Institute of Oceanology, Chinese Academy of Sciences
Article Information
- CHEN Ruoqi(陈若旗), LI Fangfang(李方方), LIU Jiadong(刘佳栋), ZHENG Hongye(郑红叶), SHEN Fei(沈飞), XUE Yarong(薛雅蓉), LIU Changhong(刘常宏)
- The combined effects of Dolichospermum flos-aquae, light, and temperature on microcystin production by Microcystis aeruginosa
- Chinese Journal of Oceanology and Limnology, 34(6): 1173-1182
- http://dx.doi.org/10.1007/s00343-016-5204-0
Article History
- Received Jul. 21, 2015
- accepted for publication Sep. 28, 2015
- accepted in principle Oct. 16, 2015
Microcystins (MCs) are a family of more than 90 different toxic variants, which have been identified from cyanobacteria (Schmidt et al., 2014), including Microcystis aeruginosa (Dawson, 1998), Anabaena spp.(Krishnamurthy et al., 1986), and Planktothrix spp.(Laub et al., 2002). MCs are synthesized nonribosomally by an integrated peptide-polyketide synthetase system, restricted within cells, and usually released when cells lyse (Carmichael, 1994). The cluster of microcystin synthetase genes from M. aeruginosa PCC7806, composed of 10 bidirectionally transcribed open reading frames, is arranged in two putative operons (mcyA-C and mcyD-J). The genes mcyA, mcyB, and mcyC encode nonribosomal peptide synthetases (NRPS), while mcyD codes for a polyketide synthase (PKS)(Tillett et al., 2000; Rouhiainen et al., 2004). The relative expression of mcyB and mcyD at least partially represents MC production under certain environmental conditions (Song et al., 2011). Among the MCs, MC-LR has received the most attention from regulatory agencies, such as the World Health Organization (WHO, 2003) and Health Canada (2002), as well as chemists, pharmacologists, biologists, and ecologists (Grosse et al., 2006; Butler et al., 2009), because, when released from M. aeruginosa, MCs can be a relevant hazard to aquatic organisms and human health, being considered the most toxic MC (Papadimitriou et al., 2012).
M. aeruginosa is a cosmopolitan cyanobacterium that develops toxic blooms in both temperate and tropical eutrophic freshwaters (Wu and Kong, 2008; Horst et al., 2014). The MC quota, or the toxin per cell or unit biomass, of Microcystis is a highly plastic trait among genotypes within some species (Horst et al., 2014). Several biological and/or physical factors, such as growth phase and rate (Orr and Jones, 1998), nitrogen (Scott et al., 2014), temperature (Lehman et al., 2008), light (Wiedner et al., 2003), phosphorous concentration (Oh et al., 2000), and grazers (Jang et al., 2003; PinedaMendoza et al., 2014), have been found to influence intracellular MC production in Microcystis. With respect to M. aeruginosa, it has been confirmed that the MC quota is substantially reduced under nitrogen-limited conditions relative to phosphorus-limited or nutrient-saturated conditions in both field and laboratory experiments (Horst et al., 2014). Recently, several photoautotrophic organisms have been shown to influence M. aeruginosa 's MC quota by excretion of secondary metabolites (LeBlanc et al., 2005; Li and Li, 2012; Hu et al., 2014). Among the compounds that might inhibit MC production, another cyanotoxin (cylindrospermopsin) has been indicated (Rzymski et al., 2014). However, although the effects of interspecific (Wan et al., 2007; Zhang et al., 2013; Rzymski et al., 2014) and/or intraspecific competition (Zhang and Song, 2006; Zhai et al., 2013) within cyanobacteria in natural ecosystems on MC quotas of toxigenic M. aeruginosa have been demonstrated, these effects still require further elucidation.
Our previous studies have shown that M. aeruginosa growth was inhibited while Dolichospermum (Anabaena) flos-aquae growth was promoted when the two species were grown in laboratory coculture (Zhang et al., 2014). This indicated that coculture effects might influence MC-LR biosynthesis by M. aeruginosa. Several environmental factors, such as light intensity and temperature, could enhance coculture effects on the MC-LR quota. Furthermore, genetic analyses of MC genes (mcyB and mcyD) have suggested that decreased MC-LR quotas because of competition with D. flos-aquae resulted from downregulation of genes encoding MC synthetases. In this study, coculture techniques were used to evaluate if the MC-LR quota of M. aeruginosa (Chen et al., 2003; Wu and Kong, 2008; Zhang et al., 2012) was influenced by light, temperature, and the nontoxic D. flos-aquae (Karadžić et al., 2010; Te and Gin, 2011).
2 MATERIAL AND METHOD 2.1 Algal speciesStrain PCC7820 of M. aeruginosa Kützing was kindly provided by Professor LI Pengfu at Nanjing University, China. MC-LR is the major toxic compound representing 81% of total MC (Ríos et al., 2014). Strain FACHB-245 of D. flos-aquae (Brébisson ex Bornet & Flahault) P. Wacklin, L. Hoffmann & J. Komárek was purchased from the culture collection of the Institute of Hydrobiology, Chinese Academy of Sciences, Wuhan, China; D. flos-aquae does not produce MCs. Each strain was axenically cultivated in batch culture in BG11 medium under 14/10 h dark/ light cycle with cool white light (20 µmol photon/(m2 ·s)) at 25±1℃(Zhai et al., 2013). Two methods were used to check if the two strains were axenic before and during experiments. One of these methods involved plating algal cultures on LB medium and checking for bacterial grow. The other was light microscopic examination for bacteria in algal cultures. The results showed that the test strains were axenic and devoid of bacterial contamination. The algal cells used for inoculation in experiments were taken from cultures 5 d after inoculation.
2.2 Algal cultivation and experiment designThe strains of M. aeruginosa and D. flos-aquae were cultured separately and together (1/1 mixture of both strains at their exponential growth phase) in 500- mL conical flasks containing 300 mL of BG11 medium. The initial concentration of M. aeruginosa and D. flos-aquae in each flask was 2×106 cells/mL, except where indicated otherwise. The flasks were incubated in growth chambers under the above conditions and shaken manually three times daily (Li and Li, 2012). The cell numbers of M. aeruginosa and D. flos-aquae (cells/mL) in the mono- and cocultured flasks were determined using a Neubauer Chambera hemocytometer (depth: 0.1 mm) under bright-field microscopy (Zhang et al., 2013), and the intracellular MC-LR quota (i.e., MC-LR per cell) in each flask measured by HPLC on specific days. All experiments were performed in triplicate. Previous results indicated that M. aeruginosa growth is inhibited while D. flos-aquae growth is promoted under cocultured conditions (Zhang et al., 2014).
Factors affecting the M. aeruginosa MC-LR quota in the presence of D. flos-aquae included temperature (15, 25, and 35℃), white light intensity (20, 30, 40, and 50 µmol photon/(m2 ·s)), and different ratios of initial inoculum (cell numbers) between M. aeruginosa and D. flos-aquae (M. aeruginosa / D. flos-aquae at 96/4, 90/10, 70/30, 50/50, and 30/70). For temperature tests, the light intensity was 20 µmol photon/(m2 ·s) and initial inoculation ratio at 50/50; for light intensity tests, the temperature was 25℃ and initial inoculation ratio at 50/50; for initial inoculation ratio tests, the temperature was 25℃ and light intensity at 20 µmol photon/(m2 ·s).
2.3 Extraction and quantification of MC-LRExtraction of intracellular MC-LR followed the method developed by Chen et al.(2010), with some modifications. Briefly, 100 mL of algal suspension were collected 6 d after inoculation and centrifuged at 3 500× g for 5 min. Algal cells in the resulting pellets were resuspended by adding 30 mL of 5% aqueous acetic acid (by vol), sonication for 1 h, and centrifugation at 3 500× g and 4℃ for 10 min. The pellet was then re-extracted three times using the acetic acid solution and all supernatants collected and transferred to a C18 cartridge (500 mg; Supelco, Inc., Bellefonte, PA, USA). Beforehand, the cartridge was washed with 10 mL of methanol followed by 10 mL of distilled water. After sample loading, impurities were eluted with 2 mL of methanol and MCs then eluted with 2 mL of 75% aq. methanol (by vol).
MC-LR was quantified using an HPLC (Labtech HPLC LC600, Labtech, Inc., Hopkinton, MA, USA) equipped with a reverse C18 column (250 mm× 4.6 mm). The mobile phase was composed of 40% Milli-Q water containing 0.05% trifluoroacetic acid and 60% HPLC-quality methanol (by vol). The flow rate was 1 mL/min and the eluate passed through a UV detector operated at 238 nm. The detector response was calibrated (µg/mL) against a standard curve produced from commercially purchased MCLR (>99% purity, Sigma-Aldrich, USA; Chen et al., 2010). The retention time of MC-LR was 17.4 min under these conditions.
The intracellular MC-LR quota (fg/cell) was calculated by dividing the MC-LR concentration (µg/mL) measured by HPLC by the number of M. aeruginosa (cells/mL) counted using a Neubauer Chamber under a microscope.
2.4 RNA extractionA 50-mL volume of both monoculture and coculture (initial cell numbers of M. aeruginosa, 1×106 cells/mL) were collected at day 3, 6, and 8 after inoculation and centrifuged at 3 500× g for 5 min. The precipitated cells were resuspended in 1 mL of TriPure reagent (Roche Molecular Biochemicals, Mannheim, Germany) with 0.5 g of 0.1-mm diameter glass beads (BioSpec Products, Inc., Bartlesville, OK, USA) and homogenized in a FastPrep Instrument (MP Biomedical, LLC, Solon, OH, USA) at a speed of 5 m/s for 40 s. RNA was extracted from the algal cells using a TriPure RNA Isolation Reagent Kit (Roche Molecular Biochemicals), following the manufacturer's instructions. Total RNA was resuspended in 20-50 µL of DEPC-H2 O, treated with DNase (Sangon Biotech Co., Ltd., Shanghai, China) at 37℃ for 45 min, and terminated by heating at 65℃ for 10 min. RNA integrity was verified by agarose electrophoresis with ethidium bromide staining. RNA purity was evaluated based on the OD260 /OD280 absorption ratio (>1.95) and checked by PCR analysis (Sevilla et al., 2010).
2.5 Reverse transcriptionReverse transcription of RNA was performed using the iScriptTM cDNA Synthesis Kit (Bio-Rad Laboratories, Inc., Hercules, CA, USA) with modified MMIV-derived reverse transcriptase and random hexamer primers. The reaction steps were incubation at 25℃ for 5 min, 42℃ for 30 min, 85℃ for 30 min, and finally held at 4℃.
2.6 RT-qPCRThe Micr184F/Micr431R primer pair was used to amplify a 220-bp fragment of the 16S rRNA gene, which is specific for M. aeruginosa (Saker et al., 2007). The mcyBF/mcyBR and mcyDF/mcyDR primer pairs were used to amplify 95- and 297-bp specific fragments of the MC synthetase genes, respectively, which are specific for mcyB (Yen et al., 2012) and mcyD genes (Kaebernick et al., 2000), respectively.
The RT-qPCR assay was performed in a 20-μL volume containing 10 μL of SsoFastTM EvaGreen Supermix (Bio-Rad Laboratories), 250 nmol/L of each primer, 1 μL cDNA template, and 7 μL of sterile ultrapure water. PCR was carried out in a Bio-Rad CFX-96 using the Bio-Rad CFX Manager Software (Bio-Rad Laboratories). Thermal cycling consisted of an initial cycle of 95℃ for 15 min, and then 40 cycles of 95℃ for 30 s, 60℃ for 60 s, and 72℃ for 30 s. Gene expression data from RT-qPCR were evaluated using a threshold cycle (Ct) value, and the 16S rRNA gene was used as the housekeeping gene for normalizing the expression of the target genes, as the expression of 16S rRNA is stable under various conditions (Bustin, 2000). All samples were amplified in triplicate. The relative expression concentrations of mcyB and mcyD from M. aeruginosa cells in cocultures were expressed as relative to the expression concentration of the genes in monocultures, which was quantified by the 2 - ΔΔ Ct method (Livak and Schmittgen, 2001), where ΔΔ Ct =(Ct, mcyB/D-C t, 16S rrn) coculture minus (Ct, mcyB/D-Ct, 16S rrn) monoculture, according to Bio-Rad CFX Manager Software (Bio-Rad Laboratories).
2.7 Statistical analysisData are presented as mean±SEM (standard error of mean). The results of triplicate experiments were analyzed with GraphPad Prism (version 5.0) software to estimate the significance of the differences (P<0.05 or P<0.01) using one-way ANOVA and the Dunnett test or Student's t-test. The Pearson correlation coefficient between cyanobacteria percentage and MC-LR quota was also analyzed.
3 RESULT 3.1 Effects of initial inoculation ratios onintracellular MC-LR quota The intracellular MC-LR quotas of M. aeruginosa under various ratios of M. aeruginosa to D. flos-aquae were estimated 6 d after inoculation (Fig. 1). It was clear that the MC-LR quota gradually decreased with increased initial ratio of D. flos-aquae to M. aeruginosa in the inoculum. When the ratio was greater than 90/10, the average MC-LR quota (24.2±2.5 fg/cell) was not significantly different from that in monoculture ((31.1±3.0 fg/cell, P >0.05). However, when the ratio ranged from 70/30 to 30/70, the MC-LR quotas in cocultures were significantly less than in monocultures (P<0.05). For instance, the MC-LR quota decreased to 16.2±0.0 fg/cell in cocultures when the ratio was 50/50, which was 48.4% less than in monocultures. Moreover, the intracellular MC-LR quota of M. aeruginosa was negatively correlated (R2 =0.93) with the percentages of D. flos-aquae in the initial inocula (P<0.01).
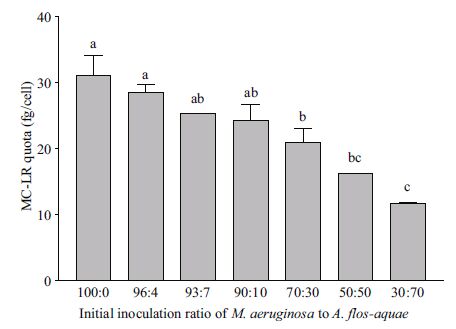
|
| Figure 1 MC-LR quotas of M. aeruginosa grown under coculture conditions for 6 d The x-axis indicates initial ratios of M. aeruginosa to D. flos-aquae. Different lowercase letters on top of bars define groups of treatments (triplicate sets) that showed significant differences among initial ratios (P<0.01) in one-way ANOVA and Dunnett tests. |
The effect of temperature on intracellular MC-LR quota of M. aeruginosa was examined under both mono- and coculture conditions 6 d after inoculation (Fig. 2). The results showed that MC-LR quotas ((108.1±6.5)-(142.1±14.8) fg/cell) in monocultures were not significantly different at a given temperature (P >0.05), while the highest quota in cocultures was 17.4±3.2 fg/cell at 25℃ and the lowest was 10.8±0.7 fg/cell at 15℃. MC-LR quotas of M. aeruginosa in cocultures were reduced by 98.8%, 54.9%, and 61.9% relative to those in monocultures at 15, 25, and 35℃, respectively. These results suggested that the effect of D. flos-aquae competition on the intracellular MC-LR quota of M. aeruginosa was enhanced at lower temperatures.

|
| Figure 2 Effect of temperature on MC-LR quotas of M. aeruginosa grown under mono- and coculture conditions Different lowercase letters on top of bars define groups of treatments (triplicate sets) that show significant differences among given temperatures (P<0.01) in one-way ANOVA and Dunnett tests. *, MC-LR quotas in cocultures that were significantly different from those in monoculture with P<0.05 in Student's t-test. |
Temperature also exhibited a significant impact on M. aeruginosa growth under both mono- and coculture conditions (Fig. 3). The optimal temperature for M. aeruginosa growth was at 25℃ and 35℃ under mono- and coculture conditions, respectively. M. aeruginosa cell numbers in monocultures at 25℃ were 5.6- and 1.8-fold more than those at 15 and 35℃, and 3.0- and 1.8-fold more at 35℃ than those at 15 and 25℃, respectively, in cocultures. However, at 15℃, coculture cell numbers were significantly less than those in monocultures at both 25 and 35℃.
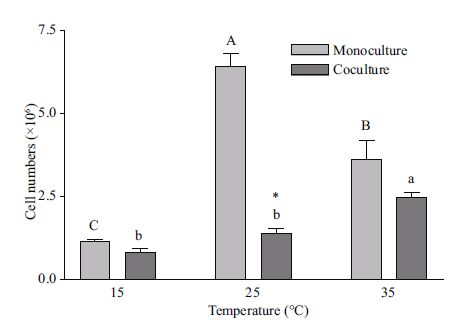
|
| Figure 3 Effect of temperature on M. aeruginosa cell numbers grown under mono- and coculture conditions Different uppercase (lowercase) letters on top of bars define cell numbers in monoculture (coculture) that showed significant differences among given temperatures (P<0.05) in one-way ANOVA and Dunnett tests. *, MC-LR quotas in cocultures that were significantly different from those in monoculture (P<0.05) in Student's t-test. |
Considering the observed differences in MC-LR quotas and cell numbers (Figs. 2 and 3, respectively) in both mono- and coculture at different temperatures, the results indicated that MC production by M. aeruginosa might not have been directly correlated with its biomass. For instance, M. aeruginosa cell numbers in monoculture were significantly different, while the MC-LR quotas were not so different, at the test temperatures.
3.3 Effect of light intensity on intracellular MC-LR quotaMC-LR quotas of M. aeruginosa in both monoculture (19.6±1.1 fg/cell) and coculture (9.0±3.1 fg/cell) at a light intensity of 20 µmol photon/(m2 ·s) were significantly higher (P<0.01) than those under other light intensities 6 d after inoculation (Fig. 4). MC-LR quotas in monoculture ((14.2±0.7)-(14.6±0.4) fg/cell) were not significantly influenced by light intensities from 30 to 50 µmol photon/(m2 ·s)(P >0.05). However, quotas in coculture ((9.7±3.0)-(0.05±0.01) fg/cell) gradually decreased with increased light intensities. The effect of light intensity on MC-LR quotas in monocultures was significantly different from that in coculture (P<0.01). In coculture, the quotas were 54.2%, 54.9%, 96.9%, and 99.7% lower than in monocultures at 20, 30, 40, and 50 µmol photon/(m2 ·s), respectively (Fig. 4). These results strongly indicated that the effect of D. flos-aquae competition on MC-LR quotas of M. aeruginosa was regulated by light intensity.
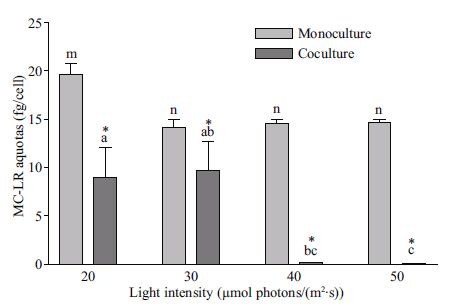
|
| Figure 4 MC-LR quotas of M. aeruginosa in both mono- and coculture under different light intensities Symbols a, b, and c indicate MC-LR quotas in coculture and m and n represent MC-LR quotas in monocultures that showed significant differences among given light intensities (P<0.01) in one-way ANOVA and Dunnett tests. *, MC-LR quotas in cocultures that were significantly different from monocultures (P<0.05) in Student's t-test. Each treatment performed in triplicate. |
Moreover, light intensity also had a significant impact on M. aeruginosa growth under both monoand coculture conditions (Fig. 5). M. aeruginosa cell numbers in cocultures at 20 µmol photon/(m2 ·s) were significantly greater than at other light intensities (30-50 µmol photon/(m2 ·s)). However, light intensity did not show significant effects on M. aeruginosa cell numbers in monocultures when it ranged from 20 to 40 µmol photon/(m2 ·s). Compared with numbers in monocultures, cell numbers were significantly reduced in cocultures at the present test light intensities.

|
| Figure 5 M. aeruginosa cell numbers in both monocultures and cocultures under different light intensities Different uppercase (lowercase) letters on top of bars define cell numbers in coculture (monoculture) that show significant differences among given light intensities (P<0.05) in one-way ANOVA and Dunnett tests. *, cell numbers in cocultures that were significantly different from monocultures (P<0.05) in Student's t- test. |
Transcriptional concentrations of mcyB and mcyD encoding MC-LR synthetases under mono- and coculture conditions were analyzed using real-time PCR assays of cDNA to test if reductions in intracellular MC-LR quotas of M. aeruginosa in cocultures resulted from gene expression inhibition. The results showed that transcriptional concentrations of these genes were significantly inhibited in cocultures (P<0.01), reduced to ~20% of concentrations in monocultures (Fig. 6).
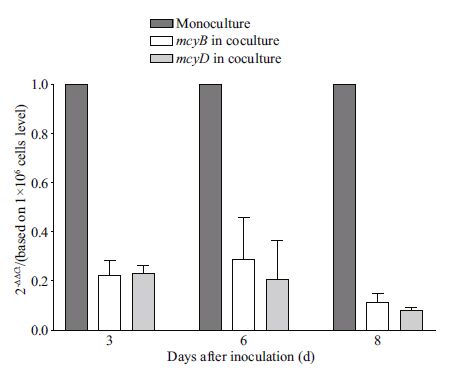
|
| Figure 6 Changes in mcyB and mcyD expression by M. aeruginosa cells grown in both monoculture and coculture, determined by real time PCR of cDNA The relative expression concentration of mcyB and mcyD from cells in coculture was calculated relative to expression in monoculture by the 2 - ΔΔ Ct method. Bars from left to right for each investigation day represent mcyB / mcyD expression in monocultures, and mcyB and mcyD in cocultures, respectively. Each treatment performed in triplicate. |
MC-LR is the most common member of the hepatotoxins, compounds that are synthetized by an integrated peptide-polyketide synthetase encoded by the mcy gene cluster (Tillett et al., 2000). It has been demonstrated that many environmental factors, including temperature, photosynthetically active radiation (PAR), pH, nutrients, light intensity, and small water clusters exhibit significant influences on the growth and MC content of Microcystis spp.(Wiedner et al., 2003; Lehman et al., 2008; Wang et al., 2013). However, study results are often contradictory and environmental factors usually induce 3 to 4-fold changes in MC quotas (Wood et al., 2011). The present data suggested that the effects of environmental factors on intracellular MC-LR quotas of M. aeruginosa vary with culture conditions. For instance, temperature (15-35℃) and light intensities (30-50 µmol photon/(m2 ·s)) did not show significant effects on MC-LR quotas in monocultures, while significantly impacting quotas in cocultures. Compared with those in monoculture, MC-LR quotas in cocultures were significantly reduced and varied at the studied temperatures (Fig. 2) and light intensities (Fig. 4). It thus appeared that biological factors, such as interspecies interactions and competition, might play more important roles than previously thought in MC biosynthesis regulation in Microcystis spp. within natural phytoplankton communities.
Despite the positive correlation between cell growth and MC quota in Microcystis spp.(Orr and Jones, 1998; Bittencourt-Oliveira et al., 2005; Briand et al., 2008; Wood et al., 2011), it remains unclear how environmental factors influence this relationship. Most researchers believe that environmental factors affect MC quotas by influencing cell growth because Microcystis spp. produces more MCs under favorable growth conditions than under growth-limiting conditions (Fahnenstiel et al., 2008; Briand et al., 2012). Contrarily, Rinta-Kanto et al.(2009) have demonstrated that toxin production by Microcystis spp. is uncoupled from cell biomass, based on qPCR analysis. Based on correlation analysis between MCLR quotas (Figs. 2 and 4) and cell numbers (Figs. 3 and 5) in both mono- and cocultures under different temperatures and light intensities, it was shown here that M. aeruginosa MC production was not strictly correlated with its biomass. For instance, M. aeruginosa cell numbers in monocultures were significant different, but MC-LR quotas were not, at the studied temperatures (Figs. 2 and 3). Similarly, cell numbers in monocultures were not correlated with MC-LR quotas at the studied light intensities (Figs. 4 and 5). Moreover, the effects of temperatures and light intensities on MC-LR production in cocultures were quite complicated. Compared to those in monocultures, all MC-LR quotas in cocultures were significantly reduced (Figs. 2 and 4), while some cell numbers significantly decreased (Figs. 3 and 5) within the tested temperatures (15-35℃) and light intensities (20-50 µmol photon/(m2 ·s)). Moreover, compared to initial cell numbers (2 × 106), cell numbers after 6 d inoculation increased by 2 to 4-fold in monocultures (expect at 15℃), and decreased by 23.8%-58.3% in cocultures (except at 35℃ and 20 µmol photon/(m2 ·s)). These results indicated that cocultured D. flos-aquae might have led to cell lysis under certain environmental conditions. It has been proposed that cell lysis might lead to increased extracellular MCs, which consequently regulates mcy genes expression and leads to increased MC quotas (Schatz et al., 2007). However, the present data suggested that this might not have been the case when M. aeruginosa was cocultured with D. flos-aquae. For example, cell number reductions in cocultures (58.5%) was not significantly different from in monocultures (42.5%), while the MC-LR quota in cocultures was significantly lower than in monocultures at 15℃. In other words, the effects of cocultured D. flos-aquae on the MC-LR quota were not directly correlated with M. aeruginosa biomass, at least at lower temperatures.
Despite extensive research on MC biosynthesis, the intracellular mechanisms that regulate MC biosynthesis remain unclear (Wood et al., 2011). Studies on Microcystis spp. have indicated that MC production is related to cell division processes or population growth (Kurmayer, 2011), colony formation (Gan et al., 2012), nitrogen availability (Ginn et al., 2010; Sevilla et al., 2010), iron (Sevilla et al., 2008), and phosphate (Kuniyoshi et al., 2013). These characteristics provide Microcystis spp. with a competitive advantage over other phytoplankton species in natural ecosystems. Using qPCR assay that allowed precise measurement of transcription of mcyB and mcyD genes encoding MC synthetases (Tillett et al., 2000) and an HPLCthat enabled the monitoring of small changes in cellular MC content, the reduction of M. aeruginosa intracellular MC-LR quota under coculture species-competition conditions was demonstrated to be caused by downregulation of mcyB and mcyD. Decreased MC-LR quotas coupled with downregulation of mcyB and mcyD under coculture conditions provided compelling evidence that this decrease was most likely caused by an external stimulus. In addition, the coculture effect was another stress factor that might have directly or indirectly regulated MC biosynthesis in M. aeruginosa.
Normally, toxic and nontoxic Microcystis genotypes coexist and change their proportions depending on diverse factors, such as nutrient concentration (Rantala et al., 2006), environmental factors (Wiedner et al., 2003; Lehman et al., 2008), and species competition (Schatz et al., 2005; Zhai et al., 2013). With the aid of a coculture technique, the competition between M. aeruginosa and other algal species, including the cyanobacteria M. flos - aquae (Zhai et al., 2013) and D. flos-aquae (Li and Li, 2012; Zhang et al., 2014) and the green alga Quadrigula chodatii (Zhang et al., 2013), as well as factors that might influence competition have been studied in many laboratories. However, results have not been consistent and in some cases even contradictory. Li and Li (2012) have reported that competition between M. aeruginosa and D. flos-aquae is mainly dependent on the initial inoculation biomasses of the two species; when the initial inoculation amounts are almost equal, D. flos-aquae begins to grow faster than M. aeruginosa. Our previous study also showed that D. flos-aquae growth is promoted while M. aeruginosa growth is inhibited under coculture conditions (Zhang et al., 2014). Moreover, growth changes in cocultures are probably caused by extracellular metabolites, such as cedrene derivatives, quinones, and phenol, diphenyl, and anthracene derivatives (Zhang et al., 2014). The clearly negative effect of increased proportions of D. flos-aquae on MC-LR production (Fig. 1) also indicated that chemicals released by D. flos-aquae might have been involved in species competition because MCs impact colony formation (Gan et al., 2012) and, thus, decreasing MC production that might play a key role in competing with M. aeruginosa. Similar results have been described by Rzymski et al.(2014). They found that cylindrospermopsin (a cyanobacterial alkaloid) contributes to interspecific competition through growth inhibition, metabolic alterations, and decreases in M. aeruginosa MC production. Recently, PinedaMendoza et al.(2014) have reported that infochemicals released by the grazer Daphnia magna also play an important role in M. aeruginosa MC synthesis. Increases in mcyA gene expression and MC production could be a defense mechanism against consumption by D. magna when the two species coexist.
Although interspecific competition was not evaluated in a completely rigorous way in this study, the effects of D. flos-aquae on MC-LR production could be speculated to have been caused by both competition and chemical release. The effect of environmental factors on M. aeruginosa 's MC quota in cocultures might have resulted from D. flos-aquae regulation that produced different varieties and/or amounts of metabolites that might have directly influenced MC biosynthesis. The real mechanism by which D. flos-aquae affects MC-LR quotas in cocultures needs to be elucidated, with many questions that need to be addressed in future studies. For example, how and to what degree do inter- and/or intraspecific competition affect the MCs produced by toxic Microcystis spp. in natural environments, which many algal species inhabit sympatrically? Which species of algae plays the most important role in Microcystis MC biosynthesis downregulation in freshwater ecosystems? By what mechanism do environmental factors, such as nitrogen and phosphorous concentrations, temperature, and pH, impose their effects on biomass and MC production by toxic Microcystis through species competition in eutrophic lakes? In summary, coculture effects are a kind of environmental stress that can significantly regulate MC biosynthesis in M. aeruginosa. Methods used for increasing the biodiversity and population of nontoxic algae could be a potential means for reducing MC contamination in lakes.
5 CONCLUSIONThe intracellular MC-LR quota and transcriptional concentrations of mcyB and mcyD genes encoding MC synthetases in the cyanobacterium M. aeruginosa were significantly reduced in cocultures with the nontoxic D. flos-aquae. This decrease in MC-LR quotas under coculture conditions was enhanced by increasing initial ratios of D. flos-aquae to M. aeruginosa in the inoculum and by increasing the temperature and light intensity during incubations. These results suggested that the effect of D. flos-aquae on MC-LR biosynthesis in M. aeruginosa could have been caused by both competition and chemical release, which was also affected by certain environmental factors, such as light and temperature.
6 ACKNOWLEDGEMENTWe thank Dr. Elizabeth Trembath-Reichert, Geological and Planetary Sciences, California Institute of Technology, for providing us with many valuable suggestions and language corrections.
| Bittencourt-Oliveira M do C, Kujbida P, Cardozo K H M, Carvalho V M, Moura A do N, Colepicolo P, Pinto E, 2005. A novel rhythm of microcystin biosynthesis is described in the cyanobacterium Microcystis panniformis Komárek et al. Biochem. Biophys. Res. Commun., 326 (3) : 687 –694. Doi: 10.1016/j.bbrc.2004.11.091 |
| Briand E, Bormans M, Quiblier C, Salencon M J, Humbert J F, 2012. Evidence of the cost of the production of microcystins by Microcystis aeruginosa under differing light and nitrate environmental conditions. PLoS One, 7 (1) : e29981 . Doi: 10.1371/journal.pone.0029981 |
| Briand E, Yéprémian C, Humbert J F, Quiblier C, 2008. Competition between microcystin-and non-microcystinproducing Planktothrix agardhii (cyanobacteria) strains under different environmental conditions. Environ.Microbiol., 10 (12) : 3 337 –3 348. Doi: 10.1111/emi.2008.10.issue-12 |
| Bustin S A, 2000. Absolute quantification of mRNA using realtime reverse transcription polymerase chain reaction assays. J. Mol. Endocrinol., 25 (2) : 169 –193. Doi: 10.1677/jme.0.0250169 |
| Butler N, Carlisle J C, Linville R, Washburn B. 2009.Microcystins:a Brief Overview of Their Toxicity and Effects, with Special Reference to Fish, Wildlife, and Livestock. Ecotoxicology Program Integrated Risk Assessment Branch Office of Environmental Health Hazard Assessment California Environmental Protection Agency, Sacramento, CA, USA, p.1-17. |
| Carmichael W W, 1994. The toxins of cyanobacteria. Sci. Am., 270 (1) : 78 –86. Doi: 10.1038/scientificamerican0194-78 |
| Chen J, Hu L B, Zhou W, Yan S H, Yang J D, Xue Y F, Shi Z Q, 2010. Degradation of microcystin-LR and RR by a Stenotrophomonas sp. strain EMS isolated from Lake Taihu, China. Int. J. Mol. Sci., 11 (3) : 896 –911. |
| Chen Y W, Qin B Q, Teubner K, Dokulil M T, 2003. Long-term dynamics of phytoplankton assemblages:microcystisdomination in Lake Taihu, a large shallow lake in China. J. Plankton Res., 25 (4) : 445 –453. Doi: 10.1093/plankt/25.4.445 |
| Dawson R M, 1998. The toxicology of microcystins. Toxicon, 36 (7) : 953 –962. Doi: 10.1016/S0041-0101(97)00102-5 |
| Fahnenstiel G L, Millie D F, Dyble J, Litaker R W, Tester P A, McCormick M J, Rediske R, Klarer D, 2008. Microcystin concentrations and cell quotas in Saginaw Bay, Lake Huron. Aquatic Ecosystem Health & Management, 11 (2) : 190 –195. |
| Gan N Q, Xiao Y, Zhu L, Wu Z X, Liu J, Hu C L, Song L R, 2012. The role of microcystins in maintaining colonies of bloom-forming Microcystis spp. Environ. Microbiol., 14 (3) : 730 –742. Doi: 10.1111/emi.2012.14.issue-3 |
| Ginn H P, Pearson L A, Neilan B A, 2010. NtcA from Microcystis aeruginosa PCC 7806 is autoregulatory and binds to the microcystin promoter. Appl. Environ.Microbiol., 76 (13) : 4 362 –4 368. Doi: 10.1128/AEM.01862-09 |
| Grosse Y, Baan R, Straif K, Secretan B, EI Ghissassi F, Cogliano V, 2006. Carcinogenicity of nitrate, nitrite, and cyanobacterial peptide toxins. Lancet Oncol., 7 (8) : 628 –629. Doi: 10.1016/S1470-2045(06)70789-6 |
| Health Canada, 2002. Guidelines for Canadian drinking water quality:supporting documentation. Cyanobacterial Toxins-Microcystin-LR. |
| Horst G P, Sarnelle O, White J D, Hamilton S K, Kaul R B, Bressie J D, 2014. Nitrogen availability increases the toxin quota of a harmful cyanobacterium, Microcystis aeruginosa. Water Res., 54 : 188 –198. Doi: 10.1016/j.watres.2014.01.063 |
| Hu X, Liu Y G, Zeng G M, Hu X J, Wang Y Q, Zeng X X, 2014. Effects of limonene stress on the growth of and microcystin release by the fresh water cyanobacterium Microcystis aeruginosa FACHB-905. Ecotoxicol.Environ. Saf., 105 : 121 –127. Doi: 10.1016/j.ecoenv.2014.01.023 |
| Jang M H, Ha K, Joo G J, Takamura N, 2003. Toxin production of cyanobacteria is increased by exposure to zooplankton. Freshwater Biology, 48 (9) : 1 540 –1 550. Doi: 10.1046/j.1365-2427.2003.01107.x |
| Kaebernick M, Neilan B A, Börner T, Dittmann E, 2000. Light and the transcriptional response of the microcystin biosynthesis gene cluster. Appl. Environ. Microbiol., 66 (8) : 3 387 –3 392. Doi: 10.1128/AEM.66.8.3387-3392.2000 |
| Karadžić V, Subakov-Simić G, Krizmanić J, Natić D, 2010. Phytoplankton and eutrophication development in the water supply reservoirs Garaši and Bukulja (Serbia). Desalination, 255 (1-3) : 91 –96. Doi: 10.1016/j.desal.2010.01.009 |
| Krishnamurthy T, Carmichael W W, Sarver E W, 1986. Toxic peptides from freshwater cyanobacteria (blue-green algae). I. Isolation, purification and characterization of peptides from Microcystis aeruginosa and Anabaena flosaquae. Toxicon, 24 (9) : 865 –873. |
| Kuniyoshi T M, Sevilla E, Bes M T, Fillat M F, Peleato M L, 2013. Phosphate deficiency (N/P 40:1) induces mcyD transcription and microcystin synthesis in Microcystis aeruginosa PCC7806. Plant Physiol. Biochem., 65 : 120 –124. Doi: 10.1016/j.plaphy.2013.01.011 |
| Kurmayer R, 2011. The toxic cyanobacterium Nostoc sp. strain 152 produces highest amounts of microcystin and nostophycin under stress conditions. J. Phycol., 47 (1) : 200 –207. |
| Laub J, Henriksen P, Brittain S M, et al, 2002. [ADMAdda5]-microcystins in Planktothrix agardhii strain PH-123(cyanobacteria)-importance for monitoring of microcystins in the environment. Environ. Toxicol., 17 (4) : 351 –357. Doi: 10.1002/(ISSN)1522-7278 |
| LeBlanc S, Pick F R, Aranda-Rondriguze R, 2005. Allelopathic effects of the toxic cyanobacterium Microcystis aeruginosa on duckweed, Lemma gibba L. Environ.Toxicol., 20 (1) : 67 –73. Doi: 10.1002/(ISSN)1522-7278 |
| Lehman P W, Boyer G, Satchwell M, Waller S, 2008. The influence of environmental conditions on the seasonal variation of Microcystis cell density and microcystins concentration in San Francisco Estuary. Hydrobiologia, 600 (1) : 187 –204. Doi: 10.1007/s10750-007-9231-x |
| Li Y X, Li D H, 2012. Competition between toxic Microcystics aeruginosa and nontoxic Microcystis wesenbergii with Anabaena PCC7120. J. Appl. Phycol., 24 (1) : 69 –78. Doi: 10.1007/s10811-010-9648-x |
| Livak K J, Schmittgen T D, 2001. Analysis of relative gene expression data using real-time quantitative PCR and the 2T-ΔΔC method. Methods, 25 (4) : 402 –408. Doi: 10.1006/meth.2001.1262 |
| Oh H M, Lee S J, Jang M H, Yoon B D, 2000. Microcystin production by Microcystis aeruginosa in a phosphorouslimited chemostat. Appl. Environ. Microbiol., 66 (1) : 176 –179. Doi: 10.1128/AEM.66.1.176-179.2000 |
| Orr P Y, Jones G J, 1998. Relationship between microcystin production and cell division rates in nitrogen-limited Microcystis aeruginosa cultures. Lancet Oceanogr., 43 (7) : 1 604 –1 614. |
| Papadimitriou T, Armeni E, Stalikas C D, Kagalou I, Leonardos I D, 2012. Detection of microcystins in Pamvotis lake water and assessment of cyanobacterial bloom toxicity. Environmental Monitoring And Assessment, 184 (5) : 3 043 –3 052. Doi: 10.1007/s10661-011-2169-5 |
| Pineda-Mendoza R M, Zúñiga G, Martínez-Jerónimo F, 2014. Infochemicals released by Daphnia magna fed on Microcystis aeruginosa affect mcyA gene expression. Toxicon, 80 : 78 –86. Doi: 10.1016/j.toxicon.2014.01.008 |
| Rantala A, Rajaniemi-Wacklin P, Lyra C, Lepistö L, Rintala J, Mankiewicz-Boczek J, Sivonen K, 2006. Detection of microcystin-producing cyanobacteria in Finnish Lakes with genus-specific microcystin synthetase gene E (mcyE)PCR and associations with environmental factors. Appl.Environ. Microbiol., 72 (9) : 6 101 –6 110. Doi: 10.1128/AEM.01058-06 |
| Rinta-Kanto J M, Konopko E A, DeBruyn J M, Bourbonniere R A, Boyer G L, Wilhelm S W, 2009. Lake Erie Microcystis:relationship between microcystin production, dynamics of genotypes and environmental parameters in a large lake. Harmful Algae, 8 (5) : 665 –673. Doi: 10.1016/j.hal.2008.12.004 |
| Ríos V, Moreno I, Prieto A I, Soria-Díaz M E, Frías J E, Cameán A M, 2014. Comparison of Microcystis aeruginosa (PCC7820 and PCC7806) growth and intracellular microcystins content determined by liquid chromatography-mass spectrometry, enzyme-linked immunosorbent assay anti-Adda and phosphatase bioassay. J. Water Health, 12 (1) : 69 –80. Doi: 10.2166/wh.2013.088 |
| Rouhiainen L, Vakkilainen T, Siemer B L, Buikema W, Haselkorn R, Sivonen K, 2004. Genes coding for hepatotoxic heptapeptides (microcystins) in the cyanobacterium Anabaena strain 90. Appl. Environ.Microbiol., 70 (2) : 686 –692. Doi: 10.1128/AEM.70.2.686-692.2004 |
| Rzymski P, Poniedziałek B, Kokociński M, Jurczak T, Lipski D, Wiktorowicz K, 2014. Interspecific allelopathy in cyanobacteria:Cylindrospermopsin and Cylindrospermopsis raciborskii effect on the growth and metabolism of Microcystis aeruginosa. Harmful Algae, 35 : 1 –8. Doi: 10.1016/j.hal.2014.03.002 |
| Saker M L, Welker M, Vasconcelos V M, 2007. Multiplex PCR for the detection of toxigenic cyanobacteria in dietary supplements produced for human consumption. Appl.Microbiol. Biotechnol., 73 (5) : 1 136 –1 142. |
| Schatz D, Keren Y, Hadas O, Carmeli S, Sukenik A, Kaplan A, 2005. Ecological implications of the emergence of nontoxic subcultures from toxic Microcystis strains. Environ.Microbiol., 7 (6) : 798 –805. Doi: 10.1111/emi.2005.7.issue-6 |
| Schatz D, Keren Y, Vardi A, Sukenik A, Carmeli S, Bӧerner T, Dittmann E, Kaplan A, 2007. Towards clarification of the biological role of microcystins, a family of cyanobacterial toxins. Environ. Microbiol., 9 (4) : 965 –970. Doi: 10.1111/emi.2007.9.issue-4 |
| Schmidt J R, Wilhelm S W, Boyer G L, 2014. The fate of Microcystins in the environment and challenges for monitoring. Toxins, 6 (12) : 3 354 –3 387. Doi: 10.3390/toxins6123354 |
| Scott L L, Downing S, Phelan R R, Downing T G, 2014. Environmental modulation of microcystin and β-Nmethylamino-L-alanine as a function of nitrogen availability. Toxicon, 87 : 1 –5. Doi: 10.1016/j.toxicon.2014.05.001 |
| Sevilla E, Martin-Luna B, Vela L, Bes M T, Fillat M F, Peleato M L, 2008. Iron availability affects mcyD expression and microcystin-LR synthesis in Microcystis aeruginosa PCC7806. Environ. Microbiol., 10 (10) : 2 476 –2 483. Doi: 10.1111/j.1462-2920.2008.01663.x |
| Sevilla E, Martin-Luna B, Vela L, Bes M T, Peleato M L, Fillat M F, 2010. Microcystin-LR synthesis as response to nitrogen:transcriptional analysis of the mcyD gene in Microcystis aeruginosa PCC7806. Ecotoxicology, 19 (7) : 1 167 –1 173. Doi: 10.1007/s10646-010-0500-5 |
| Song R F, Wang G X, Xu Y, Shao J H, Wang Z J, Liu Y, Li R H, 2011. Transcriptional response of microcystin biosynthesis gene cluster of Microcystis aeruginosa PCC7806 under Daphnia stress using real-time RT-PCR technique. J. Lake Sci., 23 (1) : 150 –154. Doi: 10.18307/2011.0122 |
| Te S H, Gin K Y H, 2011. The dynamics of cyanobacteria and microcystin production in a tropical reservoir of Singapore. Harmful Alage, 10 (3) : 319 –329. Doi: 10.1016/j.hal.2010.11.006 |
| Tillett D, Dittmann E, Erhard M, von Döhren H, Börner T, Neilan B A, 2000. Structural organization of microcystin biosynthesis in Microcystis aeruginosa PCC7806:an integrated peptide-polyketide synthetase system. Chem.Biol., 7 (10) : 753 –764. Doi: 10.1016/S1074-5521(00)00021-1 |
| Wan L, Zhu W, Zhao L F, 2007. Effect of nitrogen and phosphorus on growth and competition of M. aeruginosa and S. quadricauda. Environ. Sci., 28 (6) : 1 230 –1 235. |
| Wang J, Zhao F, Chen B H, Li Y N, Na P, Zhuo J, 2013. Small water clusters stimulate microcystin biosynthesis in cyanobacterial Microcystis aeruginosa. J. Appl. Phycol., 25 (1) : 329 –336. Doi: 10.1007/s10811-012-9867-4 |
| W HO, 2003. Cyanobacterial Toxins:Microcystin-LR in Drinking-Water. World Health Organization (WHO/SDS/WSH/03. 04/57), Geneva. |
| Wiedner C, Visser P M, Fastner J, Metcalf J S, Codd G A, Mur L R, 2003. Effects of light on the microcystin content of Microcystis strain PCC 7806. Appl. Environ. Microbiol., 69 (3) : 1 475 –1 481. Doi: 10.1128/AEM.69.3.1475-1481.2003 |
| Wood S A, Rueckert A, Hamilton D P, Cary S C, Dietrich D R, 2011. Swiching toxin production on and off:intermittent microcystin synthesis in a Microcystis bloom. Environ.Microbiol. Rep., 3 (1) : 118 –124. Doi: 10.1111/emi4.2011.3.issue-1 |
| Wu X D, Kong F X, 2008. The determination of in situ growth rates of the bloomed Microcystis in Meiliang Bay, Lake Taihu. China Environ. Sci., 28 (6) : 552 –555. |
| Yen H K, Lin T F, Tseng I C, 2012. Detection and quantification of major toxigenic Microcystis genotypes in Moo-Tan reservoir and associated water treatment plant. J. Environ.Monitor., 14 (2) : 687 –696. Doi: 10.1039/c1em10389j |
| Zhai C M, Song S, Zou S H, Liu C H, X ue, Y R, 2013. The mechanism of competition between two bloom-forming Microcystis species. Freshwater Biology, 58 (9) : 1 831 –1 839. Doi: 10.1111/fwb.2013.58.issue-9 |
| Zhang P, Zhai C M, Chen R Q, Liu C H, Xue Y R, Jiang J H, 2012. The dynamics of the water bloom-forming Microcystis aeruginosa and its relationship with biotic and abiotic factors in Lake Taihu, China. Ecol. Eng., 47 : 274 –277. Doi: 10.1016/j.ecoleng.2012.07.004 |
| Zhang P, Zhai C M, Wang X X, Liu C H, Jiang J H, Xue Y R, 2013. Growth competition between Microcystis aeruginosa and Quadrigula chodatii under controlled conditions. J. Appl. Phycol., 25 (2) : 555 –565. Doi: 10.1007/s10811-012-9890-5 |
| Zhang T, Song L R, 2006. Allelopathic effect between Microcystis aeruginosa and three filamentous cyanobacteria. J. Lake Sci., 18 (2) : 150 –156. Doi: 10.18307/2006.0208 |
| Zhang X W, Fu J, Song S, Zhang P, Yang X H, Zhang L R, Luo Y, Liu C H, Zhu H L, 2014. Interspecific competition between Microcystis aeruginosa and Anabaena flosaquae from Taihu Lake, China. Zeitschrift für Naturforschung C, 69 (1-2) : 53 –60. |
 2016, Vol. 34
2016, Vol. 34


