Institute of Oceanology, Chinese Academy of Sciences
Article Information
- WEI Jing(魏静), ZHANG Lisheng(张立胜), ZHAO Chong(赵冲), FENG Wenping(封文萍), SUN Ping(孙平), CHANG Yaqing(常亚青)
- Correlation analyses of covering and righting behaviors to fitness related traits of the sea urchin Glyptocidaris crenularis in different environmental conditions
- Chinese Journal of Oceanology and Limnology, 34(6): 1183-1190
- http://dx.doi.org/10.1007/s00343-016-5133-y
Article History
- Received Apr. 21, 2015
- accepted for publication Jun. 24, 2015
- accepted in principle Sep. 30, 2015
Animal behaviors are a function of the morphology, structures and functions of organisms and are highly affected by their complex habitat (Shang, 2005). Further, behaviors, and their relation to fitness, are far more than a one-way dependent relationship. Behavior plays an important role in the adaptive evolution of animals (Mayr, 1960; Corning, 2014). Thus, it is important to investigate the correlations between ecologically important behaviors and fitness-related traits of various representative organisms.
Sea urchins are ubiquitous and ecologically dominant in marine benthic communities as both prey and herbivorous grazers (Pearse, 2006; Lamare et al., 2011). Complex marine benthic environments shape a number of distinctive behaviors of sea urchins in the process of evolution. Covering and righting are two representative behaviors with ecological importance. Covering behavior, which is exhibited in species from both shallow (Verling et al., 2002) and deep (Pawson and Pawson, 2013) environments, refers to sea urchins using their tube feet and spines to move objects, such as shells, stones and algae fragments, onto their dorsal surface. A number of hypotheses have been tested to explain the evolutionary drives of covering behavior (Crook, 2003), including protection against exposure to solar radiation (Adams, 2001; Kehas et al., 2005; Dumont et al., 2007; Sigg et al., 2007), predation (Amsler et al., 1999; Agatsuma, 2001), desiccation (Orton, 1929), wave surge (Millott, 1976) and floating sand (Richner and Milinski, 2000), or/and as a reflexive action (Dambach and Hensel, 1970; Lawrence, 1976). Although it is considered that covering behavior is a behavioral avoidance strategy against UV radiation (Lamare et al., 2011), the fitness benefits of this behavior remains largely unknown. Correlation analysis is an important approach to investigating the fitness basis of animal behaviors. Among the six hypotheses of potential functions of this behavior (Crook, 2003), protection against suspended sand is of particular interest. Richner and Milinski (2000) reported that covering behavior was significantly enhanced in the sea urchin Paracentrotus lividus during exposure to suspended sand. However, we do not know whether a significant correlation of covering behavior exists between the sea urchins with and without suspended sand.
Righting is the behavior of an inverted sea urchin placed on its aboral surface to resume the posture with the aboral side up (Hyman, 1955). The ability to right has been commonly used as a stress indicator in sea urchins (Lawrence, 1975; Hagen, 1994; Böttger et al., 2001). However, we know of no information on the fitness-related basis of either covering or righting behaviors. Lawrence (1976) found a reversal of covering behavior by inverted Lytechinus variegatus sea urchins. This indicates a possible link between covering and righting behaviors. However, we know of no experimental correlation data on the two behaviors that further tests the possible link between covering and righting behaviors suggested by Lawrence (1976).
Fitness includes all traits related to survival, growth and reproduction (Shang, 2005). Food consumption has been identified as a factor affecting the fitness of sea urchins (i.e. Lemire and Himmelman, 1996). Somatic traits (such as test size, test robustness, body weight and gut weight) and reproductive traits (such as gonad weight) fundamentally contribute to the fitness of sea urchins. The hardness of the test and spines is important for defense against abiotic disturbance and predation, and consequently contributes to the fitness of sea urchins (Dayton et al., 1970; Amsler et al., 1999; Verling et al., 2002). Therefore, we selected these fitness-related traits for examination in the present study. Although Luo et al.(2013) previously studied the responses of fitnessrelated traits to conditions suitable for covering behavior, we know of no information on the direct correlations between a series of fitness-related traits and covering and righting behaviors in sea urchins.
Glyptocidaris crenularis is a native and dominant sea urchin species in the waters of Northern China (Chang et al., 2004). Glyptocidaris crenularis can be distinguished by their flat test and long spines. They live on rocky and sandy bottoms at depths of 5-150 m. According to our laboratory observations and a previous study (Zhao et al., 2014), G. crenularis has obvious covering and righting behaviors.
The main purposes of the present study were to investigate the potential correlations between covering and righting behaviors, between covering behavior with and without poured sand, and between covering and righting behaviors and fitness-related traits in G. Crenularis.
2 MATERIAL AND METHOD 2.1 Sea urchinsSea urchins were originally produced in Dalian Haibao Fisheries Company (38°45′43″N, 121°13′9″E) in May 2012 and transported to the Key Laboratory of Mariculture & Stock Enhancement in North China's Sea, Ministry of Agriculture, Dalian Ocean University (38°51′59″N, 121°33′55″E) in May 2013. They were then cultured in the laboratory and fed kelp Laminaria japonica ad libitum until the experiment started. The water temperature was 14.8-16.4℃ and the photoperiod was 11 h light: 13 h dark during the experiment.
2.2 Experimental designOne hundred individuals were randomly collected for the experiments. Wet food consumption was measured over the 7 days before the behavioral experiments started and was calculated by the weight of the given kelp minus the weight of the uneaten kelp (including the uneaten small fragments grazed by G. crenularis). The behavioral experiments started on 21 October 2014. Ten shells of the small bivalve Patinopecten yessoensis (shell length: 20.60±1.02 mm, shell height: 21.75±1.03 mm, shell weight: 0.29±0.06 g) were distributed evenly on the bottom of each experimental tank (10 L in volume) as potential covering material. Sea urchins were individually distributed onto the bottom of each experimental tank with still water. We recorded the time to first covering (behavioral response time) and the number of shells used for covering (behavioral ability) by individuals in the first 20 minutes after the beginning of the experiment for each of the 100 experimental tanks (n =100). On the subsequent day, we repeated the covering behavior experiment with an addition of 20 mL soft sand gently poured into the tanks. On the third day, righting response time (the time required for reversal of inverted posture to aboral side up posture) was measured.
After treatments, we measured test diameter (cm±0.01 cm), test height (cm±0.01 cm), body weight (g±0.01 g) for all experimental individuals and dissected all urchins for further analysis. Test, lantern, five gonads and gut without contents were collected and weighed as their wet weights (g±0.01 g). Maximum pressure resistance of the test and lantern were investigated using a pressure-testing instrument (Shijin Co., Jinan), which measures the minimum force necessary to break the test and lantern. This instrument includes two thick steel plates and one precise load measurement system. Tests were placed with the aboral surface up between the two steel plates, while lanterns were placed with the mouth side down.
2.3 Statistical analysisData were tested for normal distribution and homogeneity of variance before statistical analysis. Pearson correlation analysis was used to test the significance of correlations between fitness-related traits and behaviors, between covering and righting behaviors, and between covering behavior with and without poured sand in G. crenularis. All analyses were performed with SPSS 13.0 statistical software (IBM, Chicago). A probability level of P<0.05 was considered statistically significant.
3 RESULTFood consumption, fitness-related traits and behaviors of the 100 experimental individuals are summarized as mean plus or minus one standard deviation (SD) in Tables 1 and 2. Sea urchins had an average test diameter of 34.86±3.59 mm and average body weight of 15.26±4.25 g, with an average righting response time of 121.5±55.2 s. The time to first covering was 834.6±418.0 s without sand poured into the water, and slightly slower (841.1±407.4 s) with sand poured into the water.

|
|
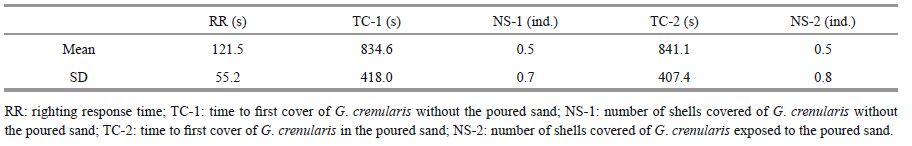
Righting response time was significantly positively correlated with test diameter (P<0.01), body weight (P<0.01), test weight (P<0.01), lantern weight (P<0.01), but was not significantly correlated with gonad and gut weights (P> 0.05, Table 3 and Fig. 1). Righting response time was significantly positively correlated with maximum pressure resistance of the lantern (P<0.01), while significantly negatively correlated with food consumption (P<0.01, Table 3).
|

|
| Figure 1 Relationship between righting response time (RR) and fitness-related traits of G. crenularis See Table 1 for abbreviations of TD, BW, TW, LW, LP and FC. |
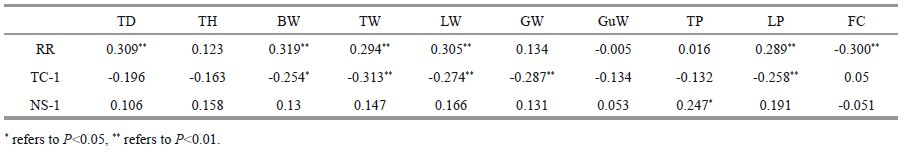
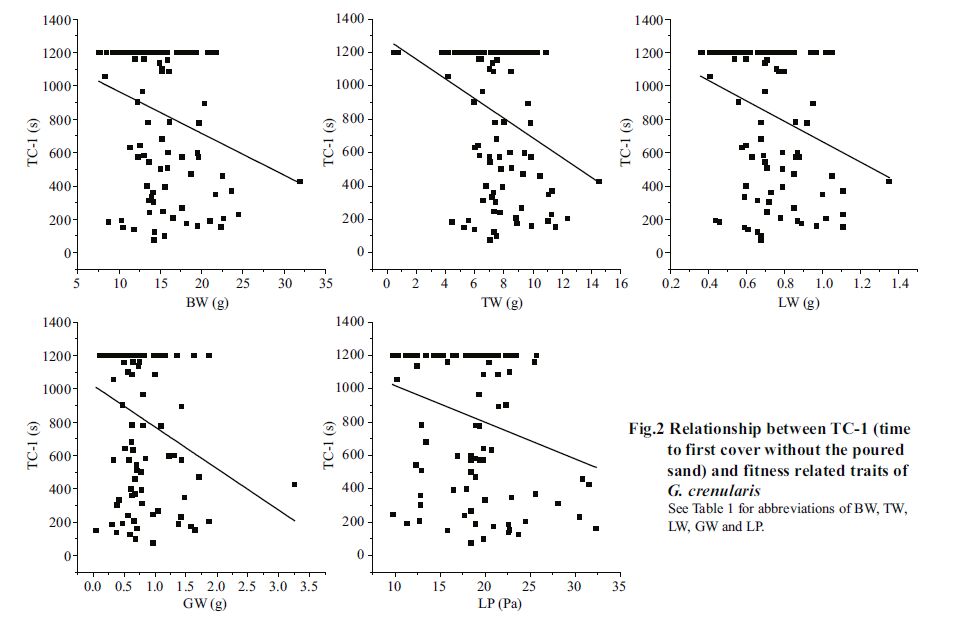
For covering behavior, time to first covering was significantly negatively correlated with body weight (P<0.05), test weight (P<0.01), lantern weight (P<0.01), gonad weight (P<0.01) and maximum pressure resistance of lantern (P<0.01), but not significantly correlated with test diameter, test height, gut weight, maximum pressure resistance of test and food consumption (P >0.05, Table 3 and Fig. 2). Additionally, the number of shells used to cover was only significantly positively correlated with maximum pressure resistance of test (P<0.05).
|
| Figure 2 Relationship between TC-1(time to first cover without the poured sand) and fitness related traits of G. crenularis See Table 1 for abbreviations of BW, TW, LW, GW and LP. |
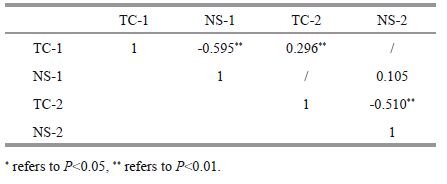
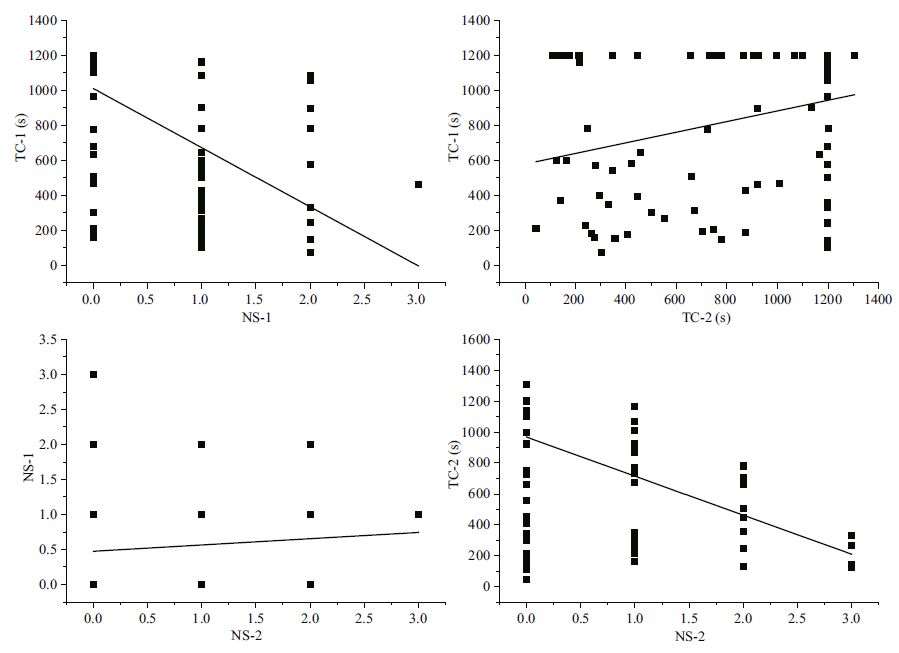
Time to first cover was significantly positively correlated with the conditions of sand poured into the water and sand not poured into water (P<0.01, Table 4 and Fig. 3). However, the number of shells used to cover showed no significant difference between individuals in the two experimental conditions (P >0.05, Table 4). In addition, the time to first covering was significantly negatively correlated with the number of shells used to cover in both experimental conditions (P<0.01, Table 4).
|
|
| Figure 3 Relationship between covering behavior of G. crenularis with and without exposure to sand See Table 2 for abbreviations of TC-1, TC-2, NS-1 and NS-2. |
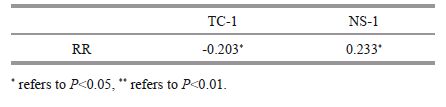
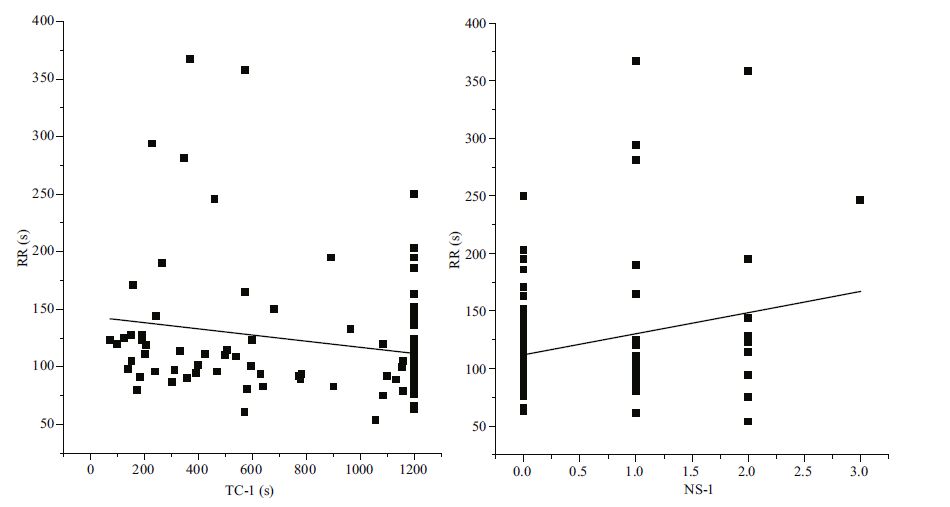
Righting response time was significantly negatively correlated with the time to first covering and significantly positively correlated with the number of shells used in covering (P<0.05, Table 5 and Fig. 4).
|
|
| Figure 4 Relationship between covering and righting behaviors of G. crenularis See Table 2 for abbreviations of RR, TC-1, NS-1. |
Although a number of environmental factors have been proposed to explain covering (Dumont et al., 2007; Lamare et al., 2011; Chang et al., 2013) and righting behaviors (Lawrence, 1975), little information is available about the fitness-related basis of these behaviors in sea urchins. The present study correlated covering and righting behaviors to a series of fitnessrelated traits in sea urchins. We found that righting response time was significantly positively correlated with body size in G. crenularis. Given that the experimental sea urchins were the same age, and had similar habitats and genetic background, we could conclude that smaller G. crenularis have a quicker righting behavior. This result is consistent with a study by Himmelman et al.(1984) showing that the sea urchin Strong y locentrotus droebachiensis showed better righting behavior at a small size when exposed to reduced salinity. One potential explanation is that less energy is required for small sea urchins to right themselves. Also, small sea urchins are more vulnerable to complex environments and predators and so need to respond quickly to adverse conditions (for example, inversion by waves) to maximize their survival (Percy, 1973). Further studies should be carried out on other species to test whether this phenomenon is common in sea urchins.
Righting behavior has been well accepted as being a stress indicator for temperature and salinity in the sea urchin Lytechinus variegatus (Lawrence, 1975; Böttger et al., 2001; Brothers and McClintock, 2015), whereas not for all stressors (Challener and McClintock, 2013). Unsurprisingly, the present study found that righting response time was significantly negatively correlated with food consumption, suggesting that sea urchins with a quicker righting behavior have better feeding activity. This indicates that sea urchins with a quick righting behavior not only have greater ability to avoid abiotic disturbance and predation, but have the direct advantage of increased food consumption. Because food consumption is among the most important factors affecting growth and reproduction in sea urchins (Lawrence, 2013), sea urchins with quick righting behavior probably have a series of advantages in fitness, although many fitness-related traits (for example, gonad development) were not measured in the present study. Further, the current results suggest that measuring righting behavior can be a simple and effective method to test the well-being of sea urchins in aquaculture (Hagen, 1994).
However, covering behavior was not significantly correlated with food consumption according to either time to first covering or the number of shells used to cover by G. crenularis. This result is consistent with our previous finding that starvation does not significantly affect covering behavior of G. crenularis (Zhao et al., 2014). Contrary to righting behavior, we found that covering behavior was not significantly correlated with body size of G. crenularis, but there was a negative correlation between covering response time and body weight. Compared with large individuals in the field, however, small sea urchins (Paracentrotus lividus) have rarely been observed to cover their oral surfaces, preferring instead to cover aboral surfaces (Barnes and Crook, 2001). The disagreement is probably because Barnes and Crook (2001) conducted a field observation on wild sea urchins, while the present study is laboratory based and uses cultured individuals. The present results clearly indicate that each behavior differs in the relationship with body size in different sea urchin species in different habitats. In addition, we found no significant correlation between righting behavior and test hardness. This is probably because flight and fight are two different defense strategies in sea urchins.
Unexpectedly, we found a significantly negative correlation between covering response time and righting response time in G. crenularis. This result indicates that covering and righting behaviors probably have different internal mechanisms in G. crenularis. This result also disagrees with Lawrence's (1976) hypothesis of covering and righting behaviors but agrees with the same study's note that a reflexive basis for covering does not affect the functional consequence of covering. Perhaps righting behavior is the result of the high stimulation of being inverted, while covering behavior does not have this artificially high stimulus. The behavioral difference among species should also be studied further.
Although time to first covering and the number of shells used to cover have been used in a number of studies of covering behavior in sea urchins (for example, Verling et al., 2002; Crook, 2003; Sigg et al., 2007), the correlation between these two representative traits has never been documented. The present study provides direct evidence of a significantly negative correlation between behavioral response time and ability of covering behavior in sea urchins.
Although Richner and Milinski (2000) successfully demonstrated that suspended sand significantly increases the covering behavior of P. lividus, we do not know whether the heavily covered sea urchins tend to cover more under different conditions of suspended sand. Our experimental results on the two consecutive days showed that the time to first cover is significantly different between the different conditions, while the number of shells used to cover is not significantly different. It indicates that G. crenularis tend to keep a quick covering response when environments change. However, individuals that are heavily covered do not maintain their cover when environments change. Because suspended sand is common in the sandy habitat of G. crenularis, the present result highlights the ecological importance of covering behavior in sea urchins.
In conclusion, righting response time of G. crenularis was significantly positively correlated with body size, but significantly negatively correlated with food consumption. Covering behavior was not significantly correlated with body size of G. crenularis, but there was a negative correlation between covering response time and body weight. A significantly negative correlation was found between covering response time and righting response time. Glyptocidaris crenularis showed a significantly positive correlation in covering response time between individuals with or without exposure to poured sand, but no significant relationship to covering ability (number of shells used to cover). It should be noted that the present study is a laboratorybased investigation on cultured sea urchins. Thus, further studies are needed to test the relationship between covering and righting behaviors to fitness of wild sea urchins in the field. The present study provides new insight into the internal mechanisms of covering and righting behaviors of sea urchins.
5 ACKNOWLEDGMENTWe are grateful to Prof. John Lawrence for academic and editorial suggestions.
| Adams N L, 2001. UV radiation evokes negative phototaxis and covering behavior in the sea urchin Strongylocentrotus droebachiensis. Mar. Ecol. Prog. Ser., 213 : 87 –95. Doi: 10.3354/meps213087 |
| Agatsuma Y, 2001. Effect of the covering behavior of the juvenile sea urchin Strongylocentrotus intermedius on predation by the spider crab Pugettia quadridens. Fisherie. Sci., 67 (6) : 1 181 –1 183. Doi: 10.1046/j.1444-2906.2001.00379.x |
| Amsler C D, McClintock J B, Baker B J, 1999. An antarctic feeding triangle:defensive interactions between macroalgae, sea urchins, and sea anemones. Mar. Ecol.Prog. Ser., 183 : 105 –114. Doi: 10.3354/meps183105 |
| Barnes D K A, Crook A C, 2001. Quantifying behavioural determinants of the coastal European sea-urchin Paracentrotus lividus. Mar. Biol., 138 (6) : 1 205 –1 212. Doi: 10.1007/s002270100543 |
| Böttger S A, McClintock J B, Klinger T S, 2001. Effects of inorganic and organic phosphates on feeding, feeding absorption, nutrient allocation, growth and righting responses of the sea urchin Lytechinus variegatus. Mar.Biol., 138 (4) : 741 –751. Doi: 10.1007/s002270000476 |
| Brothers C J, McClintock J B, 2015. The effects of climateinduced elevated seawater temperature on the covering behavior, righting response, and Aristotle's lantern reflex of the sea urchin Lytechinus variegatus. J. Exp. Mar. Biol.Ecol., 467 : 33 –38. Doi: 10.1016/j.jembe.2015.02.019 |
| Challener R C, McClintock J B, 2013. Exposure to extreme hypercapnia under laboratory conditions does not impact righting and covering behavior of juveniles of the common sea urchin Lytechinus variegatus. Mar. Freshw. Behav.Phy., 46 (3) : 191 –199. Doi: 10.1080/10236244.2013.800759 |
| Chang Y Q, Li Y X, Luo S B, Zhao C, 2013. Effects of different ecological environments in the laboratory on the covering behavior of the sea urchin Glyptocidaris crenularis. Acta Ecologica Sinica, 33 (9) : 2 754 –2 760. Doi: 10.5846/stxb |
| Chang Y, Ding J, Song J, Yang W, 2004. Biology and Aquaculture of Sea Cucumbers and Sea Urchins. Ocean Press, Beijing, China27-217. |
| Corning P A, 2014. Evolution ‘on purpose':how behaviour has shaped the evolutionary process. Biol. J. Linn. Soc., 112 (2) : 242 –260. Doi: 10.1111/bij.2014.112.issue-2 |
| Crook A C, 2003. Individual variation in the covering behaviour of the shallow water sea urchin Paracentrotus lividus. Mar. Ecol., 24 (4) : 275 –287. Doi: 10.1046/j.1439-0485.2003.00846.x |
| Dambach M, Hentschel G, 1970. Die bedeckungsreaktion von seeigeln. Neue versuche und deutungen. Mar. Biol., 6 (2) : 135 –141. |
| Dayton P K, Robilliard G A, Paine R T. 1970. Benthic faunal zo-nation as a result of anchor ice at McMurdo Sound, Antarctica. In:Holgate W M ed. Antarctic ecology.Academic Press, London, p.244-258. |
| Dumont C P, Drolet D, Deschênes I, Himmelman J H, 2007. Multiple factors explain the covering behaviour in the green sea urchin, Strongylocentrotus droebachiensis. Anim. Behav., 73 (6) : 979 –986. Doi: 10.1016/j.anbehav.2006.11.008 |
| Hagen N T. 1994. Is righting response a useful indicator of functional well-being in the green sea urchin Strongylocentrotus droebachiensis? In:David G, Feral R eds. Echinoderms Through Time. Balkema, Rotterdam.p.693-698. |
| Himmelman J H, Guderley H, Vignault G, Drouin G, Wells P G, 1984. Response of the sea urchin, Strongylocentrotus droebachiensis, to reduced salinities:importance of size, acclimation, and interpopulation differences. Can. J.Zool., 62 (6) : 1 015 –1 021. Doi: 10.1139/z84-144 |
| Hyman L H. 1955. The Invertebrates:Echinodermata. The Coelomate Bilateria, Vol IV. McGraw-Hill Book Company, New York. 763p. |
| Kehas A J, Theoharides K A, Gilbert J J, 2005. Effect of sunlight intensity and albinism on the covering response of the Caribbean sea urchin Tripneustes ventricosus. Mar.Biol., 146 (6) : 1 111 –1 117. Doi: 10.1007/s00227-004-1514-4 |
| Lamare M, Burritt D, Lister K, 2011. Ultraviolet radiation and echinoderms:past, present and future perspectives. Adv.Mar. Biol., 59 : 145 –187. Doi: 10.1016/B978-0-12-385536-7.00004-2 |
| Lawrence J M, 1975. The effect of temperature-salinity combinations on the functional well-being of adult Lytechinus variegatus (Lamarck) (Echinodermata, Echinoidea). J. Exp. Mar. Biol. Ecol., 18 (3) : 271 –275. Doi: 10.1016/0022-0981(75)90111-2 |
| Lawrence J M, 1976. Covering response in sea urchins. Nature, 262 (5568) : 490 –491. Doi: 10.1038/262490a0 |
| Lawrence J M. 2013. Sea urchin life history strategies. In:Lawrence J M ed. Sea Urchins:Biology and Ecology. 3rd edn. Academic Press, San Diego, CA. p.15-23. |
| Lemire M, Himmelman J H, 1996. Relation of food preference to fitness for the green sea urchin, Strongylocentrotus droebachiensis. Mar. Biol., 127 (1) : 73 –78. Doi: 10.1007/BF00993646 |
| Luo S B, Chang Y Q, Zhao C, Zhou H S, 2013. Effects of the covering behavior on food consumption, growth and gonad traits of the sea urchin Glyptocidaris crenularis. Acta Ecologica Sinica, 33 (2) : 402 –408. Doi: 10.5846/stxb |
| Mayr E. 1960. The emergence of evolutionary novelties. In:Tax S ed. Evolution after Darwin, vol I. University of Chicago Press, Chicago, IL. p.349-380. |
| Millott N, 1976. The photosensitivity of echinoids. Adv. Mar.Biol., 13 : 1 –52. Doi: 10.1016/S0065-2881(08)60279-5 |
| Orton J H, 1929. On the occurrence of Echinus esculentus on the foreshore in the British Isles. J. Mar. Biol. Assoc. UK., 16 (1) : 289 –296. Doi: 10.1017/S0025315400029817 |
| Pawson D L, Pawson D J, 2013. Bathyal sea urchins of the Bahamas, with notes on covering behavior in deep sea echinoids (Echinodermata:echinoidea). Deep Sea. Res.II., 92 : 207 –213. Doi: 10.1016/j.dsr2.2013.01.023 |
| Pearse J S, 2006. Ecological role of purple sea urchins. Science, 314 (5801) : 940 –941. Doi: 10.1126/science.1131888 |
| Percy J A, 1973. Thermal adaptation in the boreo-arctic echinoid, Strongylocentrotus droebachiensis (O. F.Müller, 1776). II. Seasonal acclimatization and urchin activity. Physiol. Zool., 46 (2) : 129 –138. Doi: 10.1086/physzool.46.2.30155593 |
| Richner H, Milinski M, 2000. On the functional significance of masking behaviour in sea urchins-an experiment with Paracentrotus lividus. Mar. Ecol. Prog. Ser., 205 : 307 –308. Doi: 10.3354/meps205307 |
| Shang Y C. 2005. Ethology. Peking University Press, Beijing, China. p.168-188. (in Chinese) |
| Sigg J E, Lloyd-Knight K M, Boal J G, 2007. UV radiation influences covering behaviour in the urchin Lytechinus variegatus. J. Mar. Biol. Assoc. UK., 87 (5) : 1 257 –1 261. |
| Verling E, Crook A, Barnes D, 2002. Covering behaviour in Paracentrotus lividus:is light important? Mar. Biol., 140 (2) : 391 –396. |
| Zhao C, Zhou H, Tian X F, Feng W P, Chang Y Q, 2014. The effects of prolonged food deprivation on the covering behavior of the sea urchins Glyptocidaris crenularis and Strongylocentrotus intermedius. Mar. Freshw. Behav.Phy., 47 (1) : 11 –18. Doi: 10.1080/10236244.2013.868075 |
 2016, Vol. 34
2016, Vol. 34


