Institute of Oceanology, Chinese Academy of Sciences
Article Information
- Sara PLATTO, WANG Ding(王丁), WANG Kexiong(王克雄)
- Variation in the emission rate of sounds in a captive group of false killer whales Pseudorca crassidens during feedings: possible food anticipatory vocal activity?
- Chinese Journal of Oceanology and Limnology, 34(6): 1218-1237
- http://dx.doi.org/10.1007/s00343-016-5074-5
Article History
- Received May. 15, 2015
- accepted for publication Jun. 30, 2015
- accepted in principle Sep. 2, 2015
Animals' calls have evolved to serve as communication signals partly to indicate specific emotional states. Such signals are made in the wild as well as in captivity (Mulligan et al., 1994 ; Grandin, 1998 ; Panksepp and Burgdorf, 2000 ; Burgdorf and Panksepp, 2001 ; Manteuffel et al., 2004 ; Burman et al., 2007). In fact, it has been found that sound rates, type of calls, and acoustic structure within a call may vary depending on the context of behaviors such as aggression, panic, and social separation (rodents: Goldstein et al., 1996 ; Albeck et al., 1997 ; primates: Bayart et al., 1990 ; Champoux and Suomi, 1994 ; Friedman et al., 1995 ; Laudenslager et al., 1995 ; Boinski et al., 1999 ; farm animals: von Borell and Ladewig, 1992 ; Désautés et al., 1997 ; Weary et al., 1997). Animals living in a controlled environment with predictable daily routines, experience anticipation which is a mental state that can influence their behaviors including vocalizations (Krishnamurthy, 1994 ; Knutson et al., 1998 ; Burgdorf et al., 2000 ; Waitt and Buchanan-Smith, 2001 ; Ulyan et al., 2006 ; Düpjan et al., 2008 ; Gilbert-Norton et al., 2009 ; Polizzi di Sorrentino et al., 2010).
Among the routines that are performed on captive animals, feeding represents the most important imposed event (Carlstead, 1986). Since in most facilities feeding tends to occur at predictable temporal and spatial locations (Carlstead, 1986), the animals' natural process of foraging is disrupted (Jones and Pillay, 2004). Foraging is considered a rewarding act comprised of appetitive (exploratory behaviors) and consumatory (ingestion of food) phases for which animals may have behavioral needs (Wallace, 1979 ; Jones and Pillay, 2004). The necessity to perform both phases of foraging decreases only if individuals experience the consequences of both components of this behavior (Neuringer, 1969). In captivity, animals are provided with food they do not hunt, which prevents them from fulfilling the appetitive aspect of foraging they innately require. The restrictions imposed by the controlled environment associated with their high level of motivation to express appetitive behavior in captive animals may lead them to change their arousal levels during the time prior to feeding (Mason, 1991 ; Carlstead, 1998 ; Mallapur and Chellan, 2002). These behavioral modifications are referred to as food anticipatory activity (FAA)(see review Mistlberger, 1994 ; Bassett and Buchanan-Smith, 2007), which is defined by Spruijt et al.(2001) as “responses elicited by rewarding stimuli that lead to and facilitate consumatory behaviors”(Bassett and Buchanan- Smith, 2007 ; Jensen et al., 2013).
FAA is thought to be developed by a classically conditioned process through repeated pairing of either circadian phases or external cues with food presentation (Amstrong, 1980 ; see review Mistlberger, 1994). For example, when food availability is predictable, and it occurs at the same time of the day, animals can predict the occurrence of the feeding based on their internal clock (reviewed by Mistlberger, 1994 ; Roberts, 1998). In this case, food seeking behavior (appetitive phase of foraging) comes under the control of a circadian mechanism referred to as temporal predictability (Mistlberger, 2009), which relies on the ability of the animals to estimate time intervals between events (Richelle, 1980 ; Bassett and Buchanan-Smith, 2007 ; Jensen et al., 2013). The capacity to detect and learn to use temporal information to predict intervals between food availability is a basic adaptive aspect of behavior (Richelle, 1980 ; Higa and Staddon, 1997 ; Bassett and Buchanan-Smith, 2007), which can be maintained even if the individuals are kept in an environment with either no or irregular variation in light intensity (Johanneson and Ladewig, 2000). In addition to temporal predictability, animals may develop responsiveness to signaled predictability, which is referred to when the occurrence of an event is generally preceded by specific cues defined as “safety signals”(Seligman, 1968 ; Seligman and Meyer, 1970). A safety signal is a reliable external stimulus that tells the animals when to anticipate the forthcoming event (Bassett and Buchanan-Smith, 2007).
There is no unified opinion regarding the beneficial or negative effects of predictability on the welfare of captive animals (Bassett and Buchanan-Smith, 2007). Studies have shown that feeding animals at a predictable temporal and spatial location has negative impacts that range from increased activity such as agonistic, self-directed behaviors, vocalization (Wilson and Wilson, 1968 ; Reynolds and Luscombe, 1969 ; de Waal and Hoekstra, 1980 ; Wasserman and Cruikshank, 1983 ; Krishnamurthy, 1994 ; Waitt and Buchanan, 2001), and pacing (Baldwin, 1992 ; Carlstead, 1998 ; Weller and Bennet, 2001 ; Mallapur and Chellan, 2002), to inactivity and coprophagy (Bloomsmith and Lambeth, 1995). Johanneson and Ladewig (2000) suggested that in a very predictable environment, animals may be locked into cycles of anticipating the occurrence of the routines which, in absence of control, may be more stressful than unpredictability. For example, when the anticipation of feeding is inaccurate because of delays of the session, anticipation can be a source of stress for captive animals (Waitt and Buchanan-Smith, 2001). Therefore, the introduction of unpredictable feeding schedules could provide stimulation to the individuals, enhancing the species exploratory behaviors (Hennessy et al., 1977 ; van Rooijen, 1991 ; Shepherdson et al., 1993 ; Mistlberger, 1994 ; and Lambeth, 1995 ; Waitt and Buchanan-Smith, 2001 ; Jenny and Schmid, 2002 ; Bassett and Buchanan-Smith, 2007).
Conversely, other studies have found that making feeding sessions predictable is an effective way to decrease the stress associated with both waiting for and experiencing these events (Krishnamurthy, 1994 ; Ulyan et al., 2006 ; Gottlieb et al., 2013). These findings are contrary to the common idea that animals fed on a predictable schedule undergo a high level of anticipation and stress (Bassett and Buchanan-Smith, 2007). Gottlieb et al.(2013) suggested that a predictable schedule removes the “unknown” of the session, and even though it builds anticipation, it is less stressful than not knowing when food will be delivered. Moreover, when animals are fed on a temporal unpredictable schedule, they activate responses that can be adaptive when the individuals have a degree of control over the environment, for example by increasing the amount of exploration (Reneerkens et al., 2002). In a limited space such as facilities or cages, increasing the exploration may not always be possible. The inability to respond appropriately to changes of stimuli with such adaptive behavior may cause frustration, since the motivation to perform them may not be reduced (Rosenblum and Paully, 1984 ; Hughes and Duncan, 1988 ; Andrews and Rosenblum, 1991, 1993 ; Reneerkens et al., 2002).
Furthermore, switching from fixed to variable feeding schedules for a group of animals habituated to predictable routines may be detrimental to their well-being as the events they anticipate do not occur when expected (Bassett and Buchanan-Smith, 2007). The negative consequences of delays may not be caused just by a loss of temporal predictability, but also by the loss of reliability of the safety signals accompanying the daily routines (Bassett and Buchanan-Smith, 2007). External cues may still be perceived yet not followed by the expected feeding, with a loss of signaled predictability (Carlstead, 1986). Control over one's environment is extremely important for the welfare of animals, and predictability may be critical when individuals have limited control over their housing conditions, because it provides a tool to understand and anticipate uncontrollable surroundings (Bassett and Buchanan-Smith, 2007).
Evidence of food anticipatory activity has been found in many species including rodents, bees, fish, birds, rabbits, carnivores, and primates (for review see Carlstead, 1986 ; Mistlberger, 1994 ; Azzaydi et al., 1998 ; Reebs and Lague, 2000 ; Vinke et al., 2004 ; Ulyan et al., 2006 ; Waitt and Buchanan-Smith, 2006 ; Bassett and Buchanan-Smith, 2007 ; Gilbert-Norton et al., 2009 ; Mistlberger, 2009, 2011). Yet, there is only one study that reports the presence of FAA in dolphins before they participate in feeding or training sessions (Jensen et al., 2013). Among mammals, dolphins have been found to possess good short and long term memory for actions (Mercado et al., 1999 ; Marino, 2004). This ability may allow them to predict and anticipate the occurrence of daily routines either through time estimation or via external cues that precede the sessions (Jensen et al., 2013).
Furthermore, cetaceans rely on acoustic communication to advertise information (de Waal and Tyack, 2003 ; Marino et al., 2007). The diversity of sounds produced by this class of animals in various contexts provides evidence that they serve a highly communicative function (Kaznadzei et al., 1976 ; de Waal and Tyack, 2003 ; Marino et al., 2007). In fact dolphins, like the complex primates (Manser et al., 2002 ; Seyfarth and Cheney, 2003a, b), can modify the rate of specific sounds in order to transmit information related to behavioral events (dos Santos et al., 2005 ; Hawkins and Gardside, 2009a, b, 2010). Even though there is no indication of the presence of referential signals in dolphins' sounds as has been reported in primates (Cheney and Seyfarth, 1982), previous studies have suggested that the variations in the acoustic characteristics (rate and type of call) of the vocalizations of dolphins might be related to emotional states and specific events (Tavolga, 1983 ; Johnson, 1993 ; Herzing, 1996). For example, modifications of the rate of the whistles have been observed in different dolphin species during distinct behavioral contexts such as excitement and stress in Hawaiian spinner dolphins (Stenella longirostris : Norris et al., 1994), during bow riding and feeding in common dolphins (Delphinus delphis : Busnel and Dziedzic, 1966), feeding in pilot whales (Globicephala sp.: Dreher and Evans, 1964), feeding, cooperative behavior, socialising (Acevedo- Gutiérrez and Stienessen, 2004 ; dos Santos et al., 2005), and mother/calf reunions in bottlenose dolphins (Smolker et al., 1993). The degree of exposure to human activities and interactions have also been found influential on the rate of the acoustic emissions of dolphins (Smolker et al., 1993 ; Scarpaci et al., 2000 ; Mann and Kemps, 2003 ; Morisaka et al., 2005 ; Hawkins and Gartside, 2009a, b). For example, an increase of all types of underwater sounds have been recorded in different captive cetaceans (bottlenose dolphins, Tursiops truncatus : Tyack, 1986 ; Janik et al., 1994 ; Sekiguchi and Koshima, 2003 ; Akiyama and Ohta, 2007 ; Beluga whales, Delphinatuera leuca : Fish and Mowbray, 1962 ; Pacific white-sided dolphins, Lagenorhynchus obliquidens : Brickman, 2003) during activities that were special moments for the dolphins such as training, feeding sessions, or public presentations which might have increased the level of excitement of the animals (Tyack, 1986 ; Janik et al., 1994). Immediately after all the performances were terminated the rate of the sounds emitted by the dolphins per hours declined (Brickman, 2003 ; Sekiguchi and Koshima, 2003 ; Tanchez, 2003).
The vocalizations of animals are relatively easy to record (Dawkins, 1998). Acoustic monitoring has been proven to be a valid non-invasive tool for a continuous track of the influence of captive environment on different species of farm and zoo animals (Crowell and Comuzzie, 1993 ; White et al., 1993 ; Krishnamurthy, 1994 ; Mulligan et al., 1994 ; Schapiro and Bloomsmith, 1994 ; Warris et al., 1994 ; Schwartzkopf-Genswein et al., 1997 ; Grandin, 1998 ; Ruiz-Miranda et al., 1998 ; Weary et al., 1998 ; Zimmerman and Koene, 1998 ; Taylor and Weary, 2000 ; Zimmerman et al., 2000, 2003 ; Schön et al., 2004 ; McCowan and Rommeck, 2006). Yet, the potential use of this method as a measure of wellbeing in captive dolphins is still limited (Castellote and Fossa, 2006 ; Akiyama and Ohta, 2007 ; Therrien et al., 2012).
Among the family delphinidae, false killer whales (Pseudorca crassidens) are a very vocal species (Brown et al., 1966 ; Baird et al., 2008, 2010), and despite their world-wide distribution (Stewart et al., 2002) these dolphins are one of the lesser known large Odontocetes (Stacey et al., 1994 ; Odell and McClune, 1999). The type of sounds produced by false killer whales are included either in the traditional categories such as whistles, clicks, and burst-pulses, or in the class of sounds with intermediate acoustic characteristics (graded sounds=click trains and whistles are at the opposite ends of a continuum)(Murray et al., 1998a, b). It has been suggested that sounds with graded structure may communicate the animal's behavioral states, while acoustic emissions included in well-defined categories would indicate a unique function of the signals (Lammers et al., 2003 : spotted dolphins, Stenella frontalis ; spinner dolphins, Stenella longirostris ; Herzing, 1996 : spotted dolphins, Stenella frontalis ; Rehn et al., 2007, 2011 : killer whales, Orcinus orca ; Weir et al., 2007 : sperm whales, Physeter macrocephalus ; Murray et al., 1998a, b : false killer whales, Pseudorca crassidens). Even though different studies have been performed on the characteristics of false killer whales' sounds (Busnel and Dziedzic, 1968 ; Kamminga and van Velden, 1987 ; Thomas et al., 1988 ; Au et al., 1995 ; Murray et al., 1998a, b ; Rendell et al., 1999 ; Supin et al., 2003, 2004, 2005 ; Madsen et al., 2004 ; Nachtigall and Supin, 2008), there is no information available on the effects of the controlled environment on the acoustic activity of this species of dolphin.
The aim of the current paper is to analyse the hypothesis of the presence of food anticipatory vocal activity related to predictable feeding routines in a captive group of false killer whales.
2 MATERIAL AND METHOD 2.1 Subjects and facilityThe research was conducted at Qingdao Polar Ocean World (Qingdao, China) an entertainment facility that hosts different species of marine mammals and fish. The subjects of the study, five individuals of false killer whale, three adult females (F1, F3, F4) and one adult male (F2), all arrived at the aquarium on 12th January 2012 from Japan. The initial group became five individuals on June 30th 2012 with the birth of a male calf from one of the females (F3: the mother was already pregnant before the arrival at the aquarium). According to information obtained from the place of origin, the adult male was the father of the calf. The animal treatment was carried out in strict accordance with the ASM guidelines (Sike and Gannon, 2011), and China Regulations for the Administration of Affairs Concerning Experimental Animals (1988).
The dolphins were kept in an oval-like shaped pool, 17 m long, 8 m wide, and 5 m deep, filled with salt water at a constant temperature of 15.5℃. Feeding was performed four times a day (9.40 am; 12.20 pm; 2 pm; 4 pm) where each dolphin had a different amount of fish. The species of fish used to feed the false killer whales group were herring (Clupea sp.), Japanese mackerel (Trachurus japonicus), and capelin (Mallotus villosus). According to the information given by the trainers, the diet composition and the amount of food for the dolphins changed depending on the time of the year and animals physiological conditions. During the week of the recordings, three additional feedings were introduced on three different days and at hours different than the regular food delivery schedules, in order to provide the mother (F3) and the calf with a supplementary amount of food.
During each feeding, two adult females and the adult male received their fish stationing in front of the trainers, while mother and calf were swimming around the pool and fed by tossing the fish to them. The time spent by the three adults stationed in front of the trainers was between 2 and 3 seconds for each fish intake (the stationing time was recorded during each feeding). Then the whales swam away and came back to receive other fish. Moreover, not all the individuals had the head out of the water at the same time during feeding. Precisely, when the whales stationed in front of the trainers, on many occasions they had part of the melon in the water (the dolphins lay on one side with half a head in the water, and this may imply that they could still produce sounds). For this reason, in any analysis of the sound repetition rates during feeding sessions, the group of dolphins has been treated as five individuals. After each feeding session, the trainers left the building where the pool of the false killer whales was situated until the next food delivery. During this time, the animals engaged in activities such as socializing and swimming in tight formation (Table 1). These behavioral events were characterized by significant variations of vocal activity (Mann- Whitney U : 11.000; z =-4.78; P<0.001) that ranged from the lowest total sound rates during swimming in tight formation (1.38±SE 0.68) to the highest during socializing (14.33±SE 3.11).
 |
The acoustic recordings were carried out over 7 days, from December 28th 2012 until January 3rd 2013. The daily schedule of the data collection was from 9:00 am until 4:30 pm, which coincided with the work activities at Qingdao Polar Ocean World. The reason why the recordings were performed for such a limited time is that the subject described in this paper was not the principal aim of the project. During the course of the study, a certain pattern in vocal activity of the false killer whale group was noticed around feeding times. Therefore, further analysis of the data was made to evaluate the hypothesis that these acoustic variations could be part of a food anticipatory activity.
A CRT omnidirectional hydrophone (CR3 model; Cetacean Research Technology, Washington, USA; sensitivity: -210 db re 1 V/μPa, frequency range from 0 to 240 kHz+2/-12 dB) was used. It was placed in the middle of the pool at 1.5 m below the water surface. The hydrophone was connected with a 30 m cable to an UMATM 1502 mixer/amplifier (Peavey Electronics Corporation, USA; frequency response 20 Hz to 20 kHz±1.0 dB) and a TASCAM US 144mkII (TEAC America, Inc.; USB2.0 Audio/MIDI interface with S/ PDIF I/O, 96 kHz/24-bit resolution). The data were acquired using SeaWave 2.0 software (G. Pavan/ CIBRA, 2008 -2011) with 48 kHz sample rate. Equipment setting was the same throughout all recordings.
2.3 Data analysisA total of 32 hours and 41 minutes of underwater signals were collected. The recordings were edited and each of them was divided into five intervals: NT (No Trainers); Trans (Transition); 20PrFeed (20 minutes pre-feeding); Feed (Feeding); 20PtFeed (20 minutes post-feeding)(Table 2). Feeding was an imposed event of which the length depended on the time spent by the trainers for food provisioning, with a mean duration (±SE) of 11.09 minutes (±0.72). Even though feeding sessions were performed at established times, delays and anticipation could occur. According to the recording chart, the mean delay (±SE) of feeding schedules was 15.57 minutes (±4.385) while the mean time (±SE) of anticipation was 7.25 minutes (±1.03).
Spectrograms were inspected using Cool Edit Pro 2.0(Syntrillium Phoenix, AZ, USA; Hanning window, Fast Fourier Transform (FFT)1024), and SeaWave 2.0(G. Pavan/CIBRA, 2008 -2011; Hanning window; temporal grid spacing 10.67 with an overlap of 50%; frequency resolution 93.75 Hz with a window and FFT size 1 024, producing a 3-dB filter bandwidth of 135 Hz) to count the total number of whistles and burst pulses. All whistles included in the categories “fair”(whistles with main contour distinguishable and can be ascribed to a specific tonal type), “good”(whistles with a very clear contour and with the beginning and ending points well defined), and “fine”(whistles with a good signal to noise ratio over 20 dB) were counted for each of the five intervals (Díaz López, 2011 ; Wang et al., 2013). Successive contours were considered as one whistles if the gap between them was smaller than 200 ms and shorter than the duration of both whistles, with the former ending and the latter beginning section extended to form a linkage, with the ending-beginning frequency difference less than 3 kHz (Bazúa-Durán and Au, 2002). Whistles that overlapped were excluded from the analysis as well as the fragments of the recording containing dolphins vocalizing in the air. The total number of clicks was counted using MatLab R2010b (MathWorks, Inc., MA, USA). Acoustic activity was measured both as the total number of vocalizations (whistles+click trains+burst pulses) and total number of each sound class recorded during each interval.
A tonal, narrow band signal was considered as a whistle; a click train was defined as a series of distinct and rapid clicks with ICI (defined as the portion of the echolocation signal that returns to the dolphin before the production of another click)(Melcón et al., 2012) from 1 to 200 ms. Burst pulses were defined as many clicks in such a rapid succession that to human ear it was perceived as single buzz (Caldwell et al., 1966 ; Acevedo-Gutiérrez and Stienessen, 2004 ; Díaz López, 2011 ; Wang et al., 2013)(Fig. 1). The recordings included the entire false killer whales group and a single hydrophone was used with no concurrent video footage, which did not allow the identification of the dolphin that produced the sounds.
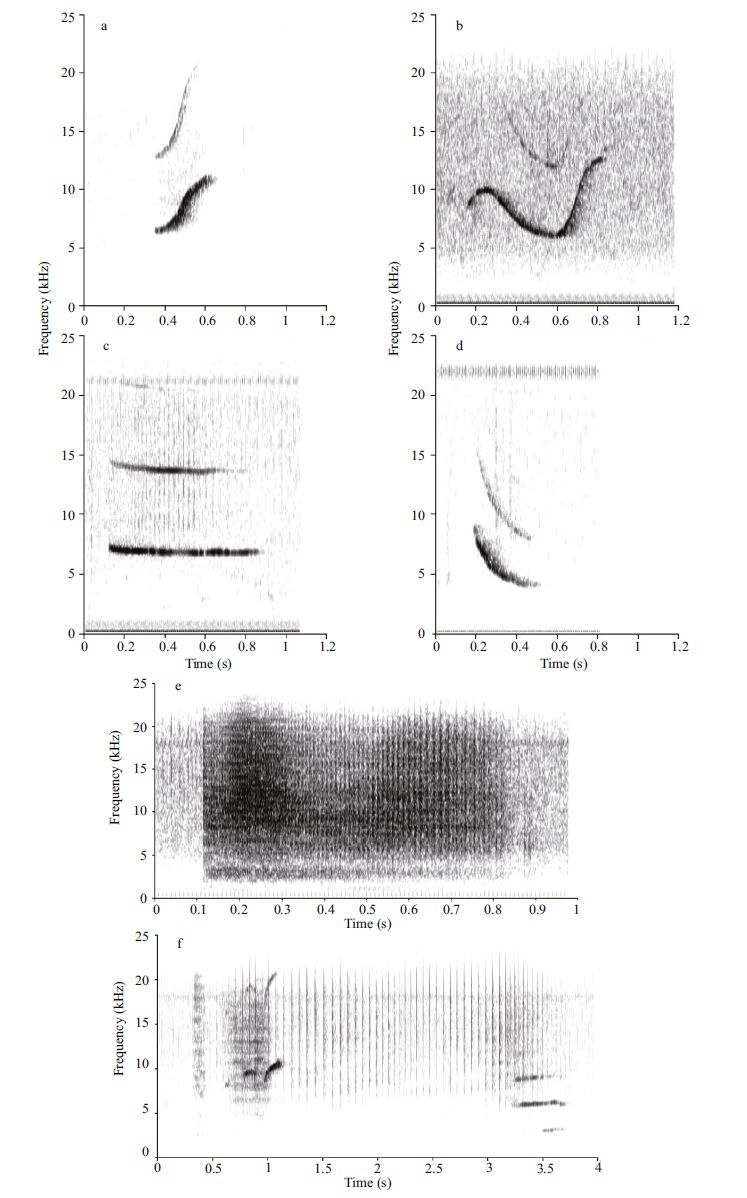
|
| Figure 1 Examples of spectrogram representation of the whistles (a, b, c, d), burst pulses (e), and clicks (f) emitted by False Killer Whales (Pseudorca crassidens) using SIGNAL/RTS™ 4.03 software Window type: Hanning; spectrogram configuration: temporal grid resolution 2.67 ms; overlap samples per frame 75%; frequency grid spacing 93.75 Hz ; window size 2 048; FFT size 2 048. |
The total sounds and sound classes' repetition rates were calculated by adding the total number of sounds recorded during each interval and dividing this value by the duration (minutes) of each interval and the number of individuals of the group. Since the data were not normally distributed and had unequal variances, nonparametric statistical procedures were adopted using SPSS 16.0(Zar, 1996)(SPSS Inc., Chicago, U.S.A.). Kruskal-Wallis one-way ANOVA test (Zar, 1996) was used with the pooled data to determine the presence of significant variations of total sounds and sound classes' rates among the five intervals considered (NT, Trans, 20PrFeed, Feed, 20PtFeed), in order to verify the possible presence of FAA. Furthermore, the same test was also used to assess the presence of variations of the total sound rates during the five intervals for each day of the recordings, the total sound and sound classes' rates for each daily feeding schedule, and the presence of differences in both total sounds and sound classes' rates among the 20PrFeeds, Feeds, and 20PtFeeds intervals following the four daily schedules. Dunn's multiple comparison procedure was used to determine the presence of significant differences for each test used. The sounds recorded during the additional, delayed, and anticipated feedings were excluded from all analysis because of the limited data available.
3 RESULTA total of 103 275 sounds were counted (35 467 whistles, 64 696 click trains, and 3 132 burst pulses), and the mean number of vocalizations emitted per minute per dolphin was 11.82(±SE 1.10).
3.1 Total sound rate during five intervalsThe false killer whales group at Qingdao Polar Ocean World showed a significant variation in the total sound production rates among the five intervals considered (Kruskal-Wallis ANOVA. χ2: 50.066; df: 4; P<0.001). Precisely, from the Transition interval (5-10 minutes before the arrival of the trainers) that showed a mean sound rates (±SE) of 3.40 per minute per dolphin (s/m/d)(±1.18), the acoustic emission increased with the trainers' arrival during 20PreFeeding to a mean sound rates (±SE) of 13.08 s/m/d (±2.15), reaching the maximum mean value during Feeding (25.12 SE±2.57). After food delivery (20PostFeeding) which corresponded with the end of the trainers' activities, the sound rates decreased to a mean value (±SE) of 10.03 s/m/d (±1.37). NT interval represented the moment where the dolphins were alone with no trainers around the pool, and the vocal production was influenced by the animals' activity levels (7.91±SE 1.86)(Fig. 2). Post hoc multiple comparisons showed significant differences between the intervals Trans and 20PtFeed (P=0.03), Trans and 20PrFeed (P =0.005), Trans and Feed (P<0.001), NT and Feed (P<0.001), 20PtFeed and Feed (P =0.002), and only a trend between 20PrFeed and Feed (P =0.062). No significant differences were found between Trans and NT, NT and 20Prfeed, NT and 20PtFeed, and between 20Prfeed and 20PtFeed (P = ns).

|
| Figure 2 Mean of the variation of the total rate of sounds (±SE) per minute produced by each dolphin during the five intervals considered |
The same trend was observed during the five intervals both for each day of the recordings and the four daily schedules. The daily variations of the total sound rates during the week of the study revealed significant differences only for three out of seven days (28 Dec 2012. χ2: 8.339; df: 4; P =0.08; 29 Dec 2012. χ2: 16.262; df: 4; P =0.003; 30 Dec 2012. χ2: 13.383; df: 4; P =0.01; 31 Dec 2012. χ2: 11.718; df: 4; P =0.02; 1 Jan 2013. χ2: 9.066; df: 4; P =0.059; 2 Jan 2013. χ2: 5.817; df: 4; P =0.21; 3 Jan 2013. χ2: 4.857; df: 4; P =0.302). Post hoc multiple comparison did not show significant differences in any day of the recordings. Despite this result, the sounds rate during the five intervals for each day of the recordings showed a pattern similar to that observed in the analysis of the pooled data, with an increase of the acoustic emissions as feeding approached (Fig. 3).
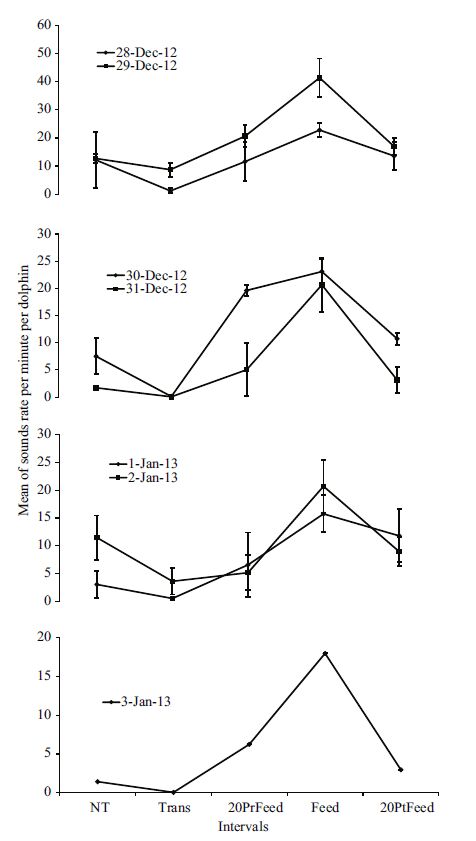
|
| Figure 3 Variations of the mean of the total rate of sounds (±SE) per minute produced by each dolphin during the five intervals for each day of the study |
On the other hand, false killer whales showed variations of the total sound rates during the five intervals for all four daily feeding schedules (Kruskal- Wallis ANOVA. 9:40 am: χ2: 12.406; df: 4; P =0.015; 12:20 pm: χ2: 15.573; df: 4; P =0.004; 2:00 pm: χ2: 13.746; df: 4; P =0.008; 4:00 pm: χ2: 14.057; df: 4; P =0.007). Post hoc multiple comparisons showed significant differences only between Transition and feeding intervals for the 2:00 pm (P =0.017), 4:00 pm schedules (P =0.002), and only a tendency for 9:40 am schedule (P =0.051); whereas differences were found between NT and feeding during 12:20 pm (P =0.004), 2:00 pm schedule (P =0.012), and a tendency for 9:40 am schedule (P =0.064). No significant differences were found for any of the other intervals during the four daily schedules (Kruskal-Wallis ANOVA. P =ns). Nevertheless, during each daily schedule the whales showed a pattern similar to that observed in the analysis of the pooled data and of each day of the recordings (Fig. 4).
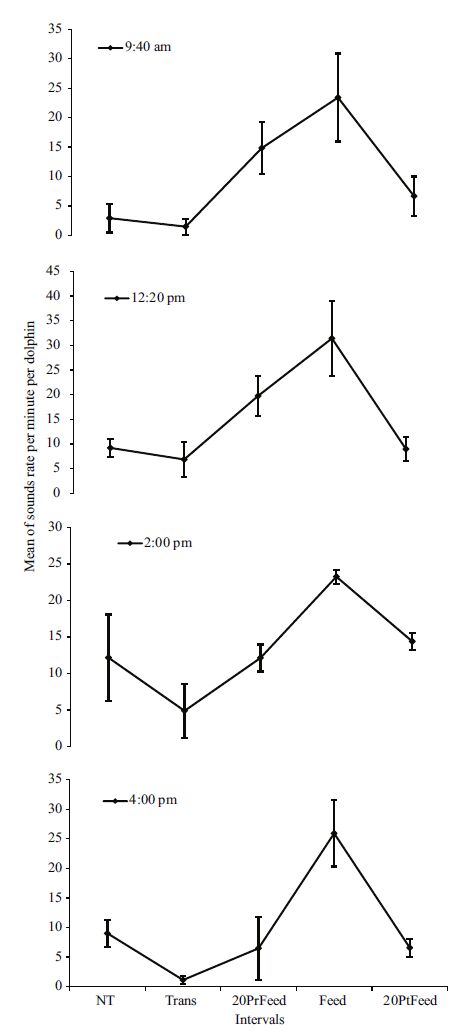
|
| Figure 4 Variation of the mean of the total rate of sounds (±SE) during the five intervals for each daily feeding schedule |
Furthermore, the comparison among the 20PrFeeds, Feeds, and 20PtFeeds following the daily schedules did not depict significant differences (Kruskal-Wallis ANOVA. P = ns). Regardless of the outcomes, false killer whales produced slightly a higher rate of sounds during 20PrFeeds of the 12:20 pm schedule (19.73±SE 4.11), followed by the 9:40 am (14.83±SE 4.40), 2:00 pm (12.12±SE 1.84), and 4:00 pm schedules (6.49±SE 5.31); whereas they produced a higher rate of sounds during the feeding interval of the 12:20 pm schedule (31.41±SE 7.61), followed by the 4:00 pm (25.93±SE 5.61), 2:00 pm (23.25±SE 0.95), and 9:40 am schedules (23.41±SE 7.47). On the other hand, the 20PtFeed interval showed highest rate of sounds during the 2:00 pm schedule (14.37±SE 1.14), followed by the 12:20 pm (8.96±SE 2.39), 9:40 am (6.66±SE 3.34), and 4:00 pm schedules (6.59±SE 1.50).
3.2 Total rates of the different sound classes during five intervalsThe analysis of the rates of the different sound classes during the five intervals showed the same pattern reported for the total sound rates. In fact, the total rates of whistles, click trains, and burst pulses depicted also variations among the five intervals considered (Kruskal-Wallis ANOVA. whistles χ2 51.066; df: 4; P<0.001; click trains χ2 44.611; df: 4; P<0.001; burst pulses χ2 45.609; df: 4; P<0.001) with an increase in their rates as feeding approached (Fig. 5). Post hoc multiple comparisons showed significant differences between the intervals Trans and Feed (whistles: P<0.001; click trains: P<0.001; burst pulses: P<0.001), Trans and PtFeed (whistles: P =0.05; click trains: P =0.022; burst pulses: P =0.012), NT and Feed (whistles: P<0.001; click trains: P<0.001; burst pulses: P<0.001), 20PtFeed and Feed (only for whistles: P =0.001, and click trains: P =0.012), 20PrFeed and Feed (only for whistles: P =0.002, and burst pulses: P<0.001), and Trans and 20PrFeed only for click trains (P =0.001). No significant differences were found for each sound class during the other intervals (P = ns).
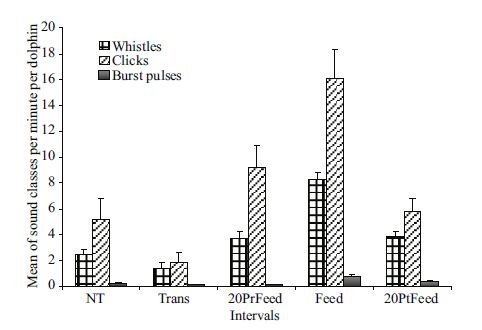
|
| Figure 5 Variation of the mean of the total rates of the sound classes (±SE) per minute per dolphins during the five intervals |
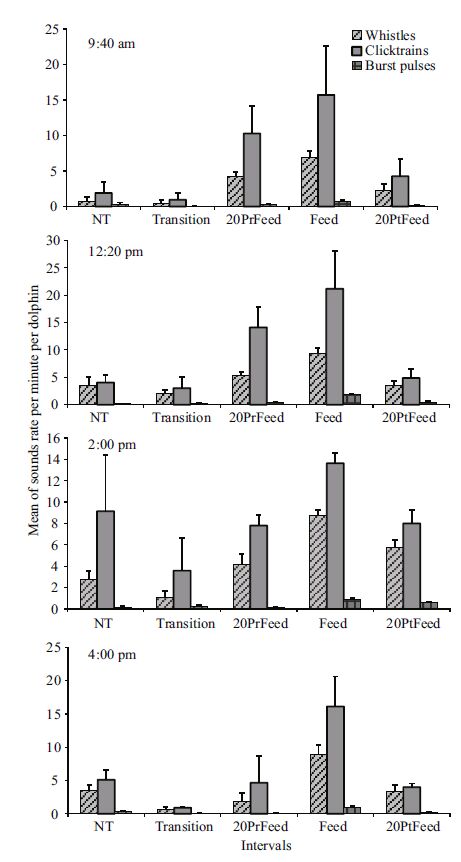
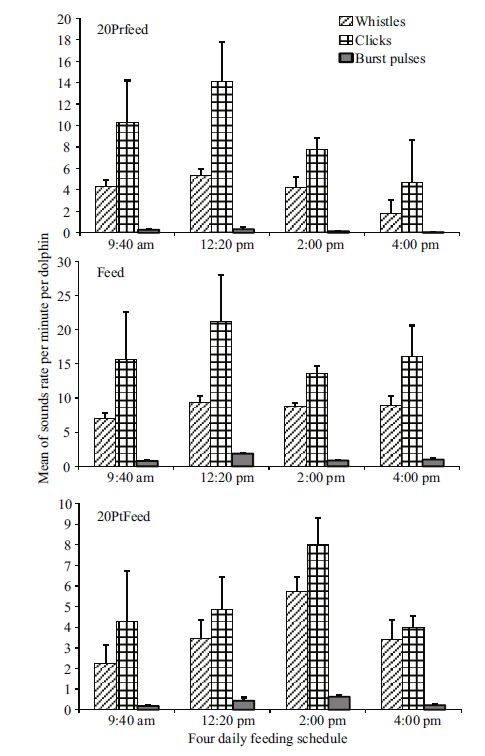
The rates of the sound classes during the five intervals following each daily feeding schedule showed significant differences only for whistles (χ2 13.773; df: 4; P =0.008) and click trains (χ2 11.415; df: 4; P =0.022) during 9:40 am, whereas significant differences were found for all the sound classes during the other daily schedules (12:20 pm: whistles χ2 16.893; df: 4; P =0.002; click trains χ2 14.584; df: 4; P =0.006; burst pulses χ2 15.237; df: 4; P =0.004; 2:00 pm: whistles χ2 18.022; df: 4; P =0.001; click trains χ2 10.145; df: 4; P =0.038; burst pulses χ2 17.067; df: 4; P =0.002; 4:00 pm: whistles χ2 13.817; df: 4; P =0.008; click trains χ2 13.390; df: 4; P =0.010; burst pulses χ2 17.179; df: 4; P =0.002). Post hoc multiple comparisons showed significant differences between Transition and Feed only during 9:40 am (whistles P =0.045), 2:00 pm (whistles P =0.003), and 4:00 pm (whistles P =0.005; click trains P =0.003; burst pulses P =0.002); between NT and Feed during 9:40 am (whistles P =0.013), 12:20 pm (whistles P =0.001; click trains P =0.014; burst pulses P =0.006), and 2:00 pm (whistles P =0.007; burst pulses P =0.018); whereas differences were found between 20PrFeed and Feed only during 4:00 pm schedule for bust pulses (P =0.014). Despite the results, the rates of the sound classes showed a similar pattern to that observed in the previous analysis of the total sound rates during the four daily schedules, with an increase in the rate of whistles, click trains and burst pulses when feeding interval approached (Fig. 6). In addition, the comparison of the four 20Prfeeds, Feeds, and 20PtFeeds following the four daily schedules did not show significant differences (Kruskal-Wallis ANOVA. P = ns). Nevertheless, false killer whales produced slightly higher rates of the sound classes during the 20PrFeed and Feed intervals of the 12:20 pm schedule (20PrFeed: whistles 5.38±SE 0.56; click trains 14.12±SE 3.72; burst pulses 0.33±SE 0.17. Feed: whistles 9.39±SE 0.90; click trains 21.16±SE 6.86; burst pulses 1.86±SE 0.15) compared to the same intervals of the other schedules; whereas among the 20PtFeed intervals the highest rate of the sound classes was recorded during the 2:00 pm schedule (whistles 5.73±SE 0.71; click trains 8.01±SE 1.30; burst pulses 0.63±SE 0.06)(Fig. 7).
|
| Figure 6 Variation of the mean of the total rates of the sound rates (±SE) per minute per dolphin during the five intervals for each daily feeding schedule |
|
| Figure 7 Comparison of the variation of the mean of the rates of the different sound classes (±SE) among the pre-feeding (20PrFeed), feeding, and post-feeding (20PtFeed) intervals for each daily schedule |
Significant changes in the total rates of the sounds and sound classes of false killer whales (Pseudorca crassidens) were found during the five intervals considered. From the Transition interval that showed the lowest vocal activity, dolphins increased their acoustic emissions rate upon the arrival of the trainers. The high rate was maintained or increased throughout the feeding sessions, and it decreased immediately after the animals were fed. This trend was observed both during the four daily feeding schedules and each day of the study. The changes in the vocalization rates of false killer whales around feeding times may support the hypothesis regarding the presence of food anticipatory vocal activity. Moreover, these preliminary results may add evidence to the existing body of literature that acoustic recording could be considered a useful method to monitor the influence of daily routines on captive cetaceans.
Several studies have shown that during the time prior to feeding sessions, animals vary their activity levels from arousal to inaction, and these behaviors are referred to as food anticipatory activity (FAA)(see review, Mistlberger, 1994). Amongst the variety of behavioral changes that are considered indicative of FAA (Howell et al., 1993 ; Carlstead, 1998 ; Jensen et al., 2013), the variation of vocalizations is also a factor influenced by the schedule of feeding sessions (Krishnamurthy, 1994 ; Waitt and Buchanan-Smith, 2001 ; Ulyan et al., 2006 ; Gilbert-Norton et al., 2009 ; Polizzi di Sorrentino et al., 2010 ; Gottlieb et al., 2013).
The false killer whales' group at Qingdao Polar Ocean World showed changes in acoustic activity approximately 30 minutes before the feeding sessions. In fact, a decrease of the vocal rate was reported during the passage from NT to Transition interval, and this variation was observed both during the four daily feeding schedules and each day of the recordings. These results are in accordance with the findings reported in the studies on FAA in bottlenose dolphins (Tursiops truncatus)(Jensen et al., 2013) and chimpanzees (Pan troglodytes)(Bloomsmith and Lambeth, 1995). These studies also recorded a drop in the activity levels of the animals approximately 20- 30 minutes before the feeding sessions, with the individuals becoming more vigilant towards the movements of the keepers as the animals seemed to “wait” for the event to happen (Bloomsmith and Lambeth, 1995 ; Jensen et al., 2013). Thus, the first drop in the sound rates of Qingdao false killer whales recorded during the Transition interval could be considered a sign that the whales started to be aware of the approaching feeding session, and they possibly became more attentive toward any movement of the trainers (Jensen et al., 2013).
Moreover, at Qingdao Polar Ocean World no trainers were present around the pool of false killer whales during the period between feedings (NT interval), and the vocal activity levels of the animals varied from the highest sound rates during socializing to the lowest vocal emissions during swimming in tight formation. Jensen et al.(2013) also reported that bottlenose dolphins (Tursiops truncatus) showed a steady activity level during the interval between training/feeding sessions, with behaviors ranging from rest (logging) to increased arousal, the latter mainly related to social interaction among individuals.
Considering the theory of Bassett and Buchanan- Smith (2007), we could speculate that the first change of the vocal activity of false killer whales might be either a result of signaled or temporal predictability (see review Mistlberger, 1994). The food deliveries at Qingdao Polar Ocean World were performed at fixed time schedules, which may allow the whales to estimate the moment when the feedings would have occurred. Therefore, it could be suggested that temporal predictability might have played a role in the variation of the vocal activity of false killer whales (Jensen et al., 2013). In fact animals, including dolphins (Mercado et al., 1999 ; Marino, 2004 ; Jensen et al., 2013), possess the ability to use temporal information (Richelle, 1980 ; Higa and Staddon, 1997) to estimate intervals between food availability, which is critical for foraging (Bassett and Buchanan-Smith, 2007). On the other hand, the false killer whales' group of Qingdao aquarium could have relied on external cues (Seligman, 1968 ; Seligman and Meyer, 1970) which informed the animals when to anticipate the expected feeding routines (Waitt and Buchanan- Smith, 2001 ; Bassett and Buchanan-Smith, 2007). This phenomenon is an example of signaled predictability (Waitt and Buchanan-Smith, 2001 ; Bassett and Buchanan-Smith, 2007 ; Jensen et al., 2013). In the current study, approximatively 30 minutes before the feeding session a filtration pump was activated (the activity of the engine was detected by the recording system with a continuous line at 15 kHz of frequency). In addition to that, it was also reported an increase of the movements of trainers in and out of the building where the pool of the dolphins was held. These two events could have represented cues that informed the animals about the timing of the food delivery. This period coincided with the Transition interval when the vocal activity of the false killer whales showed a reduction from the previous one (NT). Unfortunately, the time prior to feedings is characterized by a variety of stimuli, and it was not clear which cues (temporal or signaled) false killer whales used to anticipate the forthcoming sessions. Further experiments should be carried out to test whether false killer whales relied either on a temporal or signaled predictability or both to anticipate food deliveries (Jensen et al., 2013).
In the current study, the first rise of the acoustic rate of false killer whales occurred upon the arrival of the trainers for food preparation during the prefeeding interval (20PrFeed). This result concurs with the findings reported from previous studies on primates (Krishnamurthy, 1994 ; Waitt and Buchanan- Smith, 2001 ; Polizzi di Sorrentino et al., 2010) and dolphins (Tanchez, 2003 ; Sekiguchi and Kohshima, 2003 ; Akiyama and Ohta, 2007), in which vocal activity increased during the time immediately prior to feedings when animals heard the arrival of the keepers for food preparation. For example, it was reported that when chimpanzees saw the caregivers carrying the meal, the animals responded with loud food vocalizations and engaged in various physical contacts such as kissing and embracing in a sort of celebration for the imminent feeding session (de Waal, 1993 ; Bloomsmith and Lambeth, 1995). Similarly, the trainers at Qingdao Polar Ocean World reported that when they arrived at the false killer whales' pool (20PrFeed), the whales performed high speed swimming around the tank with sudden splashes (trainer personal communication). It might be possible that the arrival of the trainers could have raised the excitement of the dolphins for the expected feeding and social interaction, since it has been reported that these animals perceive the activity with their keepers as positive events (Brando, 2010 ; Jensen et al., 2013). This hypothesis could be supported by the overall increase of the rates of all sound classes during the pre-feeding intervals. Higher rates of whistles and burst pulses have been reported in dolphins during different behavioral contexts characterized by high arousal levels (Busnel and Dziedzic, 1966 ; Smolker et al., 1993 ; Norris et al., 1994 ; Herzing, 1996 ; Acevedo-Gutiérrez and Stienessen, 2004 ; dos Santos et al., 2005). Moreover, clicks are normally used by dolphins during inquisitive behaviors (Supin et al., 2004, 2005 ; Nachtigall and Supin, 2008). The rise in the rate of these sounds encountered in our recordings during the trainers' arrival could be explained by an escalation of the scanning activity of false killer whales toward the hydrophone that swung in the water because of the animals' high speed swimming. Another possible explanation for the high rate of clicks could be related to the graded structure of some of the false killer whales' vocalizations (Murray et al., 1998a, b). These sounds lie along a continuum with pulse trains at one end and whistles at the other end (Murray et al., 1998a, b). It has been suggested that signals with a graded structure could convey information on the status, motivation, behavioral context, and individual features of the sender (Murray et al., 1998a, b ; Scheer, 2013). According to this supposition, it could be possible that the rise in the rate of clicks reported in the false killer whales of Qingdao aquarium during the pre-feeding intervals might be explained by an increase in whistles with graded structure which could indicate an escalation of the whales' excitement (Nemiroffand Whitehead, 2009 ; Sayigh et al., 2013 ; Scheer, 2013). However, a detailed analysis of whistle types to verify the presence of whistles with a graded structure was outside the scope of this paper. In addition, Akiyama and Ohta (2007) suggested that the increase in the rate of sound in dolphins recorded during the pre-feeding intervals might represent a demand for food. This hypothesis was explained by the authors with the presence in their recordings of low frequency sounds that could reflect the emotion of sadness related to the dolphins' hunger (Akiyama and Ohta, 2007). Therefore, further analysis of the current data will be made to gather additional information such as the presence of specific calls related to the intervals around feeding sessions which may give a better understanding of the emotional states of the dolphins (Akiyama and Ohta, 2007).
During our study, a further rise of false killer whales' acoustic rates was recorded during feeding sessions, which showed the highest rate of total sounds and sound classes among all intervals considered. These findings are in accordance with previous observations carried out on captive beluga whales (Delphinaptera leuca, Fish and Mowbray, 1962 ; Tanchez, 2003), bottlenose dolphins (Tursiops truncatus, Sekiguchi and Kohshima, 2003), and Pacific white-sided dolphins (Lagenorhynchus obliquidens, Brickman, 2003), in which the highest vocal rate was recorded during feeding sessions.
The results obtained during feeding at Qingdao Polar Ocean World could be explained as an increase of the excitement of the animals because of the activities with the trainers and the modality of food delivery. During feeding, part of the fish was tossed in the water to feed the mother and calf, a method which might have triggered a heightened production of clicks by the whales (Dreher, 1966 ; dos Santos and Almada, 2004) that used the fish as a target. Moreover, the fish tossed into the tank might have developed competition among the animals with an increase in production of burst pulses (Overstrom, 1983 ; Connor and Smolker, 1996 ; Herzing, 1996 ; Lammers et al., 2003 ; Nowacek, 2005). Moreover, Ridgway et al.(2014) suggested that burst pulses produced during feeding sessions might be considered as food calls with emotional content. Nevertheless, more substantial experiments involving the different aspects of food delivery should be carried out (food quantity and quality, hunger levels, and interaction with the keepers)(Dittus, 1984 ; Caine at al., 1995; Di Bitetti, 2005 ; Slocombe and Zuberbühler, 2006 ; Clay and Zuberbühler, 2009) to assess the presence of a referential content in the calls of dolphins (Ralston and Herman, 1989 ; Herzing, 2000 ; Akiyama and Ohta, 2007 ; Therrien et al., 2012). For example, a behavioral sequence analysis would help to define the function of the vocalizations in relation to the reaction of the conspecifics to the phonating individuals (Slooten, 1994). In addition, a hydrophone array recording system should be used to facilitate the identification of the individuals who emit the calls and thus to discriminate between sender and receiver.
The current results differ from previous studies on food anticipatory activity in fish (Sánchez et al., 2009), minks (Hansen and Jeppesen, 2006), primates (Waitt and Buchanan-Smith, 2001 ; Ulyan et al., 2006), captive canids (Gilbert-Norton et al., 2009), and captive felines (Carlstead, 1998), where animals increased their activity level prior to feeding sessions and then decreased it as soon as they were fed, when the routine was performed on time. Conversely, a delay in the feeding produced an increased behavioral and vocal activity prior to the session, and the high rate was maintained or even intensified throughout food delivery (Waitt and Buchanan-Smith, 2001). Thus, it could be speculated that the high vocal rates encountered in Qingdao false killer whales during feedings might have been caused by a variation of the routine schedules. If the expectation of these events became inaccurate because of their delay according to the temporal or signaled predictability, anticipation may have caused frustration in the whales with consequent variations of their vocal activity (Amsel, 1958 ; Johanneson and Ladewig, 2000 ; Waitt and Buchanan-Smith, 2001 ; Galhardo et al., 2011). This assumption could be taken into account to explain the higher vocal rate encountered in our recordings during the 12:20 pm pre- and feeding intervals compared to all the other schedules. In fact, this hour coincided with the peak of activities with an increase of the movement of people around the building housing the false killer whales which could have been taken by the animals as a cue that food delivery was imminent. Frustration may have been built up as the whales heard staffactivities but feeding did not occur, thus causing an increase of vocal rate. This could also explain the highest rates of whistles and burst pulses recorded during the 12:20 pm feeding, which might have been caused by the escalation of the animals' arousal due to the delayed routine. Unfortunately, even though delays and anticipations of the feedings of false killer whales were reported, the time variations of the daily schedules were too limited to be considered for further analysis. In addition, Bloomsmith and Lambeth (1995) and Waitt and Buchanan-Smith (2001) asserted that feeding schedules should be separated over a long interval of the order of 150 minutes in order to consider them delayed or anticipated, because feedings within 30- 45 minutes from the original schedule still seem to make the session semi-predictable. Therefore, further experiments should be set up to assess how on-time, delayed, and anticipated food deliveries compared to the moment of the onset of the specific signaled cues could influence the vocal and behavioral activity of captive cetaceans. Moreover, it should also be taken into consideration that different captive groups, as well as individuals, can show variations in acoustic and behavioral activity during the daily routines with a consequent variation in the anticipatory behaviors (Galhardo et al., 1996).
Immediately after feedings, the false killer whales of Qingdao aquarium showed a drop in their sounds' rate which corresponded to the moment when the trainers ended their activities and left the pool of the whales. Our result is consistent with previous studies on FAA in primates, where animals decreased their vocalizations and behavioral activities immediately after the keepers left the feeding areas (Krishnamurthy, 1994 ; Bloomsmith and Lambeth, 1995 ; Waitt and Buchanan- Smith, 2001 ; Bassett and Buchanan-Smith, 2007 ; Polizzi di Sorrentino et al., 2010 ; Jensen et al., 2013). In addition, studies on captive cetaceans reported similar observations where dolphins dropped the acoustic activity when the staffmembers left after feeding/training sessions (Brickman, 2003 ; Tanchez, 2003). Moreover, in the current study the false killer whales' group showed a higher vocal rate during the post-feeding interval following the 2:00 pm food delivery compared to the same intervals following the other daily feeding schedules. This time coincided with the moment when one trainer dived into the pool of false killer whales to check for fish left by the animals on the bottom of the tank. It is possible that the presence of the trainer might have increased the excitement of the whales, or they could have used their ultrasound to check out the keeper causing an increase of the vocal rate (Akiyama and Ohta, 2007). These two hypotheses could be supported by the overall increase of whistle, click train and burst pulse rates during the 20PtFeed following the 2:00 pm schedule compared to the same intervals following the other daily times.
5 CONCLUSIONThis is the first report on the monitoring of the vocal activity of a captive group of false killer whales during feeding routines. Despite the limitations of a study with restricted amounts of data, the findings described in this paper may support the hypothesis that false killer whales anticipated feeding sessions with a gradual change in their vocal activity. Although sound rates may not give detailed information regarding referential aspects of animals' communication (Clay et al., 2012), it might still shed light about the general arousal levels of the individuals during different conditions (Weary and Fraser, 1995 ; Grandin, 1998 ; Frohoffet al., 2004 ; Monticelli et al., 2004). Among mammals, dolphins possess a very complex communication system (Caldwell and Caldwell, 1968 ; Puente and Dewbury, 1976 ; Sjare and Smith, 1986 ; Dawson and Thorpe, 1990 ; Weilgart and Whitehead, 1990 ; Norris et al., 1994 ; Barrett- Lennard et al., 1996 ; Herzing, 1996) and like primates (Owing and Virginia, 1978 ; Mitami and Nishida, 1993 ; Rendall et al., 2000), they can convey information regarding behavioral or environmental changes either by producing particular types of calls (Esch et al., 2009 ; Hawkins and Gardside, 2010) or with the variations of their rates. Therefore, acoustic monitoring should represent a tool to gather information on activity patterns, health conditions of captive cetaceans in order to improve the quality of their life (Castellote and Fossa, 2006 ; Akiyama and Ohta, 2007 ; Therrien et al., 2012).
6 ACKNOWLEDGEMENTWe thank Mr. YAO Baocheng, Deputy Director of Qingdao Polar Ocean World, for hosting the research at this facility; Mr LI Xin, senior veterinarian, Mrs Cici, staffveterinarian, and all the trainers for their support and patience during the recordings. Special thanks to ZHANG Lin and Prof. SUN Xiulian for SPSS software guide and statistic help, and to Amy Spencer from Ohio Dominican University for the English review. Thanks for the encouragement and guidance to all the members of the Research Group on Conservation Biology of Aquatic Animals, Institute of Hydrobiology, Chinese Academy of Sciences. The study was supported by grants from the Institute of Hydrobiology, Chinese Academy of Sciences, Wuhan, China.
| Acevedo-Gutièrrez A, Stienessen S C, 2004. Bottlenose dolphins (Tursiops truncatus) increase number of whistles when feeding. Aquatic Mammals, 30 (3) : 357 –362. Doi: 10.1578/AM.30.3.2004.357 |
| Akiyama J, Ohta M, 2007. Increased number of whistles of bottlenose dolphins, Tursiops truncatus, arising from interaction with people. J. Vet. Med. Sci., 69 (2) : 165 –170. Doi: 10.1292/jvms.69.165 |
| Albeck D S, McKittrick C R, Blanchard D C, Blanchard R J, Nikulina J, McEwen B S, Sakai R R, 1997. Chronic social stress alters levels of corticotropin-releasing factor and arginine vasopressin mRNA in rat brain. The Journal of Neuroscience, 17 (12) : 4 895 –4 903. |
| Amsel A, 1958. The role of frustrative nonreward in noncontinuous reward situations. Psychol. Bull., 55 (2) : 102 –199. Doi: 10.1037/h0043125 |
| Amstrong S, 1980. A chronometric approach to the study of feeding behavior. Neurosci. Biobehav. Rev., 4 (1) : 27 –53. Doi: 10.1016/0149-7634(80)90024-X |
| Andrews M W, Rosenblum L A, 1991. Attachment in monkey infants raised in variable-and low-demand environments. Child Dev., 62 (4) : 686 –693. Doi: 10.2307/1131170 |
| Andrews M W, Rosenblum L A, 1993. Assessment of attachment in differentially reared infant monkeys(Macaca radiata):response to separation and a novel environment. J. Comp. Psychol., 107 (1) : 84 –90. Doi: 10.1037/0735-7036.107.1.84 |
| Au W W L, Pawloski J L, Nachtigall P E, Blonz M, Gisner R C, 1995. Echolocation signals and transmission beam pattern of a false killer whale (Pseudorca crassidens). J.Acoust. Soc. Am., 98 (1) : 51 –59. Doi: 10.1121/1.413643 |
| Azzaydi M, Madrid J A, Zamora S, Sánchez-Vázquez F J, Martínez F J, 1998. Effect of three feeding strategies(automatic, ad libitum demand-feeding and time-restricted demand-feeding) on feeding rhythms and growth in European sea bass (Dicentrarchus labrax L. ). Aquaculture, 163 (3-4) : 285 –296. Doi: 10.1016/S0044-8486(98)00238-5 |
| Baird R W, Gorgone A M, McSweeney D J, Webster D L, Salden D R, Deakos M H, Ligon A D, Schorr G S, Barlow J, Mahaffy S D, 2008. False killer whales (Pseudorca crassidens) around the main Hawaiian Islands:long-term site fidelity, inter-island movements, and association patterns. Marine Mammal Science, 24 (3) : 591 –612. Doi: 10.1111/mms.2008.24.issue-3 |
| Baird R W, Schorr G S, Webster D L, McSweeney D J, Hanson M B, Andrews R D, 2010. Movements and habitat use of satellite-tagged false killer whales around the main Hawaiian Islands. Endangered Species Research, 10 : 107 –121. Doi: 10.3354/esr00258 |
| Baldwin R F. 1992. Behavior of Carnivores in Outdoor Exhibits at the National Zoological Park. George Mason University, Fairfax, Virginia. 154p. |
| Barrett-Lennard L G, Ford J K B, Heise K A, 1996. The mixed blessing of echolocation:differences in sonar use by fisheating and mammal-eating killer whales. Anim. Behav., 51 (3) : 553 –565. Doi: 10.1006/anbe.1996.0059 |
| Bassett L, Buchanan-Smith H M, 2007. Effects of predictability on the welfare of captive animals. Appl. Anim. Behav.Sci., 102 (3-4) : 223 –245. Doi: 10.1016/j.applanim.2006.05.029 |
| Bayart F, Hayashi K T, Faull K F, Barchas J D, Levine S, 1990. Influence of maternal proximity on behavioral and physiological responses to separation in infant rhesus monkeys (Macaca mulatta). Behavioral Neuroscience, 104 (1) : 98 –107. Doi: 10.1037/0735-7044.104.1.98 |
| Bazúa-Durán C, Au W W L, 2002. Whistles of Hawaiian spinner dolphins. J. Acoustic. Soc. Am., 112 (5) : 3 064 –3 072. |
| Bloomsmith M A, Lambeth S P, 1995. Effects of predictable versus unpredictable feeding schedules on chimpanzee behavior. Appl. Anim. Behav. Sci., 44 (1) : 65 –74. Doi: 10.1016/0168-1591(95)00570-I |
| Boinski S, Gross T S, Davis J K, 1999. Terrestrial predator alarm vocalizations are a valid monitor of stress in captive brown capuchins (Cebus apella). Zoo Biology, 18 (4) : 295 –312. Doi: 10.1002/(SICI)1098-2361(1999)18:4<>1.0.CO;2-# |
| Brando S, 2010. Advances in husbandry training in marine mammal care programs. Int. J. Comp. Psychol., 23 (4) : 777 –791. |
| Brickman J J. 2003. Factors Influencing Whistle Usage of the Pacific White-sided Dolphin, Lagenorhynchus obliquidens, at the John G. Shedd Aquarium. Western Illinois University, Quad Cities, Moline. 135p. |
| Brown D H, Caldwell D K, Caldwell M C, 1966. Observations on the behavior of wild and captive false killer whales, with notes on associated behavior of other genera of captive delphinids. Contributions in Science, 95 : 1 –32. |
| Burgdorf J, Knutson B, Panksepp J, 2000. Anticipation of rewarding electrical brain stimulation evokes ultrasonic vocalization in rats. Behavioral Neuroscience, 114 (2) : 320 –327. Doi: 10.1037/0735-7044.114.2.320 |
| Burgdorf J, Panksepp J, 2001. Tickling induces reward in adolescent rats. Physiology and Behavior, 72 (1-2) : 167 –173. Doi: 10.1016/S0031-9384(00)00411-X |
| Burman O H P, Ilyat A, Jones G, Mendl M, 2007. Ultrasonic vocalizations as indicators of welfare for laboratory rats(Rattus norvegicus). Appl. Anim. Behav. Sci., 104 (1-2) : 116 –129. Doi: 10.1016/j.applanim.2006.04.028 |
| Busnel R G, Dziedzic A. 1966. Acoustic signals of the Pilot whale, Globicephala melaena, and of the porpoise Delphinus delphis and Phocoena phocoena. In:Norris K S ed. Whales, Dolphins and Porpoises. Univ. California Press, Berkley, USA. p.607-648. |
| Busnel R G, Dziedzic A, 1968. Caracteristiques physiques des signaux acoustiques de Pseudorca crassidens owen(cetacea odontocete). Mammalia, 32 (1) : 1 –5. Doi: 10.1515/mamm.1968.32.1.1 |
| Caine N G, Addington R L, Windfelder T L, 1995. Factors affecting the rates of food calls given by red-bellied tamarins. Anim. Behav., 50 (1) : 53 –60. Doi: 10.1006/anbe.1995.0220 |
| Caldwell D K, Prescott J H, Caldwell M C, 1966. Production of pulsed sounds by the pigmy sperm whale, Kogia breviceps. Bulletin Southern California Academy of Science, 65 : 245 –248. |
| Caldwell M C, Caldwell D K, 1968. Vocalization of naïve captive dolphins in small groups. Science, 159 (3819) : 1 121 –1 123. Doi: 10.1126/science.159.3819.1121 |
| Carlstead K, 1986. Predictability of feeding:its effect on agonistic behaviour and growth in grower pigs. Appl.Anim. Behav. Sci., 16 (1) : 25 –38. Doi: 10.1016/0168-1591(86)90037-7 |
| Carlstead K. 1998. Determining the causes of stereotypic behaviors in zoo carnivores:towards developing appropriate enrichment strategies. In:Shepherdson D J, Mellen J D, Hutchins M eds. Second Nature:Environmental Enrichment for Captive Animals.Smithsonian Institution Press, Washington DC, USA.p.172-183. |
| Castellote M, Fossa F, 2006. Measuring acoustic activity as a method to evaluate welfare in captive beluga whales(Delphinapterus leucas). Aquatic Mammals, 32 (3) : 325 –333. Doi: 10.1578/AM.32.3.2006.325 |
| Champoux M, Suomi S J, 1994. Behavioral and adrenocortical responses of rhesus macaque mothers to infant separation in an unfamiliar environment. Primates, 35 (2) : 191 –202. Doi: 10.1007/BF02382054 |
| Cheney D L, Seyfarth R M, 1982. How vervet monkeys perceive their grunts:field playback experiments. Animal Behaviour, 30 (3) : 739 –751. Doi: 10.1016/S0003-3472(82)80146-2 |
| Clay Z, Smith C L, Blumstein D T, 2012. Food-associated vocalizations in mammals and birds:what do these calls really mean? Anim. Behav., 83 (2) : 323 –330. Doi: 10.106/j.anbehav.2011.12.008 |
| Clay Z, Zuberbühler K, 2009. Food-associated calling sequences in bonobos. Animal Behaviour, 77 (6) : 1 387 –1 396. Doi: 10.1016/j.anbehav.2009.02.016 |
| Connor R C, Smolker R A, 1996. ‘Pop' goes the dolphin:a vocalization male bottlenose dolphins produce during consortships. Behaviour, 133 (9) : 643 –662. Doi: 10.1163/156853996X00404 |
| Crowell D, Comuzzie D K, 1993. Baboon vocalizations as measures of psychological well-being. Laboratory Primate Newsletter, 32 : 5 –6. |
| Dawkins M S, 1998. Evolution and animal welfare. Q. Rev.Biol., 73 (3) : 305 –328. Doi: 10.1086/420307 |
| Dawson S M, Thorpe C W, 1990. A quantitative analysis of the sounds of Hector's dolphin. Ethology, 86 (2) : 131 –145. |
| de Waal F B M. 1993. Reconciliation among primates:a review of empirical evidence and unresolved issue. In:Mason W A, Mendoza S P eds. Primate Social Conflict, State University of New York Press, Albany. p.111-143. |
| de Waal F B M, Hoekstra J A, 1980. Contexts and predictability of aggression in chimpanzees. Anim. Behav., 28 (3) : 929 –937. Doi: 10.1016/S0003-3472(80)80155-2 |
| de Waal F B M, Tyack P L. 2003. Animal Social Complexity:Intelligence, Culture, and Individualized Societies.Harvard University Press, Cambridge, MA, US. p.342-362. |
| Désautés C, Bidanel J P, Mormède P, 1997. Genetic study of behavioral and pituitary-adrenocortical reactivity in response to an environmental challenge in pigs. Physiology & Behavior, 62 (2) : 337 –345. |
| Di Bitetti M S, 2005. Food-associated calls and audience effects in tufted capuchin monkeys, Cebus apella nigritus. Anim. Behav., 69 (4) : 911 –919. Doi: 10.1016/j.anbehav.2004.05.021 |
| Díaz López B, 2011. Whistle characteristics in free-ranging bottlenose dolphins (Tursiops truncatus) in the Mediterranean Sea:influence of behaviour. Mammalian Biology-Zeitschrift für Säugetierkunde, 76 (2) : 180 –189. Doi: 10.1016/j.mambio.2010.06.006 |
| Dittus W G, 1984. Toque macaque food calls:semantic communication concerning food distribution in the environment. Anim. Behav., 32 (2) : 470 –477. Doi: 10.1016/S0003-3472(84)80283-3 |
| dos Santos M E, Almada V C. 2004. A case for passive sonar:foraging by bottlenose dolphins in a turbid estuary. In:Thomas J, Moss C, Vater M eds. Advances in the Study of Echolocation:Comparison between Bats and Dolphins.University of Chicago Press, Chicago. p.400-403. |
| dos Santos M E, Louro S, Couchinho M, Brito C, 2005. Whistles of bottlenose dolphins (Tursiops truncatus) in the Sado estuary, Portugal:characteristics, production rates, and long-term contour stability. Aquatic Mammals, 31 (4) : 453 –462. Doi: 10.1578/AM.31.4.2005.453 |
| Dreher J J, Evans W E. 1964. Cetacean communication. In:Tavolga W N ed. Marine Bio-Acoustics. Pergamon, Oxford. p.373-393. |
| Dreher J J. 1966. Cetacean communication:small group experiment. In:Norris K S ed. Whales, Dolphins, and Porpoise. University of California, Berkeley. p.529-541. |
| Düpjan S, Schön P C, Puppe B, Tuchscherer A, Manteuffel G, 2008. Differential vocal responses to physical and mental stressors in domestic pigs (Sus scrofa). Appl. Anim.Behav. Sci., 114 (1-2) : 105 –115. Doi: 10.1016/j.applanim.2007.12.005 |
| Esch H C, Sayigh L S, Blum J E, Wells R S, 2009. Whistles as potential indicators of stress in bottlenose dolphins(Tursiops truncatus). Journal of Mammalogy, 90 (3) : 638 –650. Doi: 10.1644/08-MAMM-A-069R.1 |
| Fish M P, Mowbray W H, 1962. Production of underwater sound by the white whale or beluga, Delphinapterus leucas (Pallus). Journal of Marine Research, 20 : 149 –162. |
| Friedman E M, Boinski S, Coe C L, 1995. Interleukin-1 induces sleep-like behavior and alters call structure in juvenile rhesus macaques. American Journal of Primatology, 35 (2) : 143 –155. Doi: 10.1002/(ISSN)1098-2345 |
| Frohoff T G. 2004. Stress in Dolphins. In:Bekoff M ed.Encyclopedia of Animal Behavior. Greenwood Press, Westport, CT. p.1 158-1 164. |
| Galhardo L, Appleby M C, Waran N K, Dos Santos M E, 1996. Spontaneous activities of captive performing bottlenose dolphins (Tursiops truncatus). Animal Welfare, 5 (4) : 373 –389. |
| Galhardo L, Vital J, Oliveira R F, 2011. The role of predictability in the stress response of a cichlid fish. Physiology & Behavior, 102 (3-4) : 367 –372. |
| Gilbert-Norton L B, Leaver L A, Shivik J A, 2009. The effect of randomly altering the time and location of feeding on the behaviour of captive coyotes (Canis latrans). Appl.Anim. Behav. Sci., 120 (3-4) : 179 –185. Doi: 10.1016/j.applanim.2009.06.007 |
| Goldstein L E, Rasmusson A M, Bunney B S, Roth R H, 1996. Role of the amygdala in the coordination of behavioral, neuroendocrine, and prefrontal cortical monoamine responses to psychological stress in the rat. The Journal of Neuroscience, 16 (15) : 4 787 –4 798. |
| Gottlieb D H, Coleman K, McCowan B, 2013. The effects of predictability in daily husbandry routines on captive Rhesus macaques (Macaca mulatta). Appl. Anim. Behav.Sci., 143 (2-4) : 117 –127. Doi: 10.1016/j.applanim.2012.10.010 |
| Grandin T, 1998. The feasibility of using vocalization scoring as an indicator of poor welfare during cattle slaughter. Appl. Anim. Behav. Sci., 56 (2-4) : 121 –128. Doi: 10.1016/S0168-1591(97)00102-0 |
| Hansen S W, Jeppesen L L, 2006. Temperament, stereotypies and anticipatory behaviour as measures of welfare in mink. Applied Animal Behaviour Science, 99 (1-2) : 172 –182. Doi: 10.1016/j.applanim.2005.10.005 |
| Hawkins E R, Gartside D F, 2009a. Patterns of whistles emitted by wild Indo-Pacific bottlenose dolphins (Tursiops aduncus) during a Provisioning Program. Aquat. Mamm., 35 (2) : 171 –186. Doi: 10.1578/AM.35.2.2009.171 |
| Hawkins E R, Gartside D F, 2009b. Interactive behaviours of bottlenose dolphins (Tursiops aduncus) during encounters with vessels. Aquat. Mamm., 35 (2) : 259 –268. Doi: 10.1578/AM.35.2.2009.259 |
| Hawkins E R, Gartside D F, 2010. Whistle emissions of IndoPacific bottlenose dolphins (Tursiops aduncus) differ with group composition and surface behaviors. J. Acoustic Soc.Am., 127 (4) : 2 652 –2 663. Doi: 10.1121/1.3308465 |
| Hennessy J W, King M G, McClure T A, Levine S, 1977. Uncertainty, as defined by the contingency between environmental events, and the adrenocortical response of the rat to electric shock. J. Comp. Physiol. Psychol., 91 (6) : 1 447 –1 460. Doi: 10.1037/h0077408 |
| Herzing D L, 1996. Vocalizations and associated underwater behavior of free-ranging Atlantic spotted dolphins, Stenella frontalis and bottlenose dolphins, Tursiops truncatus. Aquat Mamm., 22 (2) : 61 –79. |
| Herzing D L. 2000. Acoustics and social behavior of wild dolphins:implications for a sound society. In:Au W W L, Fay R R, Popper A N eds. Hearing by Whales and Dolphins. Springer, New York. p.225-272. |
| Higa J J, Staddon J E R. 1997. Dynamic models of rapid temporal control in animals. In:Bradshaw C M, Szabadi E eds. Time and Behaviour:Psychological and Neurobehavioural Analyses. Elsevier, Amsterdam, The Netherlands. p.1-40. |
| Howell S M, Matevia M, Fritz J, Nash L, Maki S, 1993. Prefeeding agonism and seasonality in captive groups of chimpanzees (Pan troglodytes). Animal Welfare, 2 (2) : 153 –163. |
| Hughes B O, Duncan I J H, 1988. The notion of ethological ‘need', models of motivation and animal welfare. Anim.Behav., 36 (6) : 1 696 –1 707. Doi: 10.1016/S0003-3472(88)80110-6 |
| Janik V M, Todt D, Dehnhardt G, 1994. Signature whistle variations in a bottlenosed dolphin, Tursiops truncatus. Behavioral Ecology and Sociobiology, 35 (4) : 243 –248. Doi: 10.1007/BF00170704 |
| Jenny S, Schmid H, 2002. Effect of feeding boxes on the behavior of stereotyping Amur tigers (Panthera tigris altaica) in the Zurich Zoo, Zurich, Switzerland. Zool Biol., 21 (6) : 573 –584. Doi: 10.1002/(ISSN)1098-2361 |
| Jensen A L M, Delfour F, Carter T, 2013. Anticipatory behavior in captive bottlenose dolphins (Tursiops truncatus):a preliminary study. Zoo Biology, 32 (4) : 436 –444. Doi: 10.1002/zoo.v32.4 |
| Johanneson T, Ladewig J, 2000. The effect of irregular feeding times on the behaviour and growth of dairy calves. Appl.Anim. Behav. Sci., 69 (2) : 103 –111. Doi: 10.1016/S0168-1591(00)00127-1 |
| Johnson C M. 1993. Animal communication by way of coordinated cognitive systems. In:Bateson P P G, Klopfer P H, Thompson N S eds. Perspectives in Ethology. Plenum Press, New York. p.187-205. |
| Jones M, Pillay N, 2004. Foraging in captive hamadryas baboons:implications for enrichment. Appl. Anim. Behav.Sci., 88 (1-2) : 101 –110. Doi: 10.1016/j.applanim.2004.03.002 |
| Kamminga C, van Velden J G, 1987. Investigations on cetacean sonar VIII:sonar signals of Pseudorca crassidens in comparison with Tursiops truncatus. Aquatic Mammals, 13 (2) : 43 –49. |
| Kaznadzei V V, Krechi S A, Khakhalkina E N, 1976. Types of dolphin communication signals and their organization. Soviet Physical Acoustics, 22 (6) : 484 –488. |
| Knutson B, Burgdorf J, Panksepp J, 1998. Anticipation of play elicits high-frequency ultrasonic vocalizations in young rats. Journal of Comparative Psychology, 112 (1) : 65 –73. Doi: 10.1037/0735-7036.112.1.65 |
| Krishnamurthy R, 1994. Vocalizations of captive François' langurs linked to stereotypy and possible stress. Folia Primatologica, 63 (2) : 91 –95. Doi: 10.1159/000156798 |
| Lammers M O, Au W W L, Herzing D L, 2003. The broadband social acoustic signaling behavior of spinner and spotted dolphins. J. Acoust. Soc. Am., 114 (3) : 1 629 –1 639. Doi: 10.1121/1.1596173 |
| Laudenslager M L, Boccia M L, Berger C L, Gennaro-Ruggles M M, McFerran B, Reite M L, 1995. Total cortisol, free cortisol, and growth hormone associated with brief social separation experiences in young macaques. Developmental Psychobiology, 28 (4) : 199 –211. Doi: 10.1002/(ISSN)1098-2302 |
| Madsen P T, Kerr I, Payne R, 2004. Echolocation clicks of two free-ranging, oceanic delphinids with different food preferences:false killer whales Pseudorca crassidens and Risso's dolphins Grampus griseus. J. Exp. Biol., 207 : 1 811 –1 823. Doi: 10.1242/jeb.00966 |
| Mallapur A, Chellam R, 2002. Environmental Influences on stereotypy and the activity budget of Indian leopards(Panthera pardus) in four zoos in southern India. Zoo Biology, 21 (6) : 585 –595. Doi: 10.1002/zoo.10063 |
| Mann J, Kemps C, 2003. The effects of provisioning on maternal care in wild bottlenose dolphins, Shark Bay, Australia. Books Online, 5 : 304 –320. |
| Manser M B, Seyfarth R M, Cheney D L, 2002. Suricate alarm calls signal predator class and urgency. Trend in Cognitive Sciences, 6 (2) : 55 –57. Doi: 10.1016/S1364-6613(00)01840-4 |
| Manteuffel G, Puppe B, Schön P C, 2004. Vocalization of farm animals as a measure of welfare. Applied Animal Behaviour Science, 88 (1-2) : 163 –182. Doi: 10.1016/j.applanim.2004.02.012 |
| Marino L, Connor R C, Fordyce R E, Herman L M, Hof P R, Lefebvre L, Lusseau D, McCowan B, Nimchinsky E A, Pack A A, Rendell L, Reidenberg J S, Reiss D, Uhen M D, van der Gucht E, Whitehead H, 2007. Cetaceans Have Complex Brains for Complex Cognition. Essay. PLoS Biology, 5 (5) : e139 . Doi: 10.1371/journal.pbio.0050139 |
| Marino L, 2004. Dolphin cognition. Curr. Biol., 14 (21) : R910 –R911. Doi: 10.1016/j.cub.2004.10.010 |
| Mason G J, 1991. Stereotypies:a critical review. Anim. Behav., 41 (6) : 1 015 –1 037. Doi: 10.1016/S0003-3472(05)80640-2 |
| McCowan B, Rommeck I, 2006. Bioacoustic monitoring of aggression in group-housed rhesus macaques. Journal of Applied Animal Welfare Sciences, 9 (4) : 261 –268. Doi: 10.1207/s15327604jaws0904_1 |
| Melcón M L, Failla M, Iñíguez M A, 2012. Echolocation behavior of Franciscana dolphins (Pontoporia blainvillei) in the wild. J. Acoustic. Soc. Am., 131 (6) : EL448 . Doi: 10.1121/1.4710837 |
| Mercado E III, Uyeyama R K, Pack A A, Herman L M, 1999. Memory for action events in the bottlenosed dolphin. Anim. Cogn., 2 (1) : 17 –25. Doi: 10.1007/s100710050021 |
| Mistlberger R E, 1994. Circadian food-anticipatory activity:formal models and physiological mechanisms. Neurosci.Biobehav. Rev., 18 (2) : 171 –195. Doi: 10.1016/0149-7634(94)90023-X |
| Mitani J C, Nishida T, 1993. Contexts and social correlates of long-distance calling by male chimpanzees. Anim. Behav., 45 : 735 –746. Doi: 10.1006/anbe.1993.1088 |
| Mistlberger R E, 2009. Food-anticipatory circadian rhythms:concepts and methods. European Journal of Neuroscience, 30 (9) : 1 718 –1 729. Doi: 10.1111/j.1460-9568.2009.06965.x |
| Mistlberger R E, 2011. Neurobiology of food anticipatory circadian rhythms. Physiology & Behavior, 104 (4) : 535 –545. |
| Monticelli P F, Tokumaru R S, Ades C, 2004. Isolation induced changes in Guinea Pig Cavia porcellus pup distress whistles. An. Acad. Bras. Ciênc., 76 (2) : 368 –372. Doi: 10.1590/S0001-37652004000200027 |
| Morisaka T, Shinohara M, Nakahara F, Alamatsu T, 2005. Effects of ambient noise on the whistles of indo-pacific bottlenose dolphin populations. Journal of Mammalogy, 86 (3) : 541 –546. Doi: 10.1644/1545-1542(2005)86[541:EOANOT]2.0.CO;2 |
| Mulligan B E, Baker S C, Murphy M R, 1994. Vocalizations as indicators of emotional stress and psychological wellbeing in animals. Animal Welfare Information Center Newsletter, 5 (3) : 1 –3. |
| Murray S O, Mercado E, Roitblat H L, 1998a. Characterizing the graded structure of false killer whale (Pseudorca crassidens) vocalizations. J. Acoust. Soc. Am., 104 (3) : 1 679 –1 688. Doi: 10.1121/1.424380 |
| Murray S O, Mercado E, Roitblat H L, 1998b. The neural network classification of false killer whale (Pseudorca crassidens) vocalizations. J. Acoust. Soc. Am., 104 (6) : 3 626 –3 633. Doi: 10.1121/1.423945 |
| Nachtigall P E, Supin A Y, 2008. A false killer whale adjusts its hearing when it echolocates. J Exp. Biol., 211 (11) : 1 714 –1 718. Doi: 10.1242/jeb.013862 |
| Nemiroff L, Whitehead H, 2009. Structural characteristics of pulsed calls of long-finned pilot whales, Globicephala melas. Bioacoustics, 19 (1-2) : 67 –92. Doi: 10.1080/09524622.2009.9753615 |
| Neuringer A J, 1969. Animals respond for food in the presence of free food. Science, 166 (3903) : 399 –401. Doi: 10.1126/science.166.3903.399 |
| Norris K S, Dohl T P, 1980. Behavior of the Hawaiian spinner dolphins, Stenella longirostris. Fish. Bull., 77 : 821 –849. |
| Norris K S, Würsig B, Wells R S, Würsig M. 1994. The Hawaiian Spinner Dolphin. University of California Press, Berkeley, CA. 436p. |
| Nowacek D P, 2005. Acoustic ecology of foraging bottlenose dolphins (Tursiops truncatus), habitat-species use of three sound types. Marine Mammal Science, 21 (4) : 587 –602. Doi: 10.1111/j.1748-7692.2005.tb01253.x |
| Odell D K, McClune K M. 1999. False killer whale, Pseudorca crassidens (Owen, 1846). In:Ridgway S H, Harrison R eds. Handbook of Marine Mammals. Academic Press, Orlando, FL. p. 213-243. |
| Overstrom N A, 1983. Association between burst-pulse sounds and aggressive behavior in captive Atlantic bottlenosed dolphins (Tursiops truncatus). Zoo Biology, 2 (2) : 93 –103. Doi: 10.1002/(ISSN)1098-2361 |
| Owings D H, Virginia R A, 1978. Alarm calls of California ground squirrels (Spermophilus beecheyi). Zeitschrift für Tierpsychologie, 46 (1) : 58 –70. |
| Panksepp J, Burgdorf J, 2000. 50-kHz chirping (laughter?) in response to conditioned and unconditioned tickle-induced reward in rats:effects of social housing and genetic variables. Behavioural Brain Research, 115 (1) : 25 –38. Doi: 10.1016/S0166-4328(00)00238-2 |
| Polizzi di Sorrentino E, Schino G, Visalberghi E, Aureli F, 2010. What time is it? Coping with expected feeding time in capuchin monkeys. Animal Behavior, 80 (1) : 117 –123. Doi: 10.1016/j.anbehav.2010.04.008 |
| Puente A E, Dewsbury D A, 1976. Courtship and copulatory behavior of bottlenose dolphins (Tursiops truncatus). Cetology, 21 : 1 –9. |
| Ralston J V, Herman L M. 1989. Dolphin auditory perception. In:Doolin J R, Hulse S H eds. Complex Acoustic Perception:The Comparative Psychology of Complex Acoustic Perception. Hillsdale, NJ:Lawrence Erlbaum Associates. p.295-338. |
| Reebs S G, Lague M, 2000. Daily food-anticipatory activity in golden shiners. A test of endogenous timing mechanisms.Physiol. Behav., 70 (1-2) : 35 –43. |
| Rehn N, Filatova O A, Durban J W, Foote A D, 2011. Crosscultural and cross-ecotype production of a killer whale ‘excitement' call suggests universality. Naturwissenschaften, 98 (1) : 1 –6. Doi: 10.1007/s00114-010-0732-5 |
| Rehn N, Teichert S, Thomsen F, 2007. Structural and temporal emission patterns of variable pulsed calls in free-ranging killer whales (Orcinus orca). Behaviour, 144 (3) : 307 –329. Doi: 10.1163/156853907780425703 |
| Rendall D, Cheney D L, Seyfarth R M, 2000. Proximate factors mediating ‘contact' calls in adult female baboons(Papio cynocephalus ursinus) and their infants. Journal of Comparative Psychology, 114 (1) : 36 –46. Doi: 10.1037/0735-7036.114.1.36 |
| Rendell L E, Matthews J N, Gill A, Gordon J C D, MacDonald D W, 1999. Quantitative analysis of tonal calls from five odontocete species, examining interspecific and intraspecific variation. J. Zool., 249 (4) : 403 –410. Doi: 10.1111/jzo.1999.249.issue-4 |
| Reneerkens J, Piersma T, Ramenofsky M, 2002. An experimental test of the relationship between temporal variability of feeding opportunities and baseline levels of corticosterone in a shorebird. J. Exp. Zool., 293 (1) : 81 –88. Doi: 10.1002/(ISSN)1097-010X |
| Reynolds V, Luscombe G P. 1969. Social behavior of chimpanzees in an open environment. In:Proceedings of the 1st Aeromedical Research Laboratory, Aerospace Medical Division. United States Air Force. 657p. |
| Richelle M. 1980. Time in Animal Behavior. Pergamon Press, Oxford, UK. 273p. |
| Ridgway S H, Moore P W, Carder D A, Romano T A, 2014. Forward shift of feeding buzz components of dolphins and belugas during associative learning reveals a likely connection to reward expectation, pleasure and brain dopamine activation. The Journal of Experimental Biology, 217 (16) : 2 910 –2 919. Doi: 10.1242/jeb.100511 |
| Roberts W A. 1998. Principles of Animal Cognition. McGrawHill, Boston. 480p. |
| Rosenblum L A, Paully G S, 1984. The effects of varying environmental demands on maternal and infant behavior. Child Dev., 55 (1) : 305 –314. Doi: 10.2307/1129854 |
| Ruiz-Miranda C R, Wells S A, Golden R, Seidensticker J, 1998. Vocalizations and other behavioral responses of male cheetahs (Acinonyx jubatus) during experimental separation and reunion trials. Zoo Biology, 17 (1) : 1 –16. Doi: 10.1002/(ISSN)1098-2361 |
| Sánchez J A, López-Olmeda J F, Blanco-Vives B, SánchezVázquez F J, 2009. Effects of feeding schedule on locomotor activity rhythms and stress response in sea bream. Physiology & Behavior, 98 (1-2) : 125 –129. |
| Sayigh L S, Quick N, Hastie G, Tyack P, 2013. Repeated call types in short-finned pilot whales, Globicephala macrorhynchus. Mar. Mamm. Sci., 29 (2) : 312 –324. Doi: 10.1111/mms.2013.29.issue-2 |
| Scarpaci C, Bigger S W, Corkeron P J, Nugegoda D, 2000. Bottlenose dolphins, Tursiops truncatus, increase whistling in the presence of "swim-with-dolphin"operations. J. Cetacean Res. Manage., 2 (3) : 183 –186. |
| Schapiro S J, Bloomsmith M A, 1994. Behavioral effects of enrichment on pair-housed juvenile rhesus monkeys. American Journal of Primatology, 32 (3) : 159 –170. Doi: 10.1002/(ISSN)1098-2345 |
| Scheer M, 2013. Short note:call vocalizations recorded among short-finned pilot whales (Globicephala macrorhynchus) off Tenerife, Canary Islands. Aquatic Mammals, 39 (3) : 306 –313. Doi: 10.1578/AM.39.3.2013.306 |
| Schön P C, Puppe B, Manteuffel G, 2004. Automated recording of stress vocalisations as a tool to document impaired welfare in pigs. Animal Welfare, 13 (2) : 105 –110. |
| Schwartzkopf-Genswein K S, Stookey J M, Welford R, 1997. Behavior of cattle during hot-iron and freeze branding and the effects on subsequent handling ease. Journal of Animal Science, 75 (8) : 2 064 –2 072. Doi: 10.2527/1997.7582064x |
| Sekiguchi Y, Kohshima S, 2003. Resting behaviors of captive bottlenose dolphins (Tursiops truncatus). Physiology and Behavior, 79 (4-5) : 643 –653. Doi: 10.1016/S0031-9384(03)00119-7 |
| Seligman M E, Meyer B, 1970. Chronic fear and ulcers in rats as a function of the unpredictability of safety. J. Comp.physiol. Psychol., 73 (2) : 202 –207. Doi: 10.1037/h0030219 |
| Seligman M E, 1968. Chronic fear produced by unpredictable electric shock. J. Comp. Physiol. Psychol., 66 (2) : 402 –411. Doi: 10.1037/h0026355 |
| Seyfarth R M, Cheney D L, 2003a. Meaning and emotion in animal vocalizations. Annals of the New York Academy of Sciences, 1000 : 32 –55. |
| Seyfarth R M, Cheney D L, 2003b. Signalers and receivers in animal communication. Annual Review of Psychology, 54 : 145 –173. Doi: 10.1146/annurev.psych.54.101601.145121 |
| Shepherdson D J, Carlstead K, Mellen J D, Seidensticker J, 1993. The influence of food presentation on the behavior of small cats in confined environments. Zoo Biology, 12 (2) : 203 –216. Doi: 10.1002/(ISSN)1098-2361 |
| Sikes R S, Gannon N W L, 2011. Guidelines of the American Society of Mammalogists for the use of wild mammals in research. Journal of Mammalogy, 92 (1) : 235 –253. Doi: 10.1644/10-MAMM-F-355.1 |
| Sjare B L, Smith T G, 1986. The relationship between behavioral activity and underwater vocalizations of the white whale, Delphinapterus leucas. Can. J. Zool., 64 (12) : 2 824 –2 831. Doi: 10.1139/z86-406 |
| Slocombe K E, Zuberbühler K, 2006. Food-associated calls in chimpanzees:responses to food types or food preferences?. Animal Behavior, 72 (5) : 989 –999. Doi: 10.1016/j.anbehav.2006.01.030 |
| Slooten E, 1994. Behavior of hector's dolphin:classifying behavior by sequence analysis. Journal of Mammology, 75 (4) : 956 –964. Doi: 10.2307/1382477 |
| Smolker R A, Mann J, Smuts B B, 1993. Use of signature whistles during separations and reunions by wild bottlenose dolphin mothers and infants. Behav. Ecol.Sociobiol., 33 (6) : 393 –402. |
| Spruijt B M, van den Bos R, Pijlman F T A, 2001. A concept of welfare based on reward evaluating mechanisms in the brain:anticipatory behaviour as an indicator for the state of reward systems. Appl. Anim. Behav. Sci., 7 (2) : 145 –171. |
| Stacey P J, Leatherwood S, Baird R W, 1994. Pseudorca crassidens. Mammalian Species, 456 : 1 –6. |
| Stewart B S, Clapham P J, Powell J A, Reeves R R. 2002.National Audubon Society. Guide to Marine Mammals of the World. Knopf, New York. 528p. |
| Supin A Y, Nachtigall P E, Au W W L, Breese M, 2004. The interaction of outgoing echolocation pulses and echoes in the false killer whale's auditory system:evoked-potential study. J. Acoust. Soc. Am., 115 (6) : 3 218 –3 225. Doi: 10.1121/1.1707088 |
| Supin A Y, Nachtigall P E, Au W W L, Breese M, 2005. Invariance of evoked-potential echo-responses to target strength and distance in an echolocating false killer whale:evoked potential study. J. Acoust. Soc. Am., 117 (6) : 3 928 –3 935. Doi: 10.1121/1.1914150 |
| Supin A Y, Nachtigall P E, Pawloski J L, Au W W L, 2003. Evoked potential recording during echolocation in a false killer whale Pseudorca crassidens (L). J. Acoust. Soc.Am., 113 (5) : 2 408 –2 411. Doi: 10.1121/1.1561497 |
| Tanchez E K. 2003. Factors Affecting the Diel Underwater Sound Production of Captive Belugas (Delphinapterus leucas) at the John G. Shedd Aquarium. Western Illinois University, Quad Cities, Moline. 40p. |
| Tavolga W N, 1983. Theoretical principles for the study of communication in cetaceans. Mammalia, 47 (1) : 3 –26. |
| Taylor A A, Weary D M, 2000. Vocal responses of piglets to castration:Identifying procedural sources of pain. Applied Animal Behaviour Science, 70 (1) : 17 –26. Doi: 10.1016/S0168-1591(00)00143-X |
| Therrien S C, Thomas J A, Therrien R E, Stacey R, 2012. Time of day and social change affect underwater sound production by bottlenose dolphins (Tursiops truncatus) at the Brookfield Zoo. Aquatic Mammals, 38 (1) : 65 –75. Doi: 10.1578/AM.38.1.2012.65 |
| Thomas J, Stoermer M, Bowers C, Anderson L, Garver A, 1988. Detection abilities and signal characteristics of echolocating false killer whales (Pseudorca crassidens). In:Nachtigall P E, Moore P W B eds. Animal Sonar.Springer, US, 156 : 323 –328. |
| Tyack P, 1986. Whistle repertoires of two bottlenosed dolphins, Tursiops truncatus:mimicry of signature whistles?. Behavioral Ecology and Sociobiology, 18 (4) : 251 –257.. Doi: 10.1007/BF00300001 |
| Ulyan M J, Burrows A E, Buzzell C A, Raghanti M A, Marcinkiewicz J L, Phillips K A, 2006. The effects of predictable and unpredictable feeding schedules on the behavior and physiology of captive brown capuchins(Cebus apella). Applied Animal Behaviour Science, 101 (1-2) : 154 –160. Doi: 10.1016/j.applanim.2006.01.010 |
| van Rooijen J, 1991. Predictability and boredom. Appl. Anim.Behav. Sci., 31 (3-4) : 283 –287. Doi: 10.1016/0168-1591(91)90014-O |
| Vinke C M, van den Bos R, Spruijt B M, 2004. Anticipatory activity and stereotypical behaviour in American mink(Mustela vison) in three housing systems differing in the amount of enrichments. Appl. Anim. Behav. Sci., 89 (1-2) : 145 –161. Doi: 10.1016/j.applanim.2004.06.002 |
| von Borell E, Ladewig J, 1992. Relationship between behaviour and adrenocortical response pattern in domestic pigs. Applied Animal Behaviour Science, 34 (3) : 195 –206. Doi: 10.1016/S0168-1591(05)80115-7 |
| Waitt C, Buchanan-Smith H M, 2001. What time is feeding?:how delays and anticipation of feeding schedules affect stump-tailed macaque behavior. Appl. Anim. Behav. Sci., 75 (1) : 75 –85. Doi: 10.1016/S0168-1591(01)00174-5 |
| WaittC, Buchanan-Smith H M, 2006. Perceptual considerations in the use of colored photographic and video stimuli to study nonhuman primate behavior. American Journal of Primatology, 68 (11) : 1 054 –1 067. Doi: 10.1002/ajp.20303 |
| Wallace R J, 1979. Novelty and partibility as determinants of hoarding in the albino rat. Animal Learning & Behavior, 7 (4) : 549 –554. |
| Wang Z T, Fang L, Shi W J, Wang K X, Wang D, 2013. Whistle characteristics of free-ranging Indo-Pacific humpback dolphins (Sousa chinensis) in Sanniang Bay, China. J.Amer. Acoust. Soc., 133 (4) : 2 479 –2 489. Doi: 10.1121/1.4794390 |
| Warris P D, Brown S N, Adams S J M, 1994. Relationships between subjective and objective assessments of stress at slaughter and meat quality in pigs. Meat Science, 38 (2) : 329 –430. Doi: 10.1016/0309-1740(94)90121-X |
| Wasserman F E, Cruikshank W W, 1983. The relationship between time of feeding and aggression in a group of captive hamadryas baboons. Primates, 24 (3) : 432 –435. Doi: 10.1007/BF02381988 |
| Weary D M, Braithwaite L A, Fraser D, 1998. Vocal response to pain in piglets. Applied Animal Behaviour Science, 56 (2-4) : 161 –172. Doi: 10.1016/S0168-1591(97)00092-0 |
| Weary D M, Fraser D, 1995. Calling by domestic piglets:reliable signals of need? Anim. Behav., 50 (4) : 1 047 –1 055. Doi: 10.1016/0003-3472(95)80105-7 |
| Weary D M, Ross S, Fraser D, 1997. Vocalizations by isolated piglets:a reliable indicator of piglet need directed towards the sow. Applied Animal Behaviour Science, 53 (4) : 249 –257. Doi: 10.1016/S0168-1591(96)01173-2 |
| Weilgart L S, Whitehead H, 1990. Vocalizations of the North Atlantic pilot whales (Globicephala melas) as related to behavioral contexts. Behav. Ecol. Sociobiol., 26 : 399 –402. |
| Weir C R, Frantzis A, Alexiadou P, Goold J C, 2007. The burstpulse nature of ‘squeal' sounds emitted by sperm whales(Physeter macrocephalus). Journal of the Marine Biological Association of the United Kingdom, 87 (1) : 39 –46. Doi: 10.1017/S0025315407054549 |
| Weller S H, Bennett C L, 2001. Twenty-four hour activity budgets and patterns of behavior in captive ocelots(Leopardus pardalis). Appl. Anim. Behav. Sci., 71 (1) : 67 –79. Doi: 10.1016/S0168-1591(00)00169-6 |
| White R G, Deshazer J A, Tressler C J, Borcher G M, Davey S, Waninge A, Parkhurst A M, Milanuk M J, Clemens E T, 1993. Vocalization and physiological response of pigs during castration with or without a local anesthetic. Journal of Animal Science, 73 (2) : 381 –386. |
| Wilson W L, Wilson C C. 1968. Aggressive interactions of captive chimpanzees living in a semi-free-ranging environment. Technical Report No. ARL-TR-68-9. Aeromedical Research Laboratory, Mew Mexico. p.1-10. |
| Zar J H. 1996. Biostatistical Analysis. 3rd edn. Prentice Hall, Englewood Cliffs, NJ. 960p. |
| Zimmerman P H, Koene P, van Hooff J A R, 2000. Thwarting of behaviour in different contexts and the gakel-call in the laying hen. Applied Animal Behaviour Science, 69 (4) : 255 –264. Doi: 10.1016/S0168-1591(00)00137-4 |
| Zimmerman P H, Koene P, 1998. The effect of frustrative nonreward on vocalisations and behaviour in the laying hen, Gallus gallus domesticus. Behavioural Processes, 44 (1) : 73 –79. Doi: 10.1016/S0376-6357(98)00035-7 |
| Zimmerman P H, Lundberg A, Keeling L J, Koene P, 2003. The effect of an audience on the gakel-call and other frustration behaviours in the laying hen (Gallus gallus domesticus). Animal Welfare, 12 (3) : 315 –326. |
 2016, Vol. 34
2016, Vol. 34



