoli cell line from the testis of P. olivaceus termed POSC was developed and characterized. The efficiency of transfection and expression of foreign DNA were also examined. The established cell line will be valuable in biotechnological research as an in vitro biological system.
2 MATERIAL AND METHOD 2.1 Tissue isolation and cell cultureA healthy live flounder weighing 347.5 g was purchased from Nanshan market (Qingdao, China) and temporarily kept in a 500-L aerated seawater tank at the institute aquarium with filtered seawater and fed with commercial bait every day. Before sampling, the fish was anaesthetized by 40 mg/mL 3-aminobenzoic acid ethyl ester methanesulfonate, and the outer surface of the fish was disinfected with 70% (v/v) ethanol. The testis tissue was immediately removed and immersed in DMEM/F12 medium containing 400 U/mL penicillin and 400 μg/mL streptomycin. The tissue was washed twice with phosphate-buffered saline (PBS, Hyclone, Ca2+ and Mg2+ free) and cut into small pieces ( < 1 mm3) in a new cell culture dish. The tissue fragments were then transferred into a 25 cm2 tissue culture flask containing 1 mL of DMEM/F12 with 20% fetal bovine serum (FBS), 10 ng/mL basic fibroblast growth factor (bFGF), 15 ng/mL epidermal growth factor (EGF), 100 U/mL penicillin and 100 μg/mL streptomycin. The suspension was incubated at 25℃. All animal work was conducted according to relevant national and international guidelines and approved by the Institute of Oceanology, Chinese Academy of Science. After 24 h of attachment, 1-mL DMEM/F12 complete medium was carefully added to the flask. Every 3–5 days, one-half of the medium was changed until passaged.
When cell clusters formed and stopped growing, the old medium was removed and the cell sheets were dispersed with trypsin digestion (0.25% trypsin and 0.2% EDTA in PBS) and seeded in the original culture flask again. When a complete cell monolayer was obtained, the cells were sub-cultured every 3–5 days at a split ratio of 1:2 according to the cells’ growth status.
2.2 Cryogenic preservation and recoveryCells reached up to 90% confluence at passages 15, 20, 25, 35, and 45, and were used for cryostorage. Single cell suspensions by trypsinization from each flask were collected and centrifuged at 2 200 g for 3 min. The cell pellet was re-suspended in frozen stock solution (DMEM/F12 complete medium containing 10% dimethyl sulfoxide [DMSO, Sigma, USA]) at a density of (2–3)×106 cells/mL. The cell suspension was dispensed into 2 mL sterile cryogenic vials and kept in a NALGEBE™ Cryo 1℃ Freezing Container (Invitrogen, USA) at 4℃ for 30 min, -20℃ for 2 h, and -80℃ overnight. Then these vials were transferred to liquid nitrogen (-196℃) for cryostorage. To recover and reseed the frozen cells, vials from liquid nitrogen were thawed quickly in a 42℃ water bath until all cells dissolved and centrifuged (2 200 g, 3 min). The cells were transferred to a flask and resuspended in complete DMEM/F12 medium, and cultured at 25℃.
2.3 Growth curve of cells Cellgrowth was assessed by counting cell numbers with a hemocytometer. Cells of passage 27 at a density of 3.5×104 cells/mL were seeded into two 12-well plates and incubated at 25℃. After culturing for 8 days, three wells of cells were counted everyday using a hemocytometer. The cell growth curve was plotted, and the population doubling time (PDT) was calculated according to the equation PDT=t×(lg2/[lgNt−lgN0]) (N0, the number of cells 24 h after seeding; t, incubation time; Nt, the number of cells at t h) (Pan et al., 2012).
2.4 Chromosome analysisCells at passage 31 were prepared to analyze the chromosomal karyotype. In brief, 1.0×106/mL cells were seeded into a 25 cm2 culture flask and incubated at 25℃ overnight. After 24 h, colchicine (1.0 μg/mL) was added and the cells were incubated at 25℃ for 3.5 h. The monolayer was trypsinized and harvested by centrifugation (2 200 g, 3 min). The cell pellets were suspended in 5 mL hypotonic solution of 0.075 mol/LKCl for 30 min at 37℃, then prefixed for 2 min in 1 mL of cold Carnoy’s fixative (methanol: acetic acid=3:1). After 6 min centrifugation at 1 000×g, the cells were fixed in three changes of 5 mL of cold Carnoy’s fixative, 15 min for each change. Cells were re-suspended in 0.5 mL of cold Carnoy’s fixative after the last centrifugation (1 000 g, 6 min). Slides were prepared using the conventional dropsplash technique and air dried. Chromosomes were stained with 10% Giemsa for 30 min. The chromosome numbers per spread were counted for 100 spreads under an Eclipse 80I fluorescence microscope (Nikon, Japan). Chromosomal karyotype for POSC cells was analyzed as described with some modifications according to Levan et al. (1964).
2.5 Species identification of POSCTotal genomic DNA was extracted from POSC cells at passage 34 using a DNA isolation kit (TIANGEN, China). Primers FishF1 and FishR1 of fish cytochrome oxidase subunit I (COI) (Ward et al., 2005) were used for amplification (Table 1). The COI gene was amplified in a 25-μL reaction volume containing 1 μL (100 ng) of total genomic DNA as a template, 0.5 μL of each primer (10 μmol/L), 10.5 μL nuclease-free water, and 12.5 μL of 2×Pfu MasterMix. The PCR cycling conditions involved an initial denaturation step at 94℃ for 5 min followed by 35 cycles of 94℃ for 30 s, 52℃ for 30 s and 72℃ for 1 min with a final extension phase at 72℃ for 10 min, and a holding temperature of 4℃ after completion. PCR product was electrophoresed on 1% agarose gels and visualized under a UV transilluminator. Sequencing of the PCR product was performed by Invitrogen Co. Ltd. (Shanghai, China). The PCR fragment sequence was aligned against known sequences in the National Center for Biotechnology Information (NCBI) database using the Basic Local Alignment Search Tool (BLAST, http://blast.ncbi.nlm.nih.gov/Blast.cgi).
Specific primers of the male related gene, double sex and mab-3-related transcription factor1 (dmrt1) and germ cell gene marker, vasa, were used for the RT-PCR method (Table 1). β-actin was used as the internal control (Table 1). Total RNAs were extracted from muscle, ovary, and testis tissues, and POSC cells at passage 24 using an RNA isolation kit (TIANGEN, China) and incubated with RNase-free DNase I (Promega, USA) to eliminate contaminating genomic DNA. First strand cDNAs, which were synthesized using oligodT primers (Takara, Japan) and M-MLV reverse transcriptase (Promega, USA) according to the manufacturers’ instructions, were taken as templates. PCR was carried out in a volume of 25 μL containing 1 μL (300 ng) of cDNA as the template, 0.5 μL of each primer (10 μmol/L), 10.5 μL nucleasefree water and 12.5 μL of 2 × Pfu MasterMix (CWBIO, China). Thermal cycling included an initial denaturation at 94℃ for 5 min, followed by 35 cycles of 94℃ for 30 s, 60℃ for 30 s and 72℃ for 30 s, and 72℃ for 10 min for elongation. The PCR products were analyzed by 1% agarose gel electrophoresis.
2.7 ImmunocytochemistryThe POSC cells at passage 41 were analyzed by immunocytochemical staining for Fas ligand (FasL) as a specific marker of testicular Sertoli cells. About 1.0×105 cells were inoculated in one 12-well plate and incubated at 25℃ for about 2 days. Cells were washed twice in cold PBS, fixed with paraformaldehyde (4.0% in PBS, v/v) for 15 min at room temperature, washed for 5 min in PBS, permeabilized for 15 min in cold 0.2% Triton X-100 PBS, washed twice in PBS, blocked for 30 min in 1% BSA, and then incubated with Anti-Fas Ligand antibody (2 μL dissolved in 200 μL 2% goat serum/PBS ab15285, Abcam) at 4℃ overnight. After two washes in PBS, cells were incubated with Goat Anti-Rabbit IgG HRP Conjug (2 μL dissolved in 200 μL 2% goat serum/PBS, CW0103, CWBIO, China) at 25℃ for 1.5 h, then washed twice in PBS, and detection of the primary antibodies was performed with 3, 3′N-Diaminobenzidine Tetrahydrochloride (DAB) in Tris-HCl (pH 7.6) buffer containing H2O2. Labelled cells were examined under the Eclipse 80I fluorescence microscope. Negative controls (without the primary antibody) were included in the experiment.
2.8 Expression of fluorescent protein gene in POSCPOSC cells at passage 45 were seeded at a density of 5×105 cells/mLin a 24-well plate and incubated for 18 h at 25℃. Sub-confluent monolayers were transfected with pEGFP-N3 eukaryotic expression vector (Invitrogen, USA) using lipofectamineTM 2000 (Invitrogen, USA). Briefly, 1-μL lipofectamineTM 2000 and 2-μL pEGFP-N3 (400 ng/μL) were dissolved in 49 μL and 48 μL of DMEM/F12 (without FBS or antibiotics), respectively. After 5 min, the two solutions were mixed and incubated at room temperature for 25 min. Subsequently, the mixture was added gently into the well and cultured at 25℃. After 4.5 h, the supernatant was replaced with fresh DMEM/F12 complete medium. The green fluorescence signals were observed regularly over the following week under an Eclipse TE2000-U fluorescence microscope (Nikon, Japan).
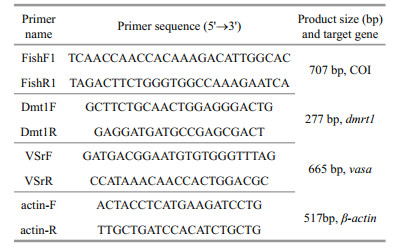
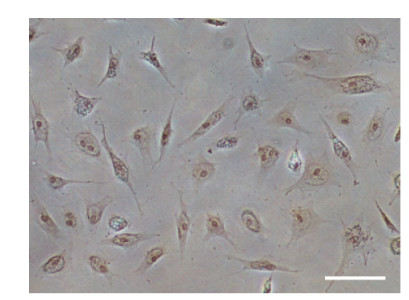
3 RESULT 3.1 Primary culture and subcultureCells began to migrate from the edges of the seeded tissue pieces 3–6 days after explanting, and confluent monolayers of cells were observed on days 8–13 (Fig. 1a). Cells grew continuously and the first subculture was conducted when they reached 90% confluence. Cells were sub-cultured in a 1:2 cell suspension to fresh medium every 3–5 days. In this way, cells were repeatedly passaged, and a new cell line from flounder testis was established (Fig. 1b). To date, the cell line has been sub-cultured for 48 passages and is designated as the P. olivaceus testis Sertoli cell (POSC) line. Morphologically, POSC are composed primarily of fibroblast-like cells and a small number of epithelial-like cells.
|
| Figure 1 Photomicrographs of the POSC cells a. primary culture on day 13 following seeding of tissue explants; b. sub-cultured at passage 25, both appeared predominantly fibroblast-like and a small number of epithelial-like cells could be seen. Scale bar: 100 μm. |
POSC cells showed 55%–65% viability when recovered from liquid nitrogen (-196℃) after storage of 4–6 months. The cells successfully grew to confluence in 10 days. No appreciable morphological changes were observed after freezing and thawing.
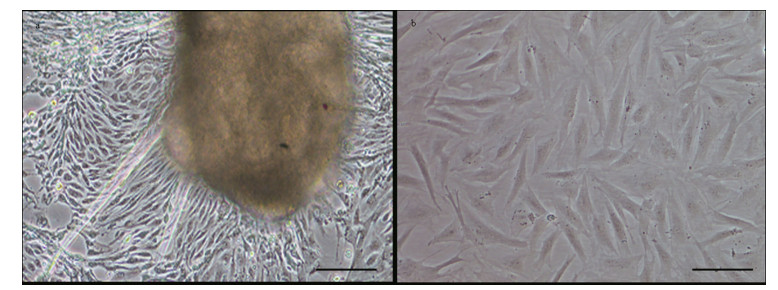
3.3 Growth curveThe growth curve of the POSC cell line had a typical “S” shape (Fig. 2). The cells adhered well to the substrate and achieved confluence in 8 days. After 5 days, the cell number reached 22.7×104 cells/mL with the starting cell number of 3.0×104 cells/mL. The doubling time was 30.10±2.80 h.
|
| Figure 2 Growth curve of POSC cells in DMEM/F12 supplied with 20% FBS at 25℃ The cell number was counted every day. The starting cell number was 3.0×104 cells/mL24 h after seeding. Values are means±SE (n=3). |
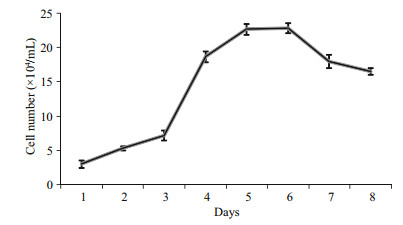
The result of chromosome counts of 100 metaphase POSC cells at passage 31 revealed that the metaphases displayed normal karyotype morphology, and the modal number was 48, which was seen in 48 of the 100 metaphase cells counted (Fig. 3), the diploid number in this organism, which meant POSC had high genetic stability.
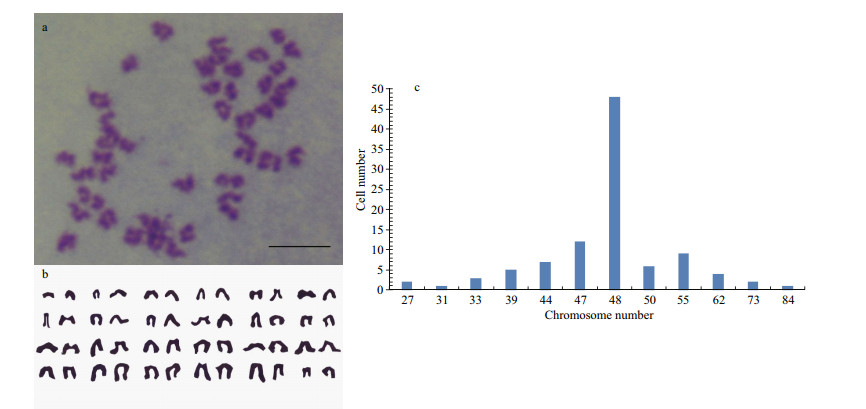
|
| Figure 3 Chromosome analysis of POSC cells Cellular chromosome of POSC cells at passage 31 arrested in metaphase (a), diploid karyotype (b) and chromosome number distribution in 100 metaphases (c). Scale bar: 10 μm. |
The origin of the POSC cell line was verified by partial sequences of mtDNA COI gene analysis. An expected 707 bp PCR product was obtained (Fig. 4). Subsequent comparative analysis of the identified sequence demonstrated a 99% match for COI with the known flounder mtDNA COI sequence (GenBank Accession No. AB028664). Therefore the POSC cell line originated from P. olivaceus.
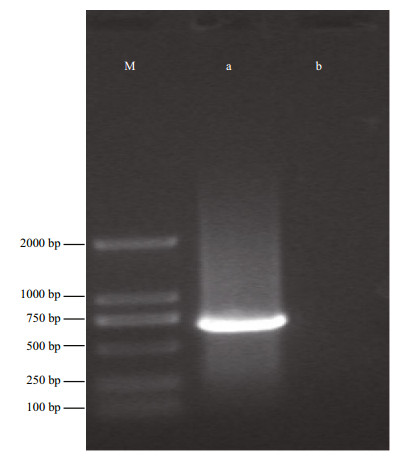
|
| Figure 4 Agarose gel electrophoresis of PCR product of COI from POSC cells M: DSTM2000 Marker; a. expression of COI in POSC cells; b. without template. |
In several fish species such as medaka, dmrt1 is defined as a gene marker of testicular somatic cells (Brunner et al., 2001; Sato et al., 2001). Unlike the dmrt1 gene, the expression of vasa was restricted to the germ cell lineage in flounder and most other fish species (Wu et al., 2014). The expected 277 bp fragment of dmrt1, 665 bp fragment of vasa, and 517 bp fragment of β-actin (Fig. 5) were analyzed by 5 1% agarose gel electrophoresis. Dmrt1 showed a high expression level in the testis and POSC cells, but low expression in the ovary, and no expression in the muscle tissue. However, vasa only appeared in the testis and ovary, and not in the muscle tissue and POSC cells. Sequencing results showed 100% match with the known flounder gene sequences. The above results showed that POSC appears to be somatic cells of the testis.
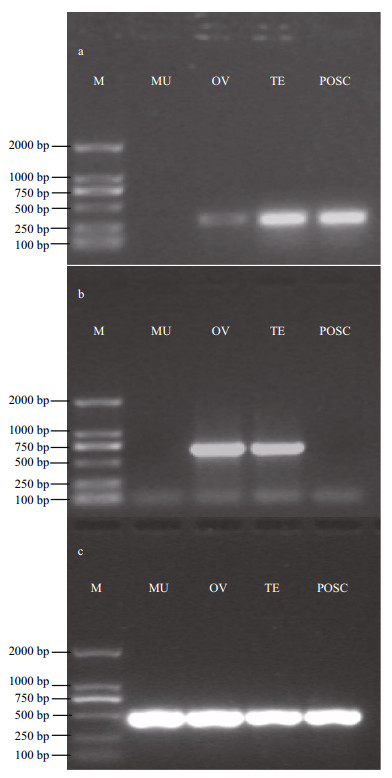
|
| Figure 5 Detection of dmrt1 and vasa expression in POSC cells a. dmrt1 expression; b. vasa expression; c. β-actin. M: DSTM2000 Marker; MU: muscle tissue; OV: ovary tissue; TE: testis tissue. |
POSC cells at passage 41 were examined for protein expression of FasL, which was specific for Sertoli cells. Positive staining for FasL of POSC cells were observed, although the intensity of the labelling varied (Fig. 6).
|
| Figure 6 Immunohistochemical detection of FasL in POSC cells POSC cells at passage 41 were characterized by protein expression of FasL. Positive staining is in brown. Scale bar: 100 μm. |
POSC cells were successfully transfected with pEGFP-N3 plasmid using lipofectamineTM2000. The expression of EGFP in these cells was detected at 24 h post transfection (Fig. 7). Transfection efficiency was 10% as determined by counting the fluorescent protein positive cells as well as total number of viable cells. Results indicated the suitability of POSC cells for transfection and gene expression studies.
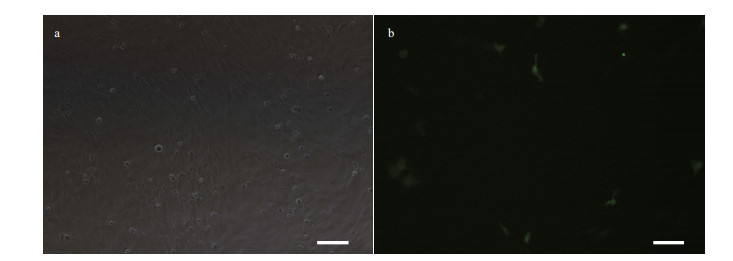
|
| Figure 7 Green fluorescent protein (GFP) expression in POSC cells at passage 45 transfected with pEGFP-N3 Scale bar: 100 μm. |
The structure of Sertoli cells was firstly reported by Enrico Sertoli in 1865 (Wessel, 2011). As mentioned by Griswold (1998), Sertoli cells have indispensable functions in the testis for spermatogenesis, and male germ cells cannot survive and mature without Sertoli cells in vivo. Thus, Sertoli cell culture systems have been established in many species, such as rat (Buzzard et al., 2002), mouse (Ahmed et al., 2009), human (Chui et al., 2011) and goat (Su et al., 2014) for the study of spermatogenesis (Komori et al., 2007), signal transduction (Zhang et al., 2012), and cytotoxicity (Lee et al., 2004; Kim et al., 2011). To date, as far as we know, there are four testicular somatic cell lines only in fish, and only one in marine fish termed CSGC from half smooth tongue sole (Zhang et al., 2011). No olive flounder Sertoli cell culture has been reported. In this study, we obtained a testis Sertoli cell line (POSC) from olive flounder (P. olivaceus). The POSC cells adapted well to grow in DMEM/F12 medium supplemented with FBS, bFGF and EGF. The cells, which have a fibroblastic-like morphology, have been sub-cultured for 48 passages.
Chromosome analysis revealed that POSC had a normal diploid karyotype of 2n=48t, identical to the modal number of FG-9307 (Tong et al., 1997b), FSP and FFN (Kang et al., 2003), and FEC (Chen et al., 2004). The various chromosome numbers and the aneuploidy were two key indicators of malignant cells (Zhou et al., 2007; Dong et al., 2008), because the chromosome number is more stable in normal cells. In addition, mitochondrial DNA, particularly COI gene sequence alignment, has been frequently used as a reliable molecular method to accurately identify the origin of established cell lines (Cooper et al., 2007; Swaminathan et al., 2012). In this study, the partial sequence data of the COI gene confirmed that the newly established POSC cell line was indeed derived from flounder, and proved the validity of the COI sequence as a marker for species identification.
Three separate identification results demonstrated that POSC was a testicular Sertoli cell line. At first, POSC expressed the testicular somatic cell marker dmrt1 but not vasa, the candidate gene in identifying germ cells. A similar result was reported in the CSGC from half smooth tongue sole that dmrt1 was used as an indicator of somatic cells to identify the cell line from testis (Zhang et al., 2011). In medaka (Brunner et al., 2001; Sato et al., 2001; Lutfalla et al., 2003) and gibel carp (Carassius auratus gibelio) (Li et al., 2014), dmrt1 showed an obviously prominent expression in Sertoli cells. In our previous study, flounder dmrt1 was also detected in Sertoli cells (Wen et al., 2014). While, in most fish species such as flounder and rare minnow (Gobiocypris rarus), vasa was used as a candidate gene in identifying germ cells in view of its specific expression in the germ cells (Cao et al., 2012; Wu et al., 2014). Secondly, POSC did have a positive reaction of FasL, which was specific for testicular Sertoli cells. The same result was observed in yak Sertoli cells (Zhang et al., 2013). The Fas system has been confirmed as a key regulator of germ cell apoptosis (Lee et al., 1997) and FasL expression is exclusively restricted to Sertoli cells (French et al., 1996; Lee et al., 1997). Furthermore, POSC cells were mainly fibroblast-like in morphology similar to the testicular Sertoli cell line RMT1 (Higaki et al., 2012), and somatic cell line CSGC (Zhang et al., 2011) and SCT1 (Qi, 2014), while germ cells were polygonal or round in culture (Hong et al., 2004).
Application of newly developed cell lines for exogenous DNA manipulation is useful for both basic research and biotechnological applications. The transfection experiment with the pEGFP-N3 vector in this study revealed that the POSC cell line was efficient in gene expression. It also showed that the CMV promoter could drive expression of the GFP gene in the POSC cells. Successful transfection of POSC cells suggested the utility of this cell line to serve as an in vitro system in recombinant constructs, gene targeting and expression research. Some relevant studies about gene targeting and expression applications using fish cells have been reported (Liu et al., 1990; Fernandez-Alonso and Coll, 1999). In our experiment, the transfection efficiency was 10%, the same efficiency of fish cell lines such as CSH (heart) (Wang et al., 2010b) and TSHKC (head kidney) (Zheng et al., 2012) from half smooth tongue sole (C. semilaevis), and higher than 5% in MAF (fin) from blunt snout bream (Megalobrama amblycephala) (Zhu et al., 2013), but lower than 27% in PCF (caudal fin) from dark mahseer (Puntius (Tor) chelynoides) (Goswami et al., 2014). In future, more attempts will be needed to improve efficiency by trying a new transfection method or changing the proportion of DNA.
5 CONCLUSIONIn this experiment, a new marine fish cell line from testis was established and well characterized. This cell line could provide an effective experimental model to study sex-related events and interactions between male germ cells and somatic cells.
ACKNOWLEDGMENT: The authors thank Professor Songlin Chen from the Yellow Sea Fisheries Research Institute, Chinese Academy of Fishery Sciences, and Professor Jianhai Xiang from the Institute of Oceanology, Chinese Academy of Sciences, for their help in the microscopic observations of cell morphology.| Ahmed E A, Barten-van Rijbroek A D, Kal H B, SadriArdekani H, Mizrak S C, van Pelt A M M, de Rooij D G, 2009. Proliferative activity in vitro and DNA repair indicate that adult mouse and human Sertoli cells are not terminally differentiated, quiescent cells. Biol. Reprod., 80 (6) : 1084 –1091. Doi: 10.1095/biolreprod.108.071662 |
| Brunner B, Hornung U, Shan Z H, Nanda I, Kondo M, ZendAjusch E, Haaf T, Ropers H H, Shima A, Schmid M, Kalscheuer V M, Schartl M, 2001. Genomic organization and expression of the doublesex-related gene cluster in vertebrates and detection of putative regulatory regions for DMRT1. Genomics, 77 (1-2) : 8 –17. Doi: 10.1006/geno.2001.6615 |
| Buzzard J J, Wreford N G, Morrison J R, 2002. Marked extension of proliferation of rat Sertoli cells in culture using recombinant human FSH. Reproduction, 124 (5) : 633 –641. Doi: 10.1530/rep.0.1240633 |
| Cao M, Yang Y H, Xu H Y, Duan J D, Cheng N, Wang J L, Hu W, Zhao H B, 2012. Germ cell specific expression of Vasa in rare minnow, Gobiocypris rarus. Comp. Biochem.Physiol. A. Mol. Integr. Physiol., 162 (3) : 163 –170. Doi: 10.1016/j.cbpa.2012.02.007 |
| Chen S L, Ren G C, Sha Z X, Hong Y H, 2005. Development and characterization of a continuous embryonic cell line from turbot (Scophthalmus maximus). Aquaculture, 249 (1-4) : 63 –68. Doi: 10.1016/j.aquaculture.2005.01.031 |
| Chen S L, Ren G C, Sha Z X, Shi C Y, 2004. Establishment of a continuous embryonic cell line from Japanese flounder Paralichthys olivaceus for virus isolation. Dis. Aquat.Organ., 60 (3) : 241 –246. |
| Cheng C Y, Mruk D D, 2012. The blood-testis barrier and its implications for male contraception. Pharmacol. Rev., 64 (1) : 16 –64. Doi: 10.1124/pr.110.002790 |
| Chui K, Trivedi A, Cheng C Y, Cherbavaz D B, Dazin P F, Huynh A L T, Mitchell J B, Rabinovich G A, NobleHaeusslein L J, John C M, 2011. Characterization and functionality of proliferative human Sertoli cells. Cell Transplantation, 20 (5) : 619 –635. Doi: 10.3727/096368910X536563 |
| Cooper J K, Sykes G, King S, Cottrill K, Ivanova N V, Hanner R, Ikonomi P, 2007. Species identification in cell culture:a two-pronged molecular approach. In Vitro Cell Dev.Biol. Anim., 43 (10) : 344 –351. Doi: 10.1007/s11626-007-9060-2 |
| Dong C F, Weng S P, Shi X J, Xu X P, Shi N, He J G, 2008. Development of a mandarin fish Siniperca chuatsi fry cell line suitable for the study of infectious spleen and kidney necrosis virus (ISKNV). Virus. Res., 135 (2) : 273 –281. Doi: 10.1016/j.virusres.2008.04.004 |
| Fernandez-Alonso M, Coll J M, 1999. Induced fusion of VHSV persistently infected fish cells. J. Fish. Dis., 22 (5) : 401 –406. Doi: 10.1046/j.1365-2761.1999.00188.x |
| French L E, Hahne M, Viard I, Radlgruber G, Zanone R, Becker K, Müller C, Tschopp J, 1996. Fas and Fas ligand in embryos and adult mice:ligand expression in several immune-privileged tissues and coexpression in adult tissues characterized by apoptotic cell turnover. J. Cell.Biol., 133 (2) : 335 –343. Doi: 10.1083/jcb.133.2.335 |
| Goswami M, Sharma B S, Yadav K, Bahuguna S N, Lakra W S, 2014. Establishment and characterization of a piscean PCF cell line for toxicity and gene expression studies as in vitro model. Tissue and Cell, 46 (3) : 206 –212. Doi: 10.1016/j.tice.2014.04.004 |
| Griswold M D, 1998. The central role of Sertoli cells in spermatogenesis. Semin. Cell. Dev. Biol., 9 (4) : 411 –416. Doi: 10.1006/scdb.1998.0203 |
| Higaki S, Koyama Y S, Shirai E, Yokota T, Fujioka Y, Sakai N, Takada T, 2012. Establishment of testicular and ovarian cell lines from Honmoroko (Gnathopogon caerulescens). Fish. Physiol. Biochem., 39 (3) : 701 –711. |
| Hong Y H, Liu T M, Zhao H B, Xu H Y, Wang W J, Liu R, Chen T S, Deng J R, Gui J F, 2004. Establishment of a normal medakafi sh spermatogonial cell line capable of sperm production in vitro. Proc. Natl. Acad. Sci. USA., 101 (21) : 8011 –8016. Doi: 10.1073/pnas.0308668101 |
| Kang M S, Oh M J, Kim Y J, Kawai K, Jung S J, 2003. Establishment and characterization of two new cell lines derived from fl ounder, Paralichthys olivaceus (Temminck & Schlegel). J. Fish. Dis., 26 (11-12) : 657 –665. Doi: 10.1046/j.1365-2761.2003.00499.x |
| Kim Y J, Chung J Y, Lee S G, Kim J Y, Park J E, Kim W R, Joo B S, Han S H, Yoo K S, Yoo Y H, Kim J M, 2011. Arsenic trioxide-induced apoptosis in TM4 Sertoli cells:the potential involvement of p21 expression and p53 phosphorylation. Toxicology, 285 (3) : 142 –151. Doi: 10.1016/j.tox.2011.04.013 |
| Komori S, Kasumi H, Sakata K, Koyama K, 2007. The role of androgens in spermatogenesis. Soc. Reprod. Fertil.Suppl., 63 : 25 –30. |
| Ku C C, Chen S N, 1992. Characterization of three cell lines derived from color carp Cyprinus carpio. J. Tiss. Cult.Meth., 14 (2) : 63 –71. Doi: 10.1007/BF01404746 |
| Ku C C, Teng Y C, Wang C S, Lu C H, 2009. Establishment and characterization of three cell lines derived from the rock fi sh grouper Epinephelus quoyanus:use for transgenic studies and cytotoxicity testing. Aquaculture, 294 (1-2) : 147 –151. Doi: 10.1016/j.aquaculture.2009.05.010 |
| Lakra W S, Swaminathan T R, Joy K P, 2011. Development, characterization, conservation and storage of fi sh cell lines:a review. Fish. Physiol. Biochem., 37 (1) : 1 –20. Doi: 10.1007/s10695-010-9411-x |
| Lee D Y, Lee S S, Joo W A, Lee E J, Kim C W, 2004. Analysis of diff erentially regulated proteins in TM4 cells treated with bisphenol A. Biosci. Biotechnol. Biochem., 68 (6) : 1201 –1208. Doi: 10.1271/bbb.68.1201 |
| Lee J, Richburg J H, Younkin S C, Boekelheide K, 1997. The Fas system is a key regulator of germ cell apoptosis in the testis. Endocrinology, 138 (5) : 2081 –2088. |
| Levan A, Fredga K, Sandberg A A, 1964. Nomenclature for centromeric position on chromosomes. Hereditas, 52 (2) : 201 –220. |
| Li X Y, Li Z, Zhang X J, Zhou L, Gui J F, 2014. Expression characterization of testicular DMRT1 in both Sertoli cells and spermatogenic cells of polyploid gibel carp. Gene, 548 (1) : 119 –125. Doi: 10.1016/j.gene.2014.07.031 |
| Liu Z J, Moav B, Faras A J, Guise K S, Kapuscinski A R, Hackett P B, 1990. Functional analysis of elements aff ecting expression of the beta-actin gene of carp. Mol.Cell. Biol., 10 (7) : 3432 –3440. Doi: 10.1128/MCB.10.7.3432 |
| Lutfalla G, Crollius H R, Brunet F G, Laudet V, Robinson-Rechavi M, 2003. Inventing a sex-specifi c gene:a conserved role of DMRT1 in teleost fi shes plus a recent duplication in the medaka Oryzias latipes resulted in DMY. J. Mol. Evol., 57 (1) : S148 –S153. |
| Miura T, Yamauchi K, Takahashi H, Nagahama Y, 1991. Hormonal induction of all stages of spermatogenesis in vitro in the male Japanese eel (Anguilla japonica). Proc.Natl. Acad. Sci. USA., 88 (13) : 5774 –5778. Doi: 10.1073/pnas.88.13.5774 |
| Pan X P, Li J Z, Du W B, Yu X P, Zhu C X, Yu C B, Cao H C, Zhang Y M, Chen Y., Li L J, 2012. Establishment and characterization of immortalized human hepatocyte cell line for applications in bioartifi cial livers. Biotechnol.Lett., 34 (12) : 2183 –2190. Doi: 10.1007/s10529-012-1025-1 |
| Qi W C. 2014. Establishment, Identifi cation and Application of Gonadal Cell Lines from Southern Catfi sh (Silurus meridionalis Chen). Southwest University, Chongqing, China. (in Chinese) |
| Saiki A, Tamura M, Matsumoto M, Katowgi J, Watanabe A, Onitake K, 1997. Establishment of in vitro spermatogenesis from spermatocytes in the medaka, Oryzias latipes. Dev.Growth. Diff er., 39 (3) : 337 –344. Doi: 10.1046/j.1440-169X.1997.t01-2-00009.x |
| Sakai N, 2002. Transmeiotic diff erentiation of zebrafi sh germ cells into functional sperm in culture. Development, 129 (14) : 3359 . |
| Sato T, Yokomizo S, Matsuda M, Hamaguchi S, Sakaizumi M, 2001. Gene-centromere mapping of medaka sex chromosomes using triploid hybrids between Oryzias latipes and O. luzonensis. Genetica, 111 (1-3) : 71 –75. |
| Sekido R, Lovell-Badge R, 2013. Genetic control of testis development. Sex. D ev., 7 (1-3) : 21 –32. Doi: 10.1159/000342221 |
| Smith B E, Braun R E, 2012. Germ cell migration across Sertoli cell tight junctions. Science, 338 (6108) : 798 –802. Doi: 10.1126/science.1219969 |
| Su H M, Luo F H, Bao J J, Wu S, Zhang X M, Zhang Y, Duo S G, Wu Y J, 2014. Long-term culture and analysis of cashmere goat Sertoli cells. In Vitro Cell Dev. Biol. Anim., 50 (10) : 918 –925. Doi: 10.1007/s11626-013-9648-7 |
| Swaminathan T R, Lakra W S, Gopalakrishnan A, Basheer V S, Kushwaha B, Sajeela K A, 2012. Development and characterization of a fi broblastic-like cell line from caudal fi n of the red-line torpedo, Puntius denisonii (Day)(Teleostei:cyprinidae). Aquac. Res., 43 (4) : 498 –508. Doi: 10.1111/j.1365-2109.2011.02854.x |
| Tong S L, Li H, Miao H Z, 1997a. Four continuous cell lines of three marine fi sh species were established. Prog ress Biot echnology, 17 (3) : 2 –3. |
| Tong S L, Li H, Miao H Z, 1997b. The establishment and partial characterization of a continuous fi sh cell line FG-9307 from the gill of fl ounder, Paralichthys olivaceus. Aquaculture, 156 (3-4) : 327 –333. Doi: 10.1016/S0044-8486(97)00070-7 |
| Wang N, Wang X L, Sha Z X, Tian Y S, Chen S L, 2010a. Development and characterization of a new marine fi sh cell line from turbot (Scophthalmus maximus). Fish.Physiol. Biochem., 36 (4) : 1227 –1234. Doi: 10.1007/s10695-010-9402-y |
| Wang X L, Wang N, Sha Z X, Chen S L, 2010b. Establishment, characterization of a new cell line from heart of half smooth tongue sole (Cynoglossus semilaevis). Fish.Physiol. Biochem., 36 (4) : 1181 –1189. Doi: 10.1007/s10695-010-9396-5 |
| Ward R D, Zemlak T S, Innes B H, Last P R, Hebert P D, 2005. DNA barcoding Australia's fi sh species. Philos. Trans. R.Soc. Lond. B. Biol. Sci., 360 (1462) : 1847 –1857. Doi: 10.1098/rstb.2005.1716 |
| Wen A Y, You F, Sun P, Li J, Xu D D, Wu Z H, Ma D Y, Zhang P J, 2014. CpG methylation of dmrt1 and cyp19a promoters in relation to their sexual dimorphic expression in the Japanese fl ounder Paralichthys olivaceus. J. Fish.Biol., 84 (1) : 193 –205. Doi: 10.1111/jfb.12277 |
| Wessel G M. 2011. Accessorizing the testis. Enrico Sertoli and the mother cell" of the testis. Mol. Reprod. Dev., 78(3):Fmi, http://dx.doi.org/10.1002/mrd.21299. |
| Wu X M, Wang Z K, Jiang J J, Gao J N, Wang J, Zhou X S, Zhang Q Q, 2014. Cloning, expression promoter analysis of vasa gene in Japanese flounder (Paralichthys olivaceus). Comp. Biochem. Physiol. B. Biochem. Mol.Biol., 167 : 41 –50. Doi: 10.1016/j.cbpb.2013.06.004 |
| Zhang B, Chen S L, 2011. Decades of researching progresses of fish cell culture and its application prospects. Marine Sciences, 35 (7) : 113 –121. |
| Zhang B, Wang X L, Sha Z X, Yang C G, Liu S S, Wang N, Chen S L, 2011. Establishment and characterization of a testicular cell line from the half smooth tongue sole, Cynoglossus semilaevis. Int. J. Biol. Sci., 7 (4) : 452 –459. |
| Zhang H, Liu B, Qiu Y, Fan J F, Yu S J, 2013. Pure cultures and characterization of yak Sertoli cells. Tissue and Cell, 45 (6) : 414 –420. Doi: 10.1016/j.tice.2013.07.004 |
| Zhang Q X, Zhang X Y, Zhang Z M, Lu W, Liu L, Li G, Cai Z M, Gui Y T, Chang C S, 2012. Identification of testosterone-/androgen receptor-regulated genes in mouse Sertoli cells. Asian. J. Androl., 14 (2) : 294 –300. Doi: 10.1038/aja.2011.94 |
| Zheng Y, Wang N, Xie M S, Sha Z X, Chen S L, 2012. Establishment and characterization of a new fish cell line from head kidney of half-smooth tongue sole(Cynoglossus semilaevis). Fish. Physiol. Biochem., 38 (6) : 1635 –1643. Doi: 10.1007/s10695-012-9660-y |
| Zhou G Z, Li Z Q, Yuan X P, Zhang Q Y, 2007. Establishment, characterization, and virus susceptibility of a new marine cell line from red spotted grouper (Epinephelus akaara). Mar. Biotechnol., 9 (3) : 370 –376. Doi: 10.1007/s10126-006-7165-3 |
| Zhu D M, Yang K, Wang W M, Song W, 2013. Establishment and characterization of a fin cell line from blunt snout bream, Megalobrama amblycephala. Fish. Physiol.Biochem., 39 (6) : 1399 –1410. Doi: 10.1007/s10695-013-9794-6 |
 2016, Vol. 34
2016, Vol. 34