Institute of Oceanology, Chinese Academy of Sciences
Article Information
- Jee-Hoon KIM, Ok Hwan YU, Eun Jin YANG, Sung-Ho KANG, Won KIM, Eun Jung CHOY
- Effects of ocean acidification driven by elevated CO2 on larval shell growth and abnormal rates of the venerid clam, Mactra veneriformis
- Chinese Journal of Oceanology and Limnology, 34(6): 1191-1198
- http://dx.doi.org/10.1007/s00343-016-5159-1
Article History
- Received Jul. 13, 2015
- accepted for publication Aug. 29, 2015
- accepted in principle Oct. 16, 2015
2 Marine Ecosystem Research Division, Korea Institute of Ocean Science and Technology, 787 Haean-ro, Sangnok-gu, Ansan 15627, Republic of Korea;
3 School of Biological Sciences, Seoul National University, 1 Gwanak-ro, Gwanak-gu, Seoul 08826, Republic of Korea
Atmospheric CO2 concentrations have been increasing since preindustrial times, resulting in changes in global temperatures, climate and ocean chemistry. The projections based on the Intergovernmental Panel on Climate Change (IPCC) emission scenario Representative Concentration Pathway (RCP)8.5 for the year 2100 indicate further increases in average pCO2 in the atmosphere of (530- 570)×10-6(Stocker et al., 2013). The oceans absorb 25%-30% of the CO2 released from the atmosphere (Fabry et al., 2008); however, this is a slow process that cannot keep pace with the current rate of pCO2 increase (Hoegh-Guldberg et al., 2007). For these reasons, in addition to causing the oceans to acidify, CO2 gas is causing global warming. Not only are the predicted changes in ocean pH substantial, but they are also expected to occur more rapidly than any pH changes experienced in the last 24 million years (Blackford and Gilbert, 2007). Despite the uncertainty surrounding the effects of ocean acidification, which is still not fully understood, it is generally assumed that ocean acidification will have significant direct and indirect impacts on marine organisms (Hoegh- Guldberg et al., 2007; Kleypas and Yates, 2009).
Calcifying organisms are already recognized as sentinel species for ocean acidification because of their ability to produce calcareous structures that could be affected by seawater carbonate chemistry (Orr et al., 2005). Among calcifying species, bivalves play an important role in marine and coastal ecosystems, providing habitats for many other invertebrates and linking the organic and inorganic carbon cycles, both as carbonate producers and as lower trophic level animals (Gutiérrez et al., 2003). Additionally, bivalves form one of the largest classes in the Mollusca, and are currently a harvestable fisheries resource for which the potential direct effects of ocean acidification are well understood (Cooley et al., 2012). For these reasons, bivalve species have been used as target species in studies examining elevated CO2(Kurihara et al., 2007, 2008; Parker et al., 2009, 2012; Talmage and Gobler, 2010, 2011, 2012; Range et al., 2012; Van Colen et al., 2012; Barros et al., 2013; Ginger et al., 2013; White et al., 2013; Scanes et al., 2014). However, the extent of the effects of elevated CO2 differs between closely related species (Parker et al., 2010), and in some cases, among different populations of the same species (Waldbusser et al., 2010; Parker et al., 2011). Although it is difficult to predict the global impact of elevated CO2 on an organism, it is useful to understand its effects on numerous species in specific regions and to anticipate the severity and effects of regional changes. The venerid clam, Mactra veneriformis Reeve 1854(Bivalvia: Mactridae), is one of the main cultured bivalve species in intertidal and shallow subtidal ecosystems along the west coast of Korea (Hur et al., 2005). Although M. veneriformis is an ecologically and economically important bivalve species, few data on its physical and ecological characteristics have been reported.
Recent studies of the effects on various life-history stages of calcifying organisms aimed to predict the impacts of CO2 -driven acidification on larval growth and development (Hendriks et al., 2010; Talmage and Gobler, 2010, 2011, 2012; Parker et al., 2012). The bivalve shell consists of calcite, aragonite, or the two mixed; however, all shells of bivalve larvae comprise only aragonite, the soluble form of CaCO3(Weiss et al., 2002), and acidification experiments have demonstrated that larvae of marine organisms are more vulnerable to ocean acidification than their adults (Kurihara, 2008; Walther et al., 2010). However, previous acidification studies on larval bivalves did not refer to specific abnormalities, and simply focused on the malformation of D-shaped stage larvae (Kurihara et al., 2007, 2008; Parker et al., 2009; Talmage and Gobler, 2010, 2011, 2012; Barros et al., 2013). Thus, abnormalities during the development from the trochophore stage to after the D-shaped (umbonate veliger and pediveliger) stage need to be investigated in much greater detail.
While it is difficult to predict the impacts of elevated pCO2 on marine organisms and their communities accurately, it is important to investigate the vulnerability of specific species and the potential impacts of acidification on a regional scale. In this study, we examined shell growth and abnormalities in the early life stages of M. veneriformis after exposure to elevated seawater pCO2, and we have categorized larval abnormality types, which previously had only been studied for D-shaped larvae, based on the shell morphology. The purpose of our study was to better understand uncertainties in the local ecological and physiological responses of larvae to increased CO2 levels, thus supporting further ocean acidification research.
2 MATERIAL AND METHOD 2.1 Experimental designD-shaped larvae of M. veneriformis were obtained from the Incheon Fisheries Research Institute through artificial spawning induced by exposure to air and elevated water temperatures. Adult M. veneriformis specimens were obtained from the west coast of Taean-gun, Chungcheongnam-do, Korea. The D-shaped larvae were kept for 1 day in an indoor (22±0.5℃) open-water cage with a continuous air supply for acclimation to the water temperature before the experiments. Initially, culture tanks were thoroughly washed with ethanol, rinsed with freshwater, and left to air-dry for 24 h. Glass fiber filter (GF/F)-treated seawater was introduced to the culture tanks and bubbled either with air (control) or with CO2 -enriched air (Multichannel Gas Mixer, Biota Korea; CO2, UHP (Ultra High Purity) CO2, purity 99.999%; N2, purity 99.999 9%) at 22±0.5℃(CO2 compensated at 400×10-6 and 1 000×10-6). Acidification was generated in the culture tanks by bubbling CO2 -enriched air at 400±25×10-6(ambient control), (800±25)×10-6(high pCO2), or (1 200±28)×10-6(extremely high pCO2); namely, the atmospheric CO2 concentrations predicted for the years 2014, 2084, and 2154, (70-year intervals or two human generations) respectively, in the RCP 8.5 scenario. The required CO2 concentrations in the CO2 -enriched air were obtained by adjusting the flow rate using a variable area flow meter/controller (Cole- Parmer, Vernon Hills, IL, USA). The gases in each culture tank during the experiment were monitored every 10 min using a CO2 Gas Analyzer (Li-820, LICOR Inc., Lincoln, NE, USA) to maintain the specific CO2 concentration, and a data logger (HOBO data logger, Onset Computer Corp., Bourne, MA, USA) logged data every 30 sec. The D-shaped larvae were distributed equally among nine experimental units/ tanks (five individuals/mL, three treatments × three replicates). Each cylindrical plastic culture tank contained 6 L of 0.45- µm filtered seawater; larval density was monitored in each culture tank during each water exchange. Salinity and nutrient concentrations of the experimental seawater in the ambient control and CO2 -enriched groups were as follows: salinity, 31±1.1; ammonia, 5.1±1.1 µmol/L; nitrate, 6.7±1.3 µmol/L; phosphate, 0.55±0.2 µmol/L; and silicate, 11±1.8 µmol/L. Each culture tank was aerated to keep the larvae dispersed in the water column. Larvae were fed live algae, Isochrysis galbana, (450 000 cells/mL) once daily.
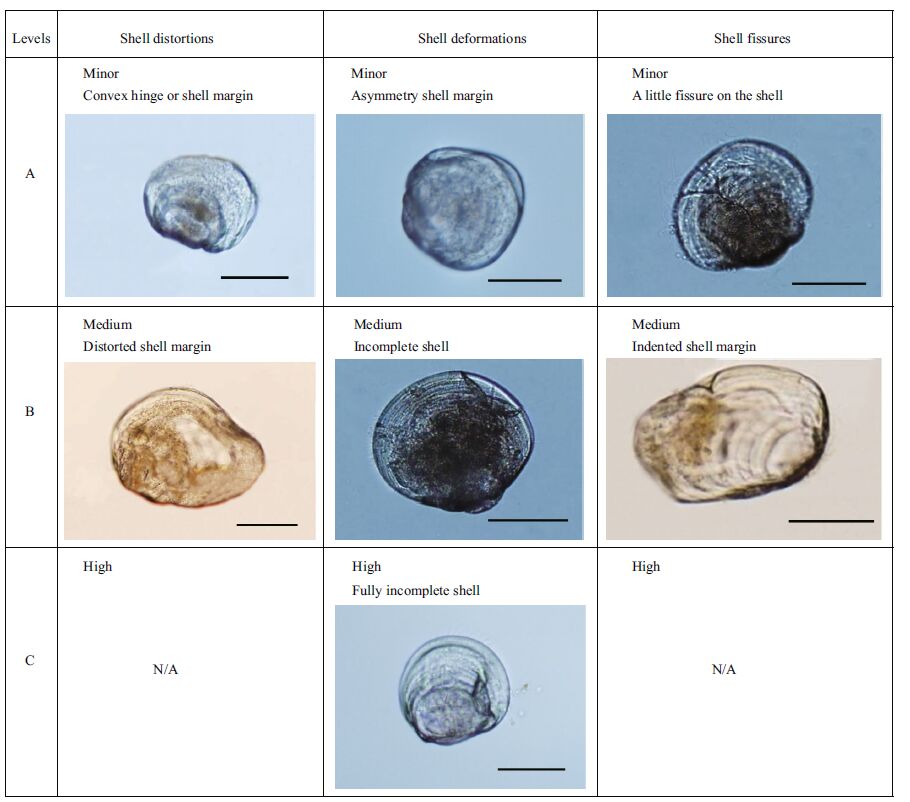
2.2 Shell growth and abnormality ratesOnce every 2 days, 90% of the seawater in each culture tank was removed and replaced with fresh seawater at the appropriate pCO2 concentration, and subsamples of >30 M. veneriformis larvae were obtained from each replicate for measurements of mean shell length (Fig. 1), mean shell height, developmental stage, and abnormality rate (Fig. 2) using image software (CellSens Standard, Olympus, Tokyo, Japan) coupled with a light microscope (BX51, Olympus). The larval shell morphological condition, which could be discerned visually, was also determined from these images. The types of abnormal M. veneriformis larvae at 9 and 13 days are summarized in Fig. 3 according to the degree of severity (minor, medium, and high), and the abnormality types, which were divided into three categories (His et al., 1997, Barros et al., 2013): shell distortions, shell deformations, and shell fissures (Figs. 3 and 4).

|
| Figure 1 Mean shell lengths of M. veneriformis larvae: 800×10-6, high pCO2 group; 1 200×10-6, extremely high pCO2 group; 400×10-6, ambient control group Asterisks denote significant differences; mean±SD; n =3 replicates (F-test; P<0.05). |
|
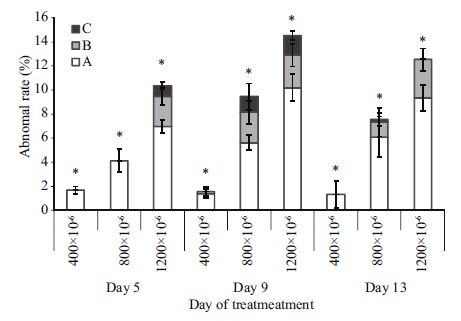
| Figure 2 Abnormality rates of M. veneriformis larvae A. minor degree abnormalities; B. medium degree abnormalities; C. high degree abnormalities; asterisks denote significant differences; mean±SD; n =3 replicates (F-test; P<0.001). |
|
| Figure 3 Levels and types of morphological abnormalities in M. veneriformis larvae Scale bar=100 µm. |
|
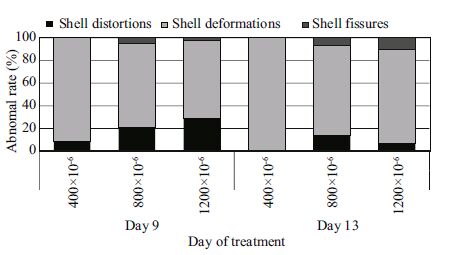
| Figure 4 The composition of morphological abnormalities in M. veneriformis larvae |
Three replicates of the experiments were carried out, each consisting of one control and two CO2 groups originating from the same larval batch. The mean from each group was used to obtain overall means and standard deviation (SD) values for statistical comparisons. The Tukey-Kramer test was applied to evaluate the effects of pCO2 on shell growth and abnormality rates. The F-test (one-way ANOVA) was used to compare the effects of pCO2 on the shell growth and percentages of larval abnormalities at each level of pCO2. The differences between mean shell length and mean abnormality rate were considered statistically significant at P<0.05 and P<0.001, respectively. The statistical analyses were performed using SPSS version 16.0(IBM Corp., Armonk, NY, USA).
3 RESULTElevated pCO2 caused a significant reduction in shell length in the M. veneriformis larvae in the high (800×10-6) and extremely high (1 200×10-6) groups when compared with the ambient control (400×10-6) group. After 9 days of exposure, statistical differences were observed between the ambient control groups and the two elevated pCO2 groups (one-way ANOVA; P<0.05; Fig. 1). The initial mean shell length of the larvae was 90.1±0.6 µm for all three treatments (Fig. 1). After 9 days of exposure, the mean shell length was 173.3±2.6 µm in the ambient control groups, in contrast to 167.5±3.3 µm (96.7%) and 155.1±3.4 µm (89.5%) in the high and extremely high pCO2 groups, respectively (one-way ANOVA, F-ratio 20.2; 2 df; P<0.05; Fig. 1). After day 9, the mean shell length remained statistically different between the ambient control groups and the two elevated pCO2 groups (one-way ANOVA, P<0.05; Fig. 1).
Abnormalities were observed in the larvae under all three conditions, but in the high and extremely high pCO2 groups, the percentages of abnormalities were increased compared with the ambient control groups (one-way ANOVA; P<0.001; Fig. 2). Only 1.7% of the larvae were abnormal in the ambient control groups by day 5, compared with 4.1% and 10.4% in the high and extremely high pCO2 groups, respectively (one-way ANOVA; F-ratio, 185.9; 2 df; P<0.001). The percentage of abnormal larvae decreased slightly to 1.4% in the control groups by day 9; however, abnormality rates increased to 9.4% and 14.5% in the high and extremely high pCO2 groups, respectively (one-way ANOVA, F-ratio, 198.5; 2 df; P<0.001). By day 13, the abnormality rates of all treatments decreased slightly to 1.32%(control), 7.6%(high CO2), and 12.5%(extremely high CO2 ; one-way ANOVA, F-ratio, 152.4; 2 df; P<0.001). Medium degree abnormalities were observed in the control group after day 9; however they appeared more rapidly (by day 5) in the extremely high pCO2 group. At both day 5 and day 9, the proportions of high abnormalities in the two elevated pCO2 groups were also higher than in the ambient control groups (one-way ANOVA; P<0.05, Figs. 2 and 3). The proportions of high abnormalities in all three treatments were markedly decreased by day 13.
The types of abnormalities were divided into three categories: shell distortions, shell deformations, and shell fissures (Fig. 3). Of these, shell deformations were the most abundant (Fig. 4). By day 9, 72% of the abnormalities (average of all three conditions) were shell deformations, while the percentage of shell distortions was 24%, and only 4% of the abnormalities were shell fissures (Fig. 4). The percentages of shell deformations and shell fissures increased to 80% and 10%, respectively, by day 13; however, the percentage of shell distortions decreased to 10%(average of all three conditions; Fig. 4). Shell distortions and shell fissures were nearly absent in the ambient control groups by days 9 and 13(Fig. 4).
4 DISCUSSIONExposure to seawater acidified through equilibration with air containing 800×10-6 or 1 200×10-6 CO2 led to significant size reductions and morphological abnormalities in the early developmental stages (D-veliger and umbonate veliger stages) of the larvae of M. veneriformis. Under acidified seawater conditions, the larvae were still able to form shells, even in seawater with extremely high pCO2 conditions (1 200×10-6), which exceeded the worst ocean acidification scenario foreshadowed by the RCP 8.5 for the year 2100(940×10-6). However, during the first 9 days of the experiment, the high and extremely high pCO2 groups exhibited reduced mean larval shell sizes (Fig. 1) and increased abnormality rates (Fig. 2) relative to the ambient control. This suggests that CO2 exposure during the early development stage is critical for shell development in M. veneriformis larvae. From days 9-13, statistically significant differences in mean shell length were observed as a result of CO2 exposure (Fig. 1), indicating that the shell growth of M. veneriformis during these early days postfertilization determines the shell size in later developmental stages.
Our results demonstrating that high pCO2 exposure impairs early larval shell size are consistent with the negative effects caused by high pCO2 exposure observed in the larval sizes of other bivalves. For example, at day 8, the larvae of the oyster Saccostrea glomerata exhibited decreased mean shell sizes of 6.3% at pH 7.8 and 8.7% at pH 7.6, relative to the ambient control treatment at pH 8.1(Watson et al., 2009). Similarly, the clams Mercenaria mercenaria and Argopecten irradians larvae grown under 390×10-6 CO2(282±5 and 449±35 μm) were significantly larger than those grown under higher (210±9 and 311±26 μm at ~1 500×10-6) levels of CO2 at day 24 and 20 respectively (Talmage and Gobler, 2010). Also, larvae of the clam Macoma balthica exhibited significant reductions in shell length at day 3 under conditions of seawater at pH 7.5 and 7.8, relative to control seawater at pH 8.1(Van Colen et al., 2012). These results combined with our present study corroborate that the shell growth of larvae exposed to high pCO2 might be impaired critically in early life-stage development, not only at one day postfertilization (D-veliger stage), but even after a week (after umbonate veliger stage).
Our findings suggest that seawater with elevated pCO2 concentrations influenced early shell-producing processes responsible for larval shell calcification. The organisms that use CaCO3 to form shells may be sensitive to chemical dissolution. The dissolution of increased CO2 in the seawater causes chemical changes leading to decrease in the availability of carbonate ions (CO32-), and this can increase the dissolution of calcium carbonate (CaCO3) minerals (Kleypas et al., 1999; Findlay et al., 2009). Although the different response of CaCO3 production to changing ocean chemistry may occur between species, the calcareous shells of these organisms start to dissolve when carbonate saturation drops below unity (Schulz et al., 2008). The calcification of mollusk shells occurs in a closed compartment into which ions can diffuse or be pumped to increase their concentrations; the organisms produce organic compounds that define the shape of the crystal, and then finally arrest its growth (Gazeau et al., 2013). The larval shells are produced during their early larval development by a shell gland, the inner part of which is transformed into the larval mantle epithelium that will define the next shell field (Marin and Luquet, 2004; Weiss and Schönitzer, 2006). The organic shell in larvae begins to be secreted at the late trochophore stage and calcification is initiated in the early veliger stage; therefore these early development stages might be more sensitive to elevated pCO2(Hayakaze and Tanabe, 1999; Kurihara et al., 2008; Parker et al., 2009).
We found here that exposure to high pCO2 led to increased frequencies and increased severity of morphological abnormalities in M. veneriformis larvae, compared with the ambient control (Fig. 2). Larval abnormalities have been reported previously in mollusks exposed to acidification caused by high pCO2(Gazeau et al., 2007; Kurihara et al., 2007, 2008; Parker et al., 2010). We also found high abnormality rates in larvae exposed to high pCO2, that are in agreement with previous studies of mollusk abnormality rates under these conditions (Fig. 2). The shell integrity is one of the most important and fundamental factors of defense for the clams, especially in their early life stages, because the shells provide physical shielding for delicate and vulnerable internal tissues and protection from predators and harmful substances (Carriker, 1986, 1996). In point of fact, these shell abnormalities can be fatal elements within the health of shellfishes, so monitoring of not only shell abnormality rates but also abnormality types and levels is needed.
In our study, light microscopy provided evidence of shell abnormalities. The morphological abnormality types, including convex hinges, indented shell margins, incomplete shells, and protrusion of the mantle, are typical criteria used to distinguish normal from abnormal larval development in mollusks (His et al., 1997; Barros et al., 2013). Previous studies focused on abnormalities in larvae in the D-veliger stage. However, we also examined larvae in the umbonate veliger and pediveliger stages. The types of abnormalities in umbonate veliger larvae were somewhat different from those in D-veliger larvae reported in previous studies (His et al., 1997; Kurihara et al., 2008; Barros et al., 2013). In the D-veliger stage, the most common abnormalities are convex hinges and mantle protuberances. However, in our results for umbonate veliger larvae, the most common abnormalities were shell deformations, distortions, and fissures, while convex hinge and mantle protuberances were absent. The two latter abnormality types are clearly fatal factors that disturb the next step in development. In addition, the abnormalities we observed under the elevated pCO2 conditions showed conspicuously different shapes compared with the larval abnormality types described by His et al.(1997). This implies that the larval abnormality types in these artificially acidified conditions differ from the natural abnormalities of larvae.
Therefore, we categorized larval abnormalities according to shell morphology in the present study— shell deformations, shell distortions, and shell fissures—to include some abnormality types that were de-emphasized in previous studies (e.g., asymmetric shell margins, distorted shells, and fissures). The reason for these classifications was that shell distortions and shell fissures occurred frequently in the elevated CO2 groups, and clearly showed features that differed from shell deformation. In addition, the abnormalities observed under high and extremely high pCO2 conditions—asymmetric shell margins, distorted shells, and fissures—are characteristics that can be fatal or can severely affect the health of the organism. Because there were no records of the shell abnormality levels, we divided the levels of morphological abnormalities as loss of the functional shell abilities. We decided on a medium degree of abnormalities when the shells lost their defense ability from the outside or formed a severe shell asymmetry, which can affect growth. If the shells maintained functionality but could have lost their function when the abnormality was more severe, the degree of abnormality was categorized as minor. Also if the inside of the larva was exposed to the outside at least 5/1, the abnormality was categorized as a high degree.
Among shell deformations, the amount of severity ranged from asymmetric shell margins (minor degree) to incomplete shells (medium degree) to fully incomplete shells (high degree). Among the shell distortions, convex hinges (found only at the D-veliger stage) or convex shell margins (minor degree) and distorted shell margins (medium degree) were observed. The fissures observed included those on the shell (minor degree) and indented shell margins (medium degree). This study revealed that at 9 and 13 days after exposure, shell deformations were the most frequently observed type resulting from seawater acidification (Fig. 4). These morphological abnormalities might arise from the inability of the larvae to produce sufficient calcium carbonate, which is crucial in the development of shells, or they could be the results of dissolution of the shell caused by corrosion from seawater (Barros et al., 2013). One possible reason for such a significant increase in shell deformations in larvae exposed to elevated pCO2 might be the disproportional production of calcium carbonate.
There is no clear evidence of elevated pCO2 -related mortalities of bivalves in natural habitats, even under extremely high pCO2 conditions that exceed the worst future scenarios for ocean acidification (Tunnicliffe et al., 2009). However, the negative effects of elevated pCO2 on the larvae of M. veneriformis could have major consequences for the adult clams at the population level. Impairment of growth and increased abnormality rates of larvae will reduce the biomass of individuals reaching the next developmental stages, and these abnormalities might also affect the swimming capability of the larvae and thus decrease their fitness (Kurihara et al., 2008). Also, weakened shells could impair functionality, increasing the vulnerability of the bivalves to predators, physical damage, and parasites, which may reduce survival rates in natural habitats and in aquaculture (Cooley et al., 2012). Even under natural conditions, larval mortality of benthic invertebrates is already extremely high (Gosselin and Qian, 1997). If these abnormalities affect viability during critical stages of development from exposure to elevated pCO2, reduced numbers might also be seen in the adult stages. Thus, ocean acidification will place an additional burden on the larval health of the venerid clam M. veneriformis by impairing development in the early life stages.
| Barros P, Sobral P, Range P, Chícharo L, Matias D, 2013. Effects of sea-water acidification on fertilization and larval development of the oyster Crassostrea gigas. Journal of Experimental Marine Biology and Ecology, 440 : 200 –206. Doi: 10.1016/j.jembe.2012.12.014 |
| Blackford J C, Gilbert F J, 2007. pH variability and CO2 induced acidification in the North Sea. Journal of Marine Systems, 64 (1-4) : 229 –241. Doi: 10.1016/j.jmarsys.2006.03.016 |
| Carriker M R, 1986. Influence of suspended particles on biology of oyster larvae in estuaries. American Malacological Bulletin, 3 : 41 –49. |
| Carriker M R. 1996. The shell and ligament. In:Kennedy V S, Newell R I E, Eble A eds. The Eastern Oyster Crassostrea virginica. Maryland Sea Grant, College Park, Maryland.p.75-168. |
| Cooley S R, Lucey N, Kite-Powell H, Doney S C, 2012. Nutrition and income from molluscs today imply vulnerability to ocean acidification tomorrow. Fish and Fisheries, 13 (2) : 182 –215. Doi: 10.1111/faf.2012.13.issue-2 |
| Fabry V J, Seibel B A, Feely R A, Orr J C, 2008. Impacts of ocean acidification on marine fauna and ecosystem processes. ICES Journal of Marine Science, 65 (3) : 414 –432. Doi: 10.1093/icesjms/fsn048 |
| Findlay H S, Kendall M A, Spicer J I, Widdicombe S, 2009. Future high CO2 in the intertidal may compromise adult barnacle Semibalanus balanoides survival and embryonic development rate. Marine Ecology Progress Series, 389 : 193 –202. Doi: 10.3354/meps08141 |
| Gazeau F, Parker L M, Comeau S, Gattuso J P, O'Connor W A, Martin S, Pörtner H O, Ross P M, 2013. Impacts of ocean acidification on marine shelled molluscs. Marine Biology, 160 (8) : 2 207 –2 245. Doi: 10.1007/s00227-013-2219-3 |
| Gazeau F, Quiblier C, Jansen J M, Gattuso J P, Middelburg J J, Heip C H R, 2007. Impact of elevated CO2 on shellfish calcification. Geophysical Research Letters, 34 (7) : L07603 . |
| Ginger K W K, Vera C B S, Dineshram R, Dennis C K S, Adela L J, Yu Z N, Thiyagarajan V, 2013. Larval and post-larval stages of pacific oyster (Crassostrea gigas) are resistant to elevated CO2. PLoS One, 8 (5) : e64147 . Doi: 10.1371/journal.pone.0064147 |
| Gosselin L A, Qian P Y, 1997. Juvenile mortality in benthic marine invertebrates. Marine Ecology Progress Series, 146 : 265 –282. Doi: 10.3354/meps146265 |
| Gutiérrez J L, Jones C G, Strayer D L, Iribarne O O, 2003. Mollusks as ecosystem engineers:the role of shell production in aquatic habitats. Oikos, 101 (1) : 79 –90. Doi: 10.1034/j.1600-0706.2003.12322.x |
| Hayakaze E, Tanabe K, 1999. Early larval shell development in mytilid bivalve Mytilus galloprovincialis. Venus, 58 : 119 –127. |
| Hendriks I E, Duarte C M, Álvarez M, 2010. Vulnerability of marine biodiversity to ocean acidification:a metaanalysis. Estuarine Coastal Shelf Science, 86 (2) : 157 –164. Doi: 10.1016/j.ecss.2009.11.022 |
| His E, Seaman M N L, Beiras R, 1997. A simplification the bivalve embryogenesis and larval development bioassay method for water quality assessment. Water Research, 31 (2) : 351 –355. Doi: 10.1016/S0043-1354(96)00244-8 |
| Hoegh-Guldberg O, Mumby P J, Hooten A J, et al, 2007. Coral reefs under rapid climate change and ocean acidification. Science, 318 (5857) : 1 737 –1 742. Doi: 10.1126/science.1152509 |
| Hur Y B, Bae J H, Hur S B, 2005. Comparison of development and larval growth of four venerid clams. Journal of the World Aquaculture Society, 36 (2) : 179 –187. |
| Kleypas J A, Buddemeier R W, Archer D, Gattuso J P, Langdon C, Opdyke B N, 1999. Geochemical consequences of increased atmospheric carbon dioxide on coral reefs. Science, 284 (5411) : 118 –120. Doi: 10.1126/science.284.5411.118 |
| Kleypas J A, Yates K K, 2009. Coral reefs and ocean acidification. Oceanography, 22 (4) : 108 –117. Doi: 10.5670/oceanog |
| Kurihara H, Asai T, Kato S, Ishimatsu A, 2008. Effects of elevated pCO2 on early development in the mussel Mytilus galloprovincialis. Aquatic Biology, 4 (3) : 225 –233. |
| Kurihara H, Kato S, Ishimatsu A, 2007. Effects of increased seawater pCO2 on early development of the oyster Crassostrea gigas. Aquatic Biology, 1 (1) : 91 –98. |
| Kurihara H, 2008. Effects of CO2-driven ocean acidification on the early developmental stages of invertebrates. Marine Ecology Progress Series, 373 : 275 –284. Doi: 10.3354/meps07802 |
| Marin F, Luquet G, 2004. Molluscan shell proteins. Comptes Rendus Palevol, 3 (6) : 469 –492. |
| Orr J C, Fabry V J, Aumont O, et al, 2005. Anthropogenic ocean acidification over the twenty-first century and its impact on calcifying organisms. Nature, 437 (7059) : 681 –686. Doi: 10.1038/nature04095 |
| Parker L M, Ross P M, O'Connor W A, 2010. Comparing the effect of elevated pCO2 and temperature on the fertilization and early development of two species of oysters. Marine Biology, 157 (11) : 2 435 –2 452. Doi: 10.1007/s00227-010-1508-3 |
| Parker L M, Ross P M, O'Connor W A, 2011. Populations of the Sydney rock oyster, Saccostrea glomerata, vary in response to ocean acidification. Marine Biology, 158 (3) : 689 –697. Doi: 10.1007/s00227-010-1592-4 |
| Parker L M, Ross P M, O'Connor W A, Borysko L, Raftos D A, Pörtner H O, 2012. Adult exposure influences offspring response to ocean acidification in oysters. Global Change Biology, 18 (1) : 82 –92. Doi: 10.1111/j.1365-2486.2011.02520.x |
| Parker L M, Ross P M, O'Connor W A, 2009. The effect of ocean acidification and temperature on the fertilization and embryonic development of the Sydney rock oyster Saccostrea glomerata (Gould 1850). Global Change Biology, 15 (9) : 2 123 –2 136. Doi: 10.1111/gcb.2009.15.issue-9 |
| Range P, Piló D, Ben-Hamadou R, Chicharo M A, Matias D, Joaquim S, Oliveira A P, Chícharo L, 2012. Seawater acidification by CO2 in a coastal lagoon environment:effects on life history traits of juvenile mussels Mytilus galloprovincialis. Journal of Experimental Marine Biology and Ecology, 424-425 : 89 –98. Doi: 10.1016/j.jembe.2012.05.010 |
| Scanes E, Parker L M, O'Connor W A, Ross P M, 2014. Mixed effects of elevated pCO2 on fertilisation, larval and juvenile development and adult responses in the mobile subtidal scallop Mimachlamys asperrima (Lamarck, 1819). PLoS One, 9 (4) : e93649 . Doi: 10.1371/journal.pone.0093649 |
| Schulz K G, Riebesell U, Bellerby R G J, Biswas H, Meyerhöfer M, Müller M N, Egge J K, Nejstgaard J C, Neill C, Wohlers J, Zöllner E, 2008. Build-up and decline of organic matter during PeECE III. Biogeosciences, 5 (3) : 707 –718. Doi: 10.5194/bg-5-707-2008 |
| Stocker T F, Qin D, Plattner G K, Tignor M M B, Allen S K, Boschung J, Nauels A, Xia Y, Bex V, Midgley P M, 2013. Climate Change 2013:The Physical Science Basis.Contribution of Working Group I to the Fifth Assessment Report of IPCC the Intergovernmental Panel on Climate Change. Cambridge University Press, Cambridge, New York. |
| Talmage S C, Gobler C J, 2010. Effects of past, present, and future ocean carbon dioxide concentrations on the growth and survival of larval shellfish. Proceedings of the National Academy of Sciences of the United States of America, 107 (40) : 17 246 –17 251. Doi: 10.1073/pnas.0913804107 |
| Talmage S C, Gobler C J, 2011. Effects of elevated temperature and carbon dioxide on the growth and survival of larvae and juveniles of three species of northwest Atlantic bivalves. PLoS One, 6 (10) : e26941 . Doi: 10.1371/journal.pone.0026941 |
| Talmage S C, Gobler C J, 2012. Effects of CO2 and the harmful alga Aureococcus anophagefferens on growth and survival of oyster and scallop larvae. Marine Ecology Progress Series, 464 : 121 –134. Doi: 10.3354/meps09867 |
| Tunnicliffe V, Davies K T A, Butterfield D A, Embley R W, Rose J M, Chadwick Jr W W, 2009. Survival of mussels in extremely acidic waters on a submarine volcano. Nature Geoscience, 2 (5) : 344 –348. Doi: 10.1038/ngeo500 |
| Van Colen C, Debusschere E, Braeckman U, Van Gansbeke D, Vincx M, 2012. The early life history of the clam Macoma balthica in a high CO2 world. PLoS One, 7 (9) : e44655 . Doi: 10.1371/journal.pone.0044655 |
| Waldbusser G G, Bergschneider H, Green M A, 2010. Sizedependent pH effect on calcification in post-larval hard clam Mercenaria spp. Marine Ecology Progress Series, 417 : 171 –182. Doi: 10.3354/meps08809 |
| Walther K, Anger K, Pörtner H O, 2010. Effects of ocean acidification and warming on the larval development of the spider crab Hyas araneus from different latitudes (54° vs. 79°N). Marine Ecology Progress Series, 417 : 159 –170. Doi: 10.3354/meps08807 |
| Watson S A, Southgate P C, Tyler P A, Peck L S, 2009. Early larval development of the Sydney rock oyster Saccostrea glomerata under near-future predictions of CO2-driven ocean acidification. Journal of Shellfish Research, 28 (3) : 431 –437. Doi: 10.2983/035.028.0302 |
| Weiss I M, Schönitzer V, 2006. The distribution of chitin in larval shells of the bivalve mollusk Mytilus galloprovincialis. Journal of Structural Biology, 153 (3) : 264 –277. Doi: 10.1016/j.jsb.2005.11.006 |
| Weiss I M, Tuross N, Addadi L, Weiner S, 2002. Mollusc larval shell formation:amorphous calcium carbonate is a precursor phase for aragonite. Journal of Experimental Zoology, 293 (5) : 478 –491. Doi: 10.1002/(ISSN)1097-010X |
| White M M, McCorkle D C, Mullineaux L S, Cohen A L, 2013. Early exposure of bay scallops (Argopecten irradians) to high CO2 causes a decrease in larval shell growth. PLoS One, 8 (4) : e61065 . Doi: 10.1371/journal.pone.0061065 |
 2016, Vol. 34
2016, Vol. 34


