Institute of Oceanology, Chinese Academy of Sciences
Article Information
- ZHAO Wen(赵文), ZHAO Yuanyi(赵元艺), WANG Qiaohan(王巧晗), ZHENG Mianping(郑绵平), WEI Jie(魏杰), WANG Shan(王珊)
- The community structure and seasonal dynamics of plankton in Bange Lake, northern Tibet, China
- Chinese Journal of Oceanology and Limnology, 34(6): 1143-1157
- http://dx.doi.org/10.1007/s00343-016-5131-0
Article History
- Received Apr. 20, 2015
- accepted for publication May. 20, 2015
- accepted in principle Sep. 6, 2015
2 R&D Center of Saline Lake and Epithermal Dep., Chinese Academy of Geological Sciences, Beijing 100037, China
Saline lakes in North Tibet are very important for resource exploitation, biodiversity protection, and monitoring global temperature changes in the northern Tibet. Saline lakes contain important raw materials for industry, agriculture, and medicine, e.g., halite, mirabilite, lithium, magnesium, boron, gypsum calcium chloride, tungsten, cesium, rubidium, strontium, hydromagnesite, and zeolite (Zheng et al., 1989). Considerable quantities of biological resources, such as halophilic organisms, e.g., Spirulina sp., Dunaliella sp., Brachionus plicatilis, Moina mongolica, and Artemia sp., which have economic and scientific value, occur in saline lakes (Zheng et al., 1989; Zhao et al., 2005, 2010b). Several limnological studies on Tibetan saline lakes have been carried out, but most have focused on physical and hydrochemical features (Yu and Tang, 1981), chemical exploitation (Chen, 1981; Zheng et al., 1989; Zheng, 1995), and either geographical or geological features. Very little is known of the biological resources in Tibetan saline lakes and no information is available on the seasonal variations in plankton communities in the saline lakes of north Tibet.
Previous reports have mentioned halosaline and freshwater halophilic species in surveys on invertebrates in the Tibetan Plateau (Wang et al., 1975; Gong, 1983; Jiang et al., 1983; Shen, 1983). Unfortunately, data on salinity, alkalinity, pH, and ionic composition were not provided. There are reports in the literature on the aquatic organisms of Qinghai Lake (14)(Wang et al., 1975) and Dali Lake (5.5)(He et al., 1981). Williams (1991) discussed the fauna of Chinese saline lakes in a review on Chinese and Mongolian saline lakes; however, many northern Chinese saline waters were not included, notably, information on zooplankton (He et al., 1984, 1989, 1993, 1994, 1996, 2001; Zhao and He, 1993, 1995, 1999; Zhao, 1992a, b; Zhao et al., 1993, 1996, 2010a; Ma, 1995; Ren et al., 1996). With the development of aquaculture, several plankton species, e.g., Artemia, Brachionus plicatilis, and Moina mongolica, have been used as food, leading to studies on their biology and ecology (An and He, 1991; Ren et al., 1996).
Zheng et al.(1989) reported on the sedimentology of Bange Lake; however, community structure and spatio-temporal dynamics were not discussed. The aim of this study is to elucidate seasonal variations in plankton species composition, biomass, and abundance in three Bange Lake sublakes in the margin of an unpopulated zone in northern Tibet, and the relationships between community structure, diversity index, and environmental factors, e.g., salinity, pH, and temperature.
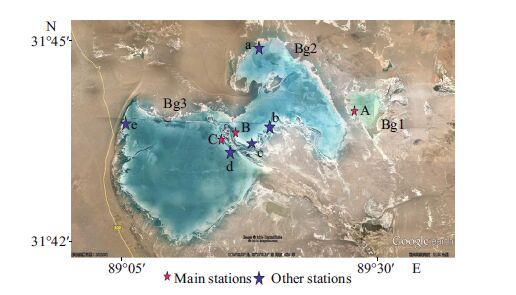
2 MATERIAL AND METHOD 2.1 Sampling locationBange Lake (or Bange Co in Tibetan, or Brang Khog Mtsho in Wylie Transliteration; Drangkhok Tso in THL standard) is located in northwestern Bange County, northern Tibet, China (Fig. 1). The geographical location is 89°29′E, 31°43′N, and 4 522 m altitude. The lake is in a cold, arid region at high altitude. It includes three sublakes, Bange Lakes 1, 2, and 3(hereafter Bg1, 2, and 3). Bg1 is a small shallow aquatic ecosystem, less than 1 km 2 in area and average depth is 2.2 m. Bg2 is 39 km 2 and 1.0 m average depth. Bg3 is 75 km 2 and 1.0 m. They contain water all year round. The climate was very cold and dry. According to local weather data, winds occur frequently, generally in the afternoon and cease at dusk. Rainfall is concentrated between June and mid- September. The annual air temperature ranges from a minimum of -37℃ in January to a maximum of 22.9℃ in July. Annual precipitation is low ~308 mm, but annual evaporation is >2 239 mm.
|
| Figure 1 Map of Bange Lake |
Sampling was undertaken one to three times (twice in most months) per month at main stations in Bg1(Station A only), Bg2(one main Station B and three supplementary Stations a-c), and Bg3(one main Station C and two supplementary Stations e and d)(Fig. 1) from June 2001 to July 2002. Air and water temperature, salinity, and pH were measured. Water temperature was measured with a mercury thermometer. Water chemistry, including pH, HCO3-, CO32-, Cl−, SO42-, Ca2+, and Mg2+ were determined by routine methods (He et al., 1989). K+ and Na+ were measured by flame atomic absorption spectrophotometry (Huang et al., 1999). Salinity was measured with a saltmeter (ATAGO S-10). Transparency was measured by Secchi disc. Total nitrogen was measured by the Kjeldahl method. Total phosphorus was measured with the phosphorus molybdenum blue spectrophotometer method.
In total, 102 plankton samples were collected, including eight qualitative samples collected on the lakeshore. Generally, only surface water samples were obtained because of the shallow water (2.2 m in depth). Phytoplankton was studied by the precipitation concentration method by preserving samples in 1.5% Lugol's solution. Macro-zooplankton were obtained by filtering 20 L of water through a plankton net (64- μm mesh size) and preserved in 5% formaldehyde. Micro-zooplankton species (e.g., protists and rotifers) were obtained from phytoplankton samples after further concentration. The phytoplankton and zooplankton biomass (mg/L) were calculated volumetrically. Shannon-Wiener and Pielou's diversity indices (H ′ and J) were calculated (He et al., 1989). Statistical analysis was performed based on SPSS 20.0.
Species were identified according to the literature (Fott, 1971; Wang, 1977; Koste, 1978; Jiang and Du, 1979; Shen, 1979; Hu and Wei, 1981; Jiang et al., 1983; Ehrlich and Dor, 1985; Li et al., 1992; Zhu and Chen, 2000). Most were identified to the species level, and a few to higher taxonomic levels.
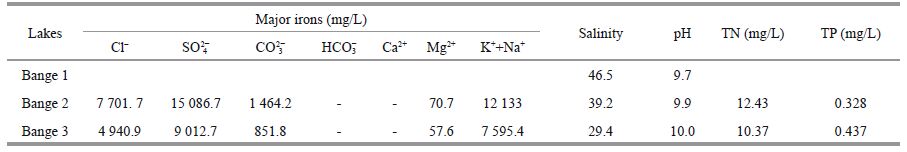
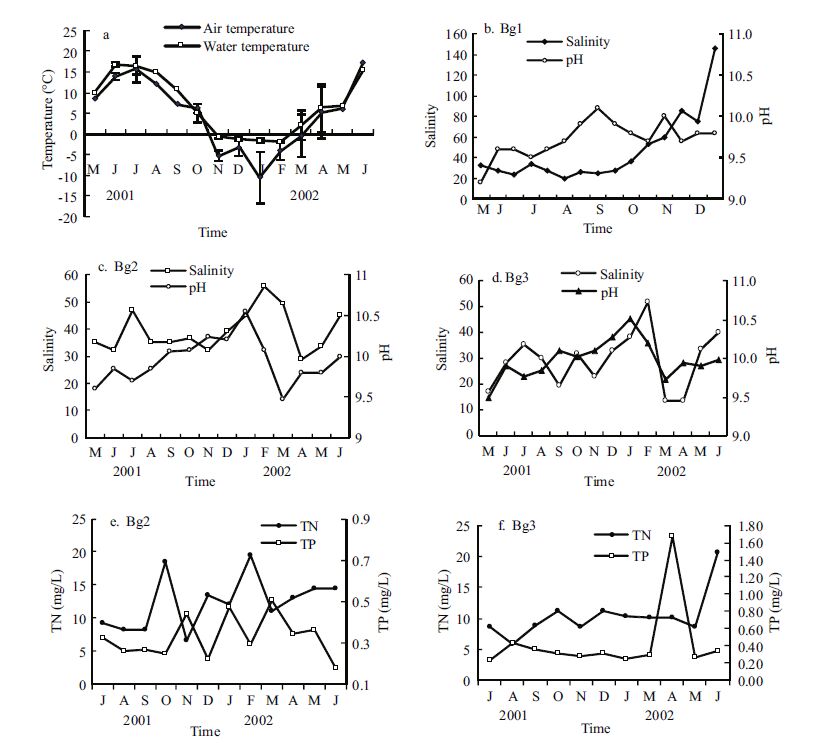
3 RESULT 3.1 Environmental conditionsThe lake water was cold and highly saline. The contribution of snow water in this region is highly variable every year (Zheng, 1989). The mean temperature of the lake in the top 0.5 m exhibited a clear seasonal pattern (Fig. 2a). The lowest temperatures were recorded from November to March (-2 to -3℃). The air temperature was lower than the water temperature for the entire year.
|
| Figure 2 Seasonal changes in temperature, salinity, pH, and the concentrations of total nitrogen and total phosphorus in Bange Lake |
The water temperature began to increase in March/ April reaching its maximum in late June (mean water temperature was 17.7℃, 22℃ in late July 2001 in Bg1) and decreased thereafter. The mean annual air and water temperatures were approximately 4.8℃ and 7.3℃ in the lake. The salinity and pH variations in the lake are shown in Fig. 2b-d. The salinity rose from 14 to 146 in Bange Lake. The mean annual salinity and the range (in bracket) of Lakes 1, 2, and 3 were 46.5(20-146), 39.2(28-55.5), and 29.4(14- 52.25), respectively. Mean annual pH values were 9.7(9.2-10.1), 9.9(9.47-10.54), and 10.0(9.5-10.5), respectively (Table 1). The three sublakes were similar in salinity with seasonal variation: high salinity in summer and winter, and lower in midspring and autumn. The mean annual salinity and pH value of the entire lake water were approximately 38 and 9.9, respectively. Total lake transparency ranged from 0.2 to 0.8 m.
Seasonal variation in total nitrogen and total phosphorus based on monthly measurements at the surface at the main stations in Lakes 2 and 3 are shown in Fig. 2e and f. The total nitrogen content was high during all sampling periods. The mean annual range was approximately 12.43 mg/L, it was lowest in August (<5 mg/L) and highest in February (10.0 mg/L). In Bg2, the total phosphorus concentration was 0.328 mg/L, it was lowest in December (<5 mg/L) and increasing gradually to the maximum in March (0.591 mg/L). In Bg3, the mean annual total nitrogen content was approximately 10.37 mg/L, it was lowest in August (5.9 mg/L) and highest in April (20.53 mg/L); the total phosphorus concentration was 0.437 mg/L, it was lowest in July (0.240 mg/L) and increased gradually to the maximum in April (1.68 mg/L).
In general, the dominant cation was sodium, followed by magnesium; carbon was the dominant anion (Table 1). The water type was classified as NaCO3SO4 according to Hammer (1986).
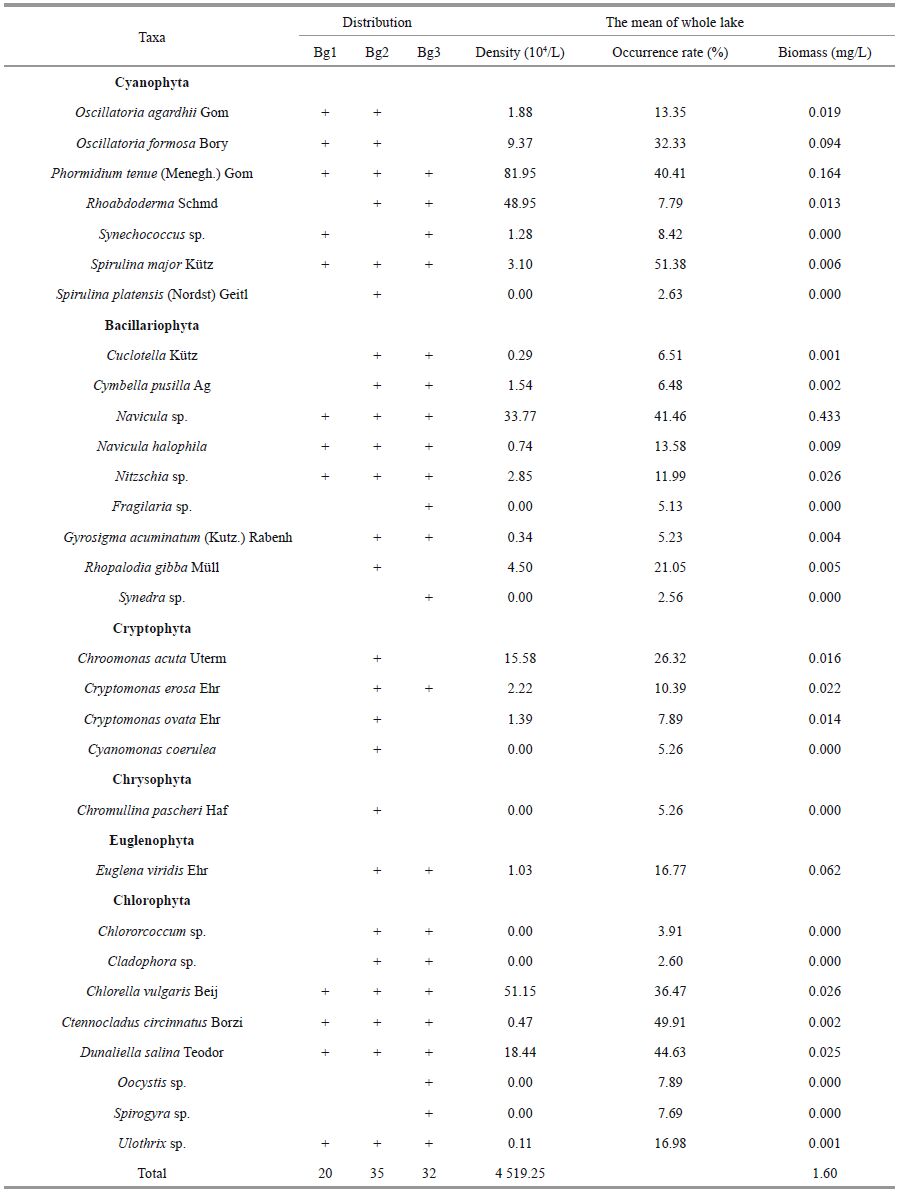
There were 41 phytoplankton taxa in Bange Lake, belonging to six phyla (Table 2). Cyanobacteria (18 species), diatoms (nine taxa), and green algae (eight taxa) were the most diverse. Other taxa included four species of Cryptophyta, one species of Euglenophyta, and one species of Chrysophyta.

|
|
The dominant species were Gloeothece linearis, Oscillatoria tenuis, Gloeocapsa punctata, Phormidium tenue, Navicula sp., Ctenocladus circinnatus, and Dunaliella salina. Most of the algae recorded are common in other saline lakes, but some are rather rare, particularly Ctenocladus circinnatus.
The seasonal variation in total phytoplankton biomass varied from 0.31 mg/L in July 2001 to 5.21 mg/L in September 2001 in Bg1(Fig. 3d); from 0.32 mg/L in summer to 2.80 mg/L in late spring in Bg2(Fig. 3e); and from 0.04 mg/L in winter to 11.69 mg/L in late spring in Bg3(Fig. 3f). The total phytoplankton biomass remained low, the annual mean phytoplankton biomass in Lakes 1, 2, and 3 were 2.02, 1.28, and 1.21 mg/L, respectively. The mean annual total phytoplankton biomass in the lake was 1.60 mg/L.
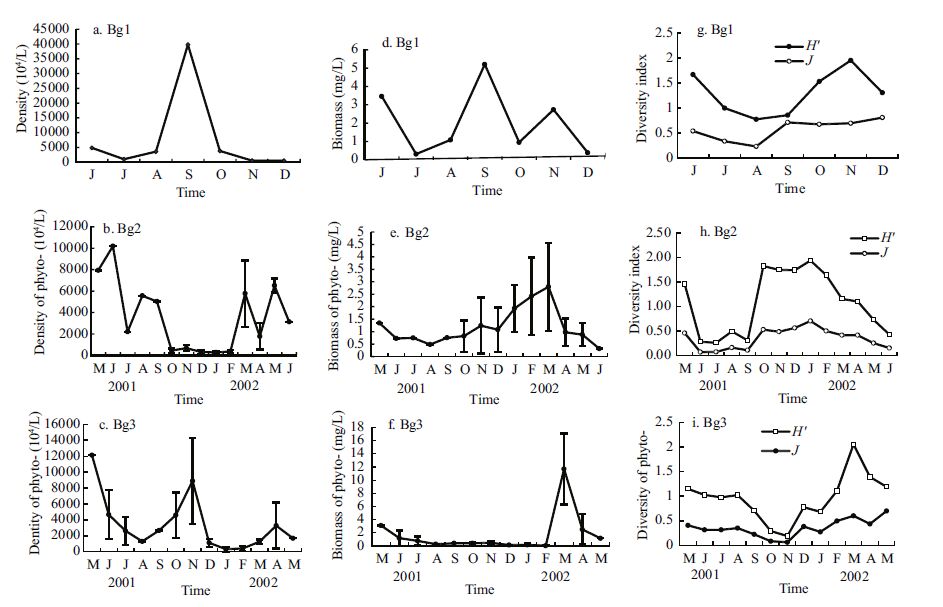
|
| Figure 3 Dynamics of the density (a-c), biomass (d-f), and diversity index (g-i) of total phytoplankton in Bange Lakes 1, 2, and 3 from May 2001 to June 2002 |
The seasonal cycle of phytoplankton abundance showed no difference from that of biomass but the patterns differed among the three sublakes. The seasonal variation in total phytoplankton density ranged from 5.20×106 cells/L in December 2001 to 3.98×108 cells/L in September 2001 in Bg1(Fig. 3a); from 2.76×106 cells/L in winter to 1.02×108 cells/L in late spring and summer in Bg2(Fig. 3b); and from 3.16×106 cells/L in winter to 1.21×108 cells/L in late spring and to late autumn 0.89×108 cells/L in Bg3(Fig. 3c). The total phytoplankton density remained high, the annual mean phytoplankton abundance of Lakes 1, 2, 3 were 7.74×107, 2.89×107, and 2.81×107 cells/L, respectively. The mean annual total phytoplankton density in the lake was 4.52×107 cells/L. Seasonal variation in the phytoplankton diversity index was characterized by the lowest value in summer and highest in winter and spring (Fig. 3g-i). The annual mean phytoplankton diversity indices H' and J(in bracket) of the three sublakes were 1.299(0.571), 1.080(0.350), and 0.966(0.358), respectively. The annual means of phytoplankton H' and J were 1.112 and 0.426, respectively.
3.3 Taxonomic composition of phytoplanktonThe seasonal changes in relative abundance of the major taxonomic groups are presented in Table 2. Cyanobacteria abundance was dominated by phytoplankton all year. The cyanobacteria Gloeothece linearis (70.3%) and Gloeocapsa punctata (21.8%) dominated the total phytoplankton density (92.1%). In Bg1, the cyanobacteria biomass dominated the phytoplankton all year (Fig. 4). In Bg2, diatoms made up a large proportion of the total phytoplankton biomass in winter and spring, and cyanobacteria in other seasons. In Bg3, cyanobacteria were the major contributor to the total phytoplankton biomass in summer and autumn, while diatoms were in winter and spring. The percentage of cyanobacteria in the three sublakes for the year was 88.3%, 39.6%, and 50.1%; and that of diatoms was 8.62%, 45.4%, and 32.6%, respectively. As far as the entire lake is concerned, the biomass percentages of Cyanophyta, Bacillariophyta, Chlorophyta, Euglenophyta, and Cryptophyta for the year were 59.3%, 28.9%, 6.57%, 3.58%, and 1.69%, respectively. Five algae species, including Navicula sp.(27.1%), G. linearis (18%), O. tenuis (11.3%), G. punctata (10.1%), and P. tenue (6.5) were the main contributors to the total phytoplankton biomass (72.9%).
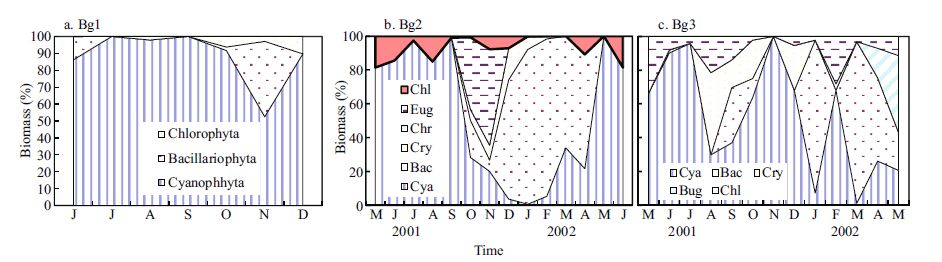
|
| Figure 4 Relative biomass frequency of the most numerous phytoplankton taxa in Bange Lake from May 2001 to July 2002 The values are means of the lakes sampled. |
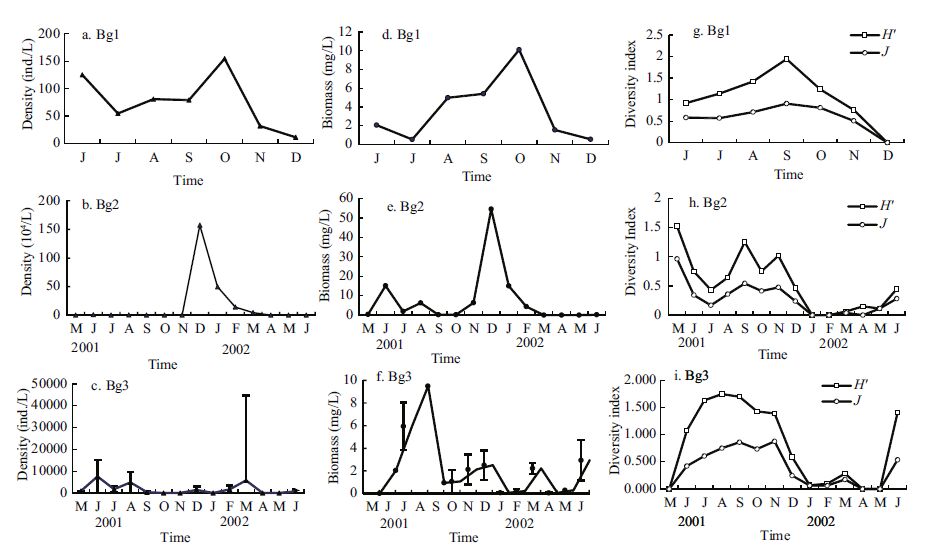
|
| Figure 5 Dynamics of the density (a-c), biomass (d-f), and diversity index (g-i) of total zooplankton in Bange Lakes 1, 2, and 3 from May 2001 to June 2002 |
In Bg1, the seasonal variation in total zooplankton biomass featured a low winter value (0 mg/L) and a maximum (3.45 mg/L) in the mid-autumn (Fig. 5d). In Bg2, it was low in winter and late autumn (0- 0.20 mg/L) and peaked (54.6 mg/L) in winter (Fig. 5e). In Bg3, it was low in late winter and spring (0- 0.10 mg/L) and reached a maximum (9.49 mg/L) in the autumn (Fig. 5f). The annual mean total zooplankton biomass in Lakes 1-3 was 1.23, 9.98, and 2.13 mg/L, respectively, with a total-lake average of 4.45 mg/L.
The seasonal succession of zooplankton abundance in Bg1 was similar to that of biomass: low winter values and a peak value in the early summer, reaching a minimum of ~0 ind./L in December and a maximum of 116 ind./L in June (Fig. 5a). In Bg2, zooplankton abundance values peaked in winter (1.57×106 ind./L) and were lowest in spring (0-200 ind./L)(Fig. 5b). In Bg3, values were low in autumn and winter (0- 50 ind./L) and high in spring (3.89×105 ind./L)(Fig. 5c). The annual mean zooplankton abundance was 52, 162, 322, and 57, 144 ind./L, in the three sublakes, respectively.
The seasonal succession of zooplankton diversity index generally reflected the variations in zooplankton abundance in the three sublakes, with a similar pattern of a winter/spring low and autumn/summer high (Fig. 5g-i). The annual mean zooplankton diversity indices H' and J(in bracket) of Lakes 1, 2, and 3 were 1.060(0.583), 0.544(0.281), and 0.814(0.381), respectively. The annual mean zooplankton H' and J were 0.662 and 0.415, respectively for the entire lake combined.
3.5 Taxonomic composition of zooplankton and benthic tychoplanktonThe zooplankton species/taxa composition and distribution are given in Table 3. A total of 21 species and taxonomic groups were identified, including 11 Protista, 4 Copepoda, 3 Rotifera, 2 Cladocerans, and 1 Anostraca. In the frequency of occurrence, biomass, and density, the major species were Holophrya actra [Protista]; Brachionus plicatilis [Rotifera]; Artemia sp. [Anostraca]; Daphniopsis tibetana and Alona quadrangularis [Cladocera]; and Cletocamptus dertersi and Arctodiaptomus salinus [Copepoda]. The number of animal species ranged 1-12, and tended to decrease with increasing salinity. Most of the animals recorded are common in other Tibetan saline lakes. The most abundant groups are discussed in more detail below.
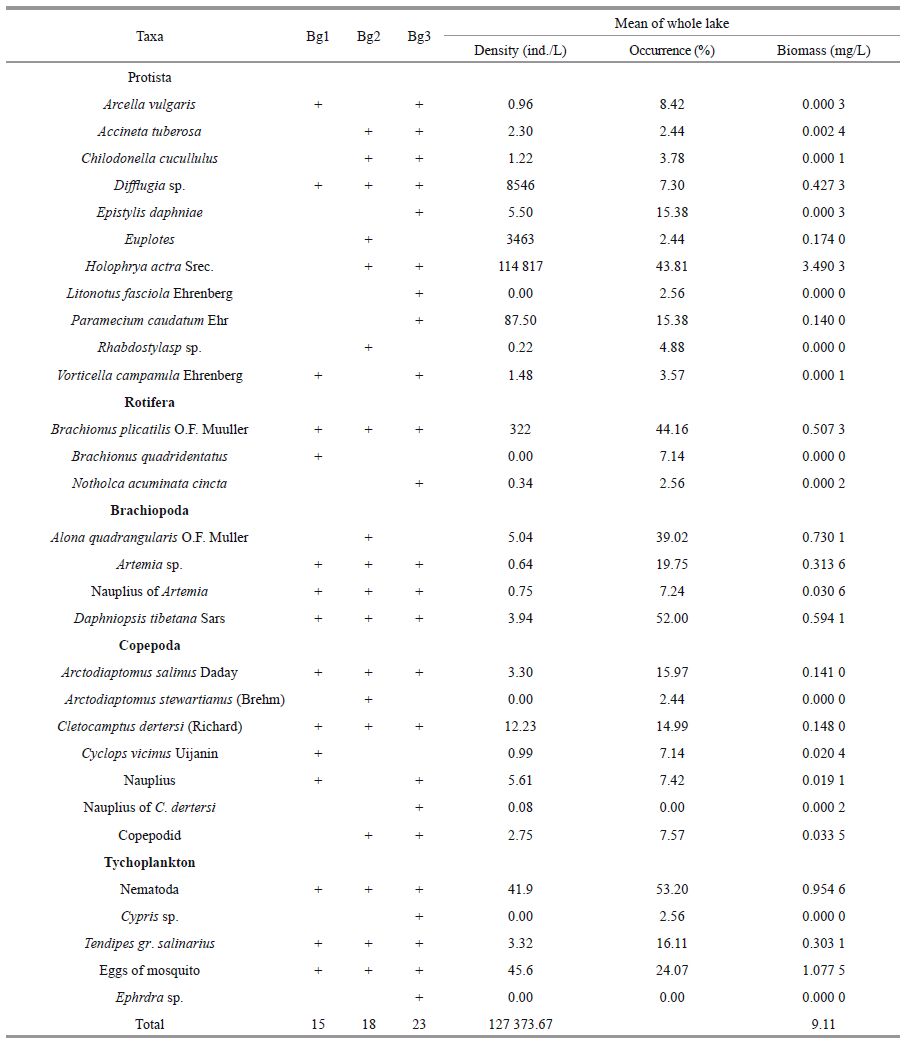
|
Four benthic tychoplankton (macroinvertebrates) taxa are listed in Table 3. They were represented by Cypris sp. [Ostracoda], chironomids Tendipus group salinarius [Diptera], Nematoda, and Ephedra sp. Moreover, mosquito eggs were dominant in the lakes. The annual mean tychoplankton abundances in Lakes 1, 2, and 3 were 47, 67, and 654 ind./L, respectively. The annual mean tychoplankton biomass was 2.36, 0.16, and 2.03 mg/L, respectively.
The seasonal changes in the relative abundance and biomass of the major taxonomic groups are illustrated in Fig. 6. Tychoplankton dominated all year in Bg1, accounting for 47% zooplankton abundance and 65.8% biomass (including Tychoplankton). Rotifera (57.2%) dominated zooplankton abundance and Branchiopoda (58.6%), such as D. tibetana, dominated the biomass (not including Tychoplankton) in Bg1(Fig. 6a, b). In Bg2, the Protista dominated zooplankton abundance and biomass in winter and spring from November 2001 to April 2002, and Rotifera dominated in summer and autumn (Fig. 6c, d). The Branchiopoda dominated in June and November 2001(Fig. 6d). Protista dominated the zooplankton, accounting for 99.4% zooplankton abundance and 68.54% biomass for the entire year. Moreover, Rotifera was the second most dominant taxonomic group (0.58% abundance and 18.8% biomass). The pattern of abundance and biomass in Bg3 was similar to that in Bg2(Fig. 6e, f). Protista dominated zooplankton abundance (85.8%) all year (Fig. 6e). Tychoplankton dominated zooplankton biomass with 48.8%(Fig. 6f).
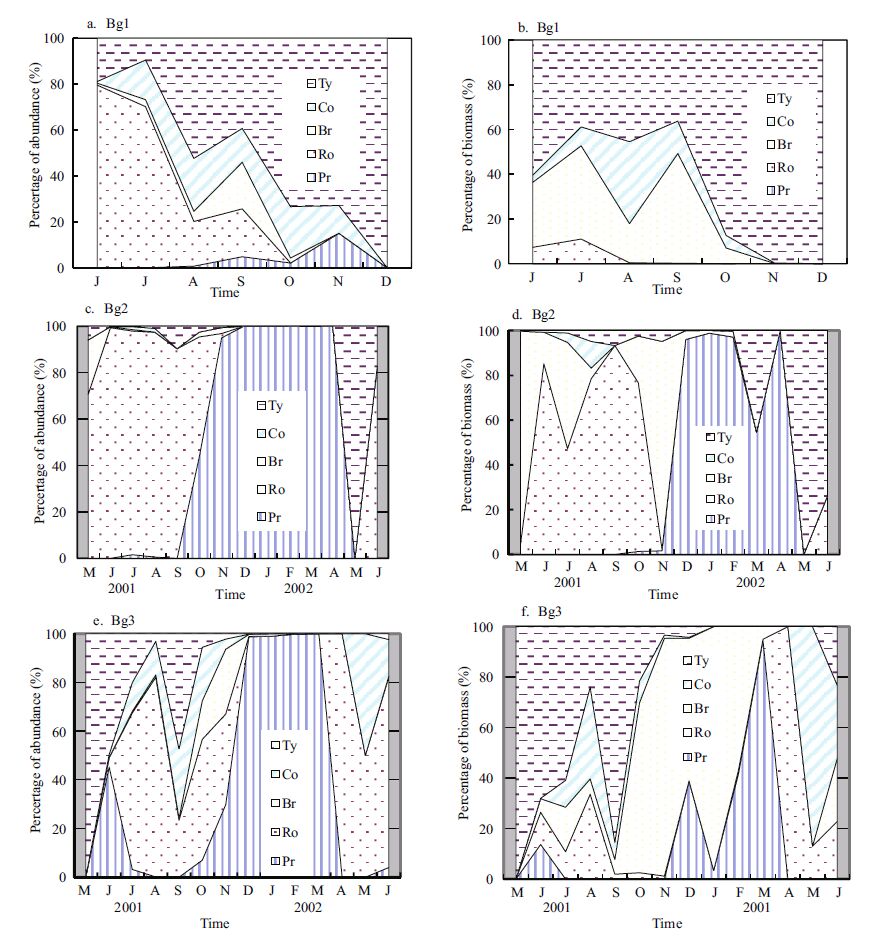
|
| Figure 6 Relative frequency of zooplankton taxa density (a, c, e) and biomass (b, d, f) in Bange Lakes 1, 2, and 3, from May 2001 to July 2002 |
The dynamics of dominant zooplankton and tychoplankton species in Bange Lake are presented in Fig. 7. Some of the dominant species in the sublakes exhibited similar patterns of variation in seasonal abundance. The numbers of Brachionus plicatilis remained low during the winter and spring months and peaked once during the summer from June to August at 100, 8 622, and 2 712 ind./L in Lakes 1-3, respectively (Fig. 7a, f, h). Daphniopsis tibetana densities remained low in the spring and summer months and peaked once during the autumn and early winter at 18, 62, and 17.4 ind./L in Lakes 1-3, respectively, and Artemia sp. numbers remained high during the summer at 2.2, 37, and 8.7 ind./L, respectively (Fig. 7b, d, j). Holophrya actra abundance peaked once (1.31×108 ind./L) in late winter in Bg2 and twice in July 2001(6 375 ind./L) and March 2002(5 791 ind./L) in Bg3. Cletocamptus dertersi peaked in August at 94 and 326 ind./L in Lakes 2 and 3, respectively. Arctodiaptomus stewartianus also peaked in August in Lakes 1-3, at 30.4, 15.6, and 41.5 ind./L, respectively. Nematoda was dominant species of benthic macroinvertebrate, with one peak in October 2001(99 ind./L, in Bg1) and one in May 2002(783 ind./L in Bg2).
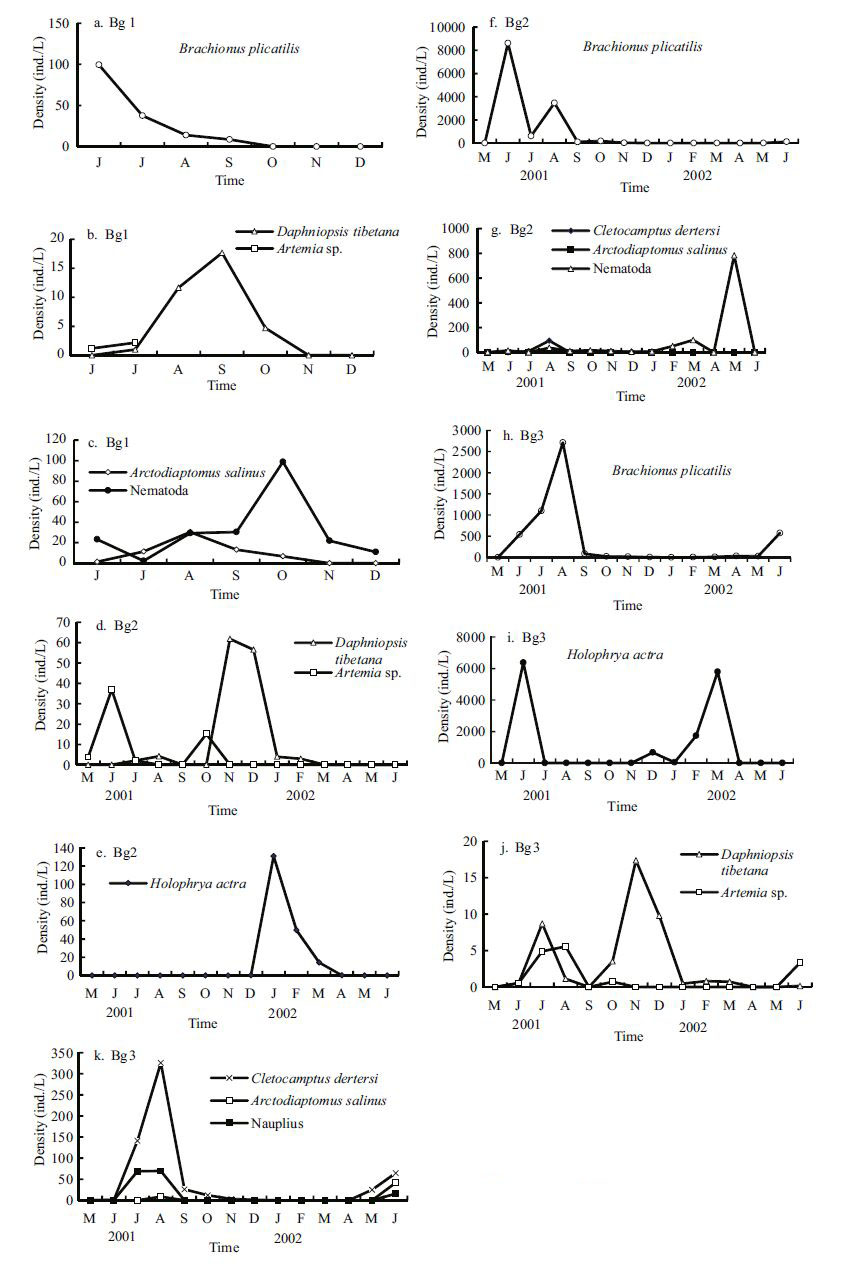
|
| Figure 7 The dynamics of the dominant zooplankton species in Bange Lake |
The phytoplankton taxa numbers, abundance, and biomass were significantly negatively correlated with salinity (R 89 =-0.175 to 0.225, P <0.05). The phytoplankton diversity index tended to increase with increasing salinity but decreased with increasing water temperature (Table 4).
The zooplankton taxa numbers, abundance, and biomass decreased with increasing salinity, but taxa numbers were significantly positively correlated with water temperature (R 103 =0.512, P <0.01). The zooplankton diversity index was significantly negatively correlated with salinity and pH (R 103 = -0.200, P <0.05 salinity and R 103 =-0.303, P <0.01 pH), but positively correlated with water temperature (R 103 =0.507 and R 103 =0.512, P <0.01)(Table 5).
4 DISCUSSIONThe annual mean salinity in the Bange Lake sublakes was 46.5, 39.2, and 29.4. The salinity reached its maximum value in late December 2001 in Bg1 and its minimum in early April 2002 in Bg2(Fig. 2) ranging from 4 to 146. The water type was NaCO3 SO4 according to the Hammer (1986) scheme. Salinity was significantly negatively correlation with water temperature (R 106 =-0.325, P <0.01). Thus, in late spring, the relative quantity of snow water melt from jokul was highest, while during spring salinity decreased as a result of the mixing of the jokul water. The mean phytoplankton biomass in Bange Lake was 1.60 mg/L, and was dominated by Cyanophytes, diatoms, and green algae. The mean zooplankton biomass was 4.45 mg/L. The ratio of zooplankton biomass to that of phytoplankton was 2.8. The predominant zooplankton members included Daphniopsis tibetana, Cletocamptus dertersi, and Arctodiaptomus stewartianus.
In general, biological composition in inland saline waters is very simple. Long-time investigations on plankton flora and fauna in Tibetan saline lakes are scarce. Wang et al.(1975) reported 53 algal genera and 26 zooplankton species in Qinghai Lake (12.5 salinity, 4 456 km 2 in area); and the sampling lasted 4 years. Ma (1995) reported 23 algal species in some northern Chinese saltpans and lakes ranging in salinity from 61.0 to 320.0. Zhao and He (1999) reported 91 algal species in saline waters and lakes in Zhangjiakou, northern Hebei Province, ranging in salinity from 0.98 to 175.2. Ren et al.(1996) reported 92 species (or genera) of algae in 23 inland saline lakes (12.4- 394.5) in NW China. He et al.(1981) reported 52 genera algae and 35 species zooplankton in Da-Li Lake located in eastern Inner Mongolia, China (5.5, 238 km 2 in area, sampling 4 times in a year). He et al.(1994) identified 42 algal genera and 62 zooplankton species in Xiaochi Lake (10.3-78.3, 15 km 2 in area, five samples in 4 years). Xu et al (2002) reported 46 algae taxa and 12 zooplankton species in Gahai Lake (119, 37.4 km 2 in area, sampling in summer 1997). Zhao et al.(2005) reported 95 algae taxa and 42 zooplankton taxa in Ali-Tibetan saline lakes in Ali, northern Tibet, China, where salinity ranged from 1 to 390.
In the present investigation, 41 phytoplankton and 21 zooplankton taxa were identified in Bange Lake during the year in bi-monthly sampling. Compared to those of other studies, the plankton species and taxa in Bange Lake were very few. In particular, there were fewer zooplankton species in Bange Lake than in Qinghai, Dalai, and Xiaochi lakes. We believe that this situation can be attributed to the salinity, ion composition, and other adverse conditions in the environment. In terms of fish fauna, there are two species of Gymnocypris przewalskii, four Nemacheilus species, crucian carp (Carassius auratus L.), and Leuciscus walekii (Dyb.) in Dalai Lake. There is only one fish species (Oryzias latipes) in Xiaochi Lake (16.3). No fish were found in Bange Lake.
Among the 41 phytoplanktonic algae taxa recorded, Dunaliella salina, Ctenocladus circinnatus, Spirulina major, Diatoma elongatum, and some others were either typical hyposaline or halobiont species, and most of them were either salt-tolerant freshwater species, euryhaline species or halophile species that are adapted to a wide range of salinities. These results are in accordance with those from other regions (Fott, 1971; Geddes et al., 1981; He et al., 1981, 1989, 1993, 1994; Hammer et al., 1983; Hammer, 1986; Ehrlich and Dor, 1985; Colburn, 1988; Wood and Tailing, 1988; Jakher et al., 1990; Servant-Vildary and Roux, 1990; Zhao, 1992b; Zhao et al., 1993; Ma, 1995; Ren et al., 1996; Zhao and He, 1999).
Of the 21 zooplankton species recorded, Brachionus plicatilis, Artemia sp., and Daphniopsis tibetana are regarded as halobiont species as per Beadle (1981), Gong (1983), Williams (1983), Hammer (1986), He et al.(1989), Alonso (1990), Zhao (1992a), Zhao and He (1993), Zhao et al.(1996, 2010b), and Ren et al.(1996). Information on protistans in inland saline waters around the world is rare (Shen, 1983; Hammer, 1986; Stephens, 1990; Zhao and He, 1995). Freshwater species were predominant in Bange Lake phytoplankton and zooplankton, but many were halobiont species. The proportion of halobiont species in both total species and taxa was 10%.
Our previous study (Zhao and He, 1995) and this one have shown that protozoans, especially ciliates, play an important role in inland saline waters, and ciliates density and biomass are usually higher in hypersaline waters where they might dominate particular communities in some seasons, they were abundant in Bg2 in January 2002. Nevertheless, as noted by other researchers, most protistan species in inland saline waters are of freshwater derivation, indicating that the Protista are very salt tolerant (Wang, 1977; Shen, 1983; Zhao and He, 1995).
D. tibetana is a halobiont Cladoceran, which lives in plateau lakes at altitudes of >4 000 m and occurs widely in hyposaline lakes in the northern Tibet, Qinghai, and Xinjiang, China (Zhao et al., 2002). Additionally, D. tibetana often occurs in Russia and India. In China, the species is endemic to the Tibet region as cited by Jiang et al.(1983); however, it was also found in Sayram Lake, Xinjiang, where temperatures ranged from -2℃ to 20℃, salinity from 9 to 35 and pH from 9.0 to 10.4(Zhao et al., 2002). In this investigation, D. tibetana was dominant above all other species at a density of 83 ind./L. This Cladoceran is a potential live food for marine fish and other economic animals in marine water acclimation.
In the northern Tibet, Ctenocladus circinnatus is widespread where there are no filamentous green algae. It can survive salinities as high as 200(e.g., Gangtang Lake). In this investigation, Ctenocladus circinnatus occurred in Rabang Lake (73-75) and Kahu Lake (300-310). Some green algal taxa are endemic to salt lakes in this region, for example, as Hu and Wei (1981) mentioned in particular, Didymonema tibeticum. Moreover, Cladophora globulina and Ulothrix implexa are two other green algal genera found in the northern Tibetan saline waters, although they are less important than C. circinnatus. A form of Dunaliella salina particularly tolerant to cold was reported from Zabuye Lake (Zheng et al., 1989). Cyanophyta in Tibetan salt lakes, according to Li (1981), are the most common taxa that can inhabit in both fresh and saline waters.
High and often variable salinity in salt lakes often reduces biodiversity. The higher the salinity, the fewer plankton species occur, resulting in smaller a diversity index and total species number, which was confirmed by our results. High alkalinities could also decrease diversity and species number (Beadle, 1981; Zhao and He, 1999). Williams et al.(1990), in a study on the macroinvertebrate fauna of 79 Australian salt lakes, suggested that although salinity per se is relatively unimportant in determining what species occur in a particular lake, species richness and composition nevertheless strongly correlate with salinity over the total salinity range. This relationship, however, becomes insignificant above an intermediate salinity range, which has been also reported by other authors (Colburn, 1988; Wood and Talling, 1988; He et al., 1989; Hammer et al., 1990; Zhao, 1992a). The implication is that many factors other than salinity may determine the distribution of aquatic organisms in salt lakes. In the present study, the saline lakes investigated have high alkalinity and pH. Again, this suggests that salinity itself is not the only important factor determining the species composition of plankton. Local climate, ionic composition, alkalinity, pH, and some biological factors are also influential (Lei et al., 1985). In this study, in terms of the number of taxa, the zooplankton diversity index was significantly negatively correlated with pH in Lakes 2 and 3. However, community structure and diversity are also strongly determined by resource interactions (Paine, 1980; Leibold et al., 1997; Vargas et al., 2006).
The mean phytoplankton biomass in Bange Lake was 1.60 mg/L, and was dominated by cyanobacteria and diatoms. The mean zooplankton biomass (including tychoplankton) was 9.11 mg/L (Table 3). The ratio of total zooplankton biomass to that of phytoplankton was 5.7:1, which was lower than the 14.8:1 reported in Namuka Lake (Zhao et al., 2010a), near Bange Lake. The zooplankton biomass (ZB) in freshwater lakes is generally much lower than the phytoplankton biomass (PB). For example, the PB/ZB ratio was 0.9:1 in Xiaochi Lake in the southern Shanxi Province, 1.2:1 in Dali Lake in Inner Mongolia; PB/ZB ratio in Namuka Lake was close to that of 10.4 in Lake Bolshoy Sanntroy in pre-Soviet Union, where the ratio of phytoplankton: bacterioplankton: zooplankton biomass was 1:2.5:10.4(Hammer, 1986).
The zooplankton biomass in Bange Lake may be higher, equal to the level of high-production fishponds (10-20 mg/L), indicating that a higher biomass of bacteria and detrital phytoplankton maintained zooplankton food resources. Moreover, higher zooplankton biomass may be the result of there being no fish feeding in the lake. Therefore, the zooplankton biomass was at the top position of communities in this aquatic ecosystem.
In Bange Lake, total nitrogen and phosphorus concentrations were very high. The nutrient characteristics were that of a typical eutrophic lake. Furthermore, the dominant phytoplankton groups were cyanobacteria, diatoms, and chlorophytes; phytoplankton density and biomass were also very high in some months, similar to other subtropical and eutrophic lakes in China (Zhao et al., 2010b). The zooplankton and phytoplankton biomass exhibited obvious seasonal variations. The maximum phytoplankton biomass in Bg2 and Bg3 occurred in March 2002, which coincided with lower TP concentrations. Meanwhile, the ratio of TN to TP was 37.9:1 for Bg2, and 23.7:1 for Bg3, which differs from the optimum TN:TP ratio (10:1-17:1) for phytoplankton growth proposed by Wetzel (1983). In winter (December), lower phytoplankton biomass likely resulted from cooler water and light deficiency, while higher phytoplankton biomass in spring (March) likely resulted from higher TP concentrations.
Some species of Arctodiaptomus, e.g., A. salinus, A. rectispinosus, and A. stewartianus, are geographically widespread in inland saline waters in the northern hemisphere; they were also a common feature of the Bange Lake fauna.
Artemia is the best-known and most economically useful salt lake species. In the present investigation, Artemia sp. occurred in Bange Lake from April to July 2002. However, the density was very low because of the low salinity of <60. Therefore, it could not compete with other zooplankton such as B. plicatilis and D. tibetana, which reproduce and grow faster than Artemia sp.
This investigation did not look at planktonic bacteria. Future research into the genetic biodiversity and resource development and utilization of saline lake organisms would be useful.
5 CONCLUSIONThis study clarifies the seasonal variations in plankton species composition, abundance, and biomass in relation to hydrography in the saline Bange Lake. Forty-one phytoplankton, 21 zooplankton, and 5 benthic/facultative zooplankton taxa were identified over 1 year. The major phytoplankton species were Gloeothece linearis, Oscillatoria tenuis, Gloeocapsa punctata, Ctenocladus circinnatus, Dunaliella salina, Spirulina major. The major zooplankton species included Holophrya actra, Brachionus plicatilis, Daphniopsis tibetana, Cletocamptus dertersi, and Arctodiaptomus salinus. The total number of plankton species was significantly negatively correlated with salinity.
6 ACKNOWLEDGEMENTThe authors wish to thank the many colleagues that assisted with the collection and analysis of water samples.
| Alonso M, 1990. Anostraca, Cladocera and Copepoda of Spanish saline lakes. Hydrobiologia, 197 (1) : 221 –231. Doi: 10.1007/BF00026952 |
| An Y X, He Z H, 1991. Toxicity of four heavy metals in marine water to Moina mongolica. Journal of Fisheries of China, 15 (4) : 273 –282. |
| Beadle L C, 1981. The Inland Waters of Tropical Africa. Langman, London, UK. |
| Chen Z M, 1981. Approaches to the changes of ecological environment of lakes in Xizang based on the upheaval of the Qinghai-Xizang plateau. Oceanologia et Limnologia Sinica, 12 (5) : 402 –411. |
| Colburn E A, 1988. Factors influencing species diversity in saline waters of Death Valley, USA. Hydrobiologia, 158 (1) : 215 –226. Doi: 10.1007/BF00026279 |
| Ehrlich A, Dor I. 1985. Photosynthetic microorganisms of the Gavish Sabkha. In:Friedman G M, Krumbein W E eds.Hypersaline Ecosystems:The Gavish Sabkha. Springer-Verlag, Berlin Heidelberg. p.296-321. |
| Fott B. 1971. Algenkunde. 2nd edn. VEB Gustav Fischer Verlag, Jena. 581p. |
| Geddes M C, de Deckker P, Williams W D, Morton D W, Topping M, 1981. On the chemistry and biota of some saline lakes in Western Australia. Hydrobiologia, 81 . |
| Gong X J. 1983. Rotifera of Tibetan Plateau. In:Jiang X Z, Shen Y F, Gong X J eds. Aquatic Invertebrates of the Tibetan Plateau. Series of the Scientific Expedition to Qinghai-Xizang Plateau. Science Press, Beijing, China.(in Chinese) |
| Green J, Mengestou S, 1991. Specific diversity and community structure of Rotifera in a salinity series of Ethiopian inland waters. Hydrobiologia, 209 (2) : 95 –106. Doi: 10.1007/BF00006921 |
| Hammer U T, Shamess J, Haynes R C, 1983. The distribution and abundance of algae in saline lakes of Saskatchewan, Canada. Hydrobiologia, 105 (1) : 1 –26. Doi: 10.1007/BF00025173 |
| Hammer U T, Sheard J S, Kranabetter J, 1990. Distribution and abundance of littoral benthic fauna in Canadian prairie saline lakes. Hydrobiologia, 197 (1) : 173 –192. Doi: 10.1007/BF00026949 |
| Hammer U T. 1986. Saline Lakes Ecosystems of the World.Junk, Dordrecht. |
| He Z H, Jiang H, Bi F S, 1996. Further research on the hydrochemistry and hydrobiologia of Dali Lake. Journal of Dalian Fisheries University, 11 (2) : 1 –13. |
| He Z H, Qin J G, Wang H Q, Wang Z H, Xia X, 1989. Studies on the saline and hypersaline zooplanktons from Jinnan and Yinchuan regions. Acta Hydrobiologic a Sinica, 13 (1) : 24 –37. |
| He Z H, Qin J G, Wang Y, Jiang H, Zhao W, 2001. Biology of Moina mongolica (Moinidae, Cladocera) and perspective as live food for marine fish larvae:review. Hydrobiologia, 457 (1-3) : 25 –37. |
| He Z H, Qin K J, Wang Y, Zhao W, 1993. Biological resources in inland saline waters from Southern Shanxi, China. Part I. Lake Xiao chi. Journal of Dalian Fisheries University, 8 (4) : 1 –16. |
| He Z H, Qin K J, Wang Y, Zhao W, 1994. Biological resources in inland saline waters from Southern Shanxi, China. Part II. Lake Bei Mentan. Journal of Dalian Fisheries University, 9 (2) : 1 –16. |
| He Z H, Xie Z H, Lei Y Z, 1981. Studies on the hydrochemistry and hydrobiologia of Da-Li Lake. Acta Hydrobiologic a Sinica, 7 (3) : 341 –357. |
| He Z H, Xie Z H, Lei Y Z, 1984. Preliminary studies on the hydrochemistry and hydrobiologia of Wuliangsuhai Lake. Journal of Dalian Fisheries University (1) : 41 –56. |
| Hu H J, Wei Y X. 1981. The characteristics of the green algal flora of Xizang and the unheaval of the plateau in relation to certain endemic species. In:Anon. ed. Geological and Ecological Studies of Qing-Xiang Plateau. Proceedings of Symposium on Qinghai-Xizang (Tibet) Plateau (Beijing, China). Science Press, Beijing, Gordon and Breach Science Publishers, Inc., NY. |
| Huang X F, Chen W M, Cai Q M, 1999. Survey, Observation and Analysis of Lake Ecology. Standards Press of China Beijing. |
| Jakher G R, Bhargava S C, Sinha R K, 1990. Comparative limnology of Sambhar and Didwana lakes (Rajasthan, NW India). Hydrobiologia, 197 (1) : 245 –256. Doi: 10.1007/BF00026954 |
| Jiang X Z, Du N S, 1979. Fauna Sinica. Science Press, Beijing. |
| Jiang X Z, Shen Y F, Gong X J, 1983. Aquatic Invertebrates of the Tibetan Plateau. Series of the Scientific Expedition to Qinghai-Xizang Plateau. Science Press, Beijing. |
| Koste W. 1978. Rotatoria. Die Radertiere Mitteleuropas. 2nd edn. Gebruder Borntraeger, Berlin and Stuttgart, 673p. |
| Lei Y Z, Dong S L, Shen C G, 1985. Study on the toxicity of carbonate-alkaline to fishes. Journal of Fisheries of China, 9 (2) : 171 –183. |
| Leibold M A, Chase J M, Shurin J B, Downing A L, 1997. Species turnover and the regulation of trophic structure. Annual Review of Ecology and Systematics, 28 : 467 –494. Doi: 10.1146/annurev.ecolsys.28.1.467 |
| Li X Y, Wei Y X, Xin S Z, Hu H J, 1992. The Algae of the Xizang Plateau. Science Press, Beijing. |
| Li Y Y. 1981. The blue-green algae of Xizang Plateau. In:Anon ed. Geological and ecological Studies of Qinghai-Xizang Plateau. Gorden & Breach, New York. p.1 131-1 134. |
| Ma Z Z, 1995. Preliminary studies on algae in salt pans and saline lakes of Northern China. Oceanologia et Limnologia Sinica, 26 (3) : 317 –322. |
| Paine R T, 1980. Food webs:Linkage, interaction strength and community infrastructure. Journal of Animal Ecology, 49 (3) : 666 –685. Doi: 10.2307/4220 |
| Ren M L, Quo Y, Wang J L, Su R, Li H, Ren B, 1996. Survey of Artemia Ecology and Resources in Inland Salt Lakes in Northwest China. Heilongjiang Science and Technology Press, Harbin. |
| Servant-Vildary S, Roux M, 1990. Multivariate analysis of diatoms and water chemistry in Bolivian saline lakes. Hydrobiologia, 197 (1) : 267 –290. Doi: 10.1007/BF00026956 |
| Shen J R, 1979. Fauna Sinica, Crustacea Freshwater Copepoda . |
| Shen Y F, 1983. Protozoa of the Tibetan Plateau. In:Jiang X Z, Shen Y F, Gong X J eds. Aquatic Invertebrates of the Tibetan Plateau. Series of the Scientific Expedition to Qinghai-Xizang Plateau. Science Press, Beijing, China. |
| Stephens D W, 1990. Changes in lake levels, salinity and the biological community of Great Salt Lake (Utah, USA), 1847-1987. Hydrobiologia, 197 (1) : 139 –146. Doi: 10.1007/BF00026946 |
| Vargas C A, Escribano R, Poulet S, 2006. Phytoplankton food quality determines time windows for successful zooplankton reproductive pulses. Ecology, 87 (12) : 2 . |
| Wang J J, 1977. Protozoa from some districes of Tibetan Plateau. Acta Zoologica Sinica, 23 (2) : 131 –160. |
| Wang J L, Zheng Y M, Xing D J, 1975. Investigation report of Qinghai Lake naked carps bait. In:Qinghai Biological Research Institute ed. Fish Fauna and Biology of Naked Carps in Qinghai Lake. Science Press, Beijing. |
| Wetzel R G. 1983. Limnology. 2nd edn. Saunders, Philadelphia.p.1-300. |
| Williams W D, 1991. Chinese and Mongolian saline lakes:a limnological overview. Hydrobiologia, 210 : 39 –66. Doi: 10.1007/BF00014322 |
| Williams W D, Boulton A J, Taaffe R G, 1990. Salinity as a determinant of salt lake fauna:a question of scale. Hydrobiologia, 197 (1) : 257 –266. Doi: 10.1007/BF00026955 |
| Williams W D, 1983. Life in Inland Waters. Blackwell Scientific Publications, Victoria, Australia. |
| Wood R B, Talling J F, 1988. Chemical and algal relationships in a salinity series of Ethiopian inland waters. Hydrobiologia, 158 (1) : 29 –67. Doi: 10.1007/BF00026266 |
| Xu M Q, Cao H, Jia Q X, Gao Y R, Chen S G, 2002. Preliminary study of plankton community diversity of the Gahai Salt Lake in the Qaidam Basin of the Qinghai Tibet Plateau. Biodiversity Science, 10 (1) : 38 –43. |
| Yu S S, Tang Y, 1981. The hydrochemical characteristics of the saline lakes on the Qinghai-Xizang Plateau. Oceanologia et Limnologia Sinica, 12 (6) : 498 –511. |
| Zhao W, He Z H, 1993. Rotifera from inland saline waters in northern China. Journal of Dalian Fisheries University, 8 (2-3) : 20 –31. |
| Zhao W, He Z H, 1995. Protozoa from inland saline waters in North China. Acta Hydrobiologic a Sinica, 19 (3) : 193 –202. |
| Zhao W, He Z H, 1999. Biological and ecological features of inland saline waters in North Hebei, China. International Journal of Salt Lake Research, 8 (3) : 267 –285. |
| Zhao W, Jiang H, He Z H, 1996. Planktonic Crustaceans of inland saline waters in Sanbei District, Northern China. Journal of Dalian Fisheries University, 11 (1) : 1 –13. |
| Zhao W, Sun Z H, Huang Q, Xia Y J, Liu C Y, Wang Y Y, 1993. A Study on the plankton of inland saline water in northwest Jilin. Journal of Jilin Agricultural University, 15 (2) : 65 –71. |
| Zhao W, Wang Q H, Zheng M P, Zhao Y Y, Wang H L, 2002. A preliminary study on the biology of Daphniopsis tebitana Sars. Journal of Dalian Fisheries University, 17 (3) : 209 –214. |
| Zhao W, Ying X W, Peng Z, et al, 2010b. Ecology of Inland Saline Lakes in China. Science Press, Beijing. |
| Zhao W, Zhao Y Y, Wang Q H, Zheng M P, Zhang P, He Z H, 2010a. Spatial and temporal patterns of plankton assemblage structure in a high altitude saline lake, Namuka Co in Northern Tibet, China. CLEAN-Soil, Air, Water, 38 (10) : 960 –968. Doi: 10.1002/clen.v38:10 |
| Zhao W, Zheng M P, Xu X Z, et al, 2005. Biological and ecological features of saline lakes in northern Tibet, China. Hydrobiologia, 541 (1) : 189 –203. Doi: 10.1007/s10750-004-5707-0 |
| Zhao W, 1992a. A review on the Cladocera in inland saline waters. Journal of Dalian Fisheries University, 6 (2) : 31 –41. |
| Zhao W, 1992b. A Study on the phytoplankton from inland saline waters in northern China. Journal of Dalian Fisheries University, 7 (2-3) : 49 –64. |
| Zheng M P, Xiang J, Wei X J, Zheng Y, 1989. Saline Lakes on the Qinghai-Xizang (Tibet) Plateau. Beijing Scientific and Technical Publishing House, Beijing, China. |
| Zheng M P, 1995. On "Saline lake agriculture". Acta Geosicientia Sinica (4) : 404 –418.. |
| Zhu H Z, Chen J Y, 2000. Bacillariophyta of the Xizang Plateau. Science Press, Beijing. |
 2016, Vol. 34
2016, Vol. 34