Institute of Oceanology, Chinese Academy of Sciences
Article Information
- LIANG Jiayuan(梁甲元), SUN Aijun(孙爱君), ZHANG Yun(张云), DENG Dun(邓盾), WANG Yongfei(王永飞), MA Sanmei(马三梅), HU Yunfeng(胡云峰)
- Functional characterization of a novel microbial esterase identified from the Indian Ocean and its use in the stereoselective preparation of (R)-methyl mandelate
- Chinese Journal of Oceanology and Limnology, 34(6): 1269-1277
- http://dx.doi.org/10.1007/s00343-016-5164-4
Article History
- Received Jun. 9, 2015
- accepted for publication Sep. 2, 2015
- accepted in principle Sep. 28, 2015
2 Key Laboratory of Tropical Marine Bio-Resources and Ecology, South China Sea Institute of Oceanology, Chinese Academy of Sciences, Guangzhou 510301, China;
3 South China Sea Bio-Resource Exploitation and Utilization Collaborative Innovation Center, Guangzhou 510275, China;
4 Department of Biotechnology, Jinan University, Guangzhou 510632, China
Optically pure α-hydroxycarboxylic acids, which bear a carbonyl group and a hydroxyl group on one chiral carbon atom, are crucial chiral intermediates for the synthesis of valuable drugs. Chiral mandelic acids and their ester derivatives are the most important types of α-hydroxycarboxylic acids and have multiple commercial uses (Gröger, 2001). For example, (R)- mandelic acid is extensively used as a chiral intermediate for the manufacture of semi-synthetic cephalosporin, penicillin and various other pharmaceuticals. Both enantiomers of mandelic acid have been successfully employed as chiral solvating agents for the resolution of racemic amines and alcohols in industry (Kinbara et al., 1996).
The great value of chiral mandelic acids and their derivatives has led to intensive efforts in the development of production methods of optically pure products. Enzymes are industrially useful biocatalysts because of their high chemical selectivity, regioselectivity, and enantioselectivity. Multiple enzymatic routes have been developed for the generation of optically pure mandelic acids and their derivatives (Scheme 1). For example, α-keto acids can be converted to mandelic acids by dehydrogenases using NADH/NADPH as cofactors (Tsuchiya et al., 1992 ; Xiao et al., 2008). A second enzymatic route is to hydrolyze cyano groups using nitrilases as the enantioselective biocatalysts (Banerjee et al., 2006 ; Zhang et al., 2010). However, the drawbacks of these two enzymatic routes are that NADH/NADPH in the enzymatic reactions catalyzed by dehydrogenases are expensive, need to be recycled, and nitrilases can be unstable.
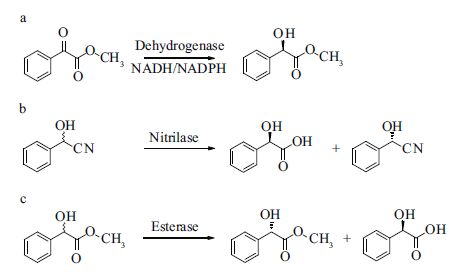
|
| Scheme 1 Enzymatic methods for the preparation of chiral mandelic acid and their derivatives a. preparation of chiral methyl mandelate by dehydrogenase; b. preparation of chiral mandelic acid by nitrilase; c. preparation of chiral methyl mandelate by esterase. |
Esterases (EC 3.1.1.1) are one class of the most commonly used enzymes in the asymmetric synthesis of chiral drug intermediates or chiral chemicals. They have good stability, a wide range of substrate specificities, do not require expensive cofactors, and most importantly, they have the ability to recognize chirality (Kim et al., 2000 ; Ma et al., 2013 ; Yao et al., 2013 ; Cao et al., 2014 ; Chen and Yang, 2014). Hence, the third method for the enzymatic generation of chiral mandelic acid is to hydrolyze inexpensive esters of mandelic acid in aqueous or biphasic media by inexpensive esterases.
At present, there are few reports about the generation of chiral methyl mandelate through stereoselective hydrolysis of racemic mandelate by lipases. Yadav and Sivakumar (2004) reported the employment of lipase Novezym 435 in the kinetic resolution of methyl mandelate.(S)-methyl mandelate was generated in this enzymatic hydrolysis reaction with an optical purity of 78% at 19% conversion. Wei and Wu (2008) reported the use of one extracellular lipase identified from Burkholderia sp. GXU56 in the resolution of racemic methyl mandelate. This extracellular lipase could convert racemic methyl mandelate to (S)-methyl mandelate and (R)-mandelic acid with an enantiomeric excess of 98.5% and a conversion of 34.5%. However, the protein sequence of this extracellular lipase was not reported. In addition, chiral mandelic acid could potentially also be prepared through enzymatic acylation of the hydroxyl group in racemic mandelic acid by lipases.
With its huge diversity of microorganisms, plants and animals, the marine environment is a great biological source. Many novel marine organisms have evolved in the special environmental conditions of the oceans. Those marine organisms, especially marine microorganisms, contain abundant novel genes and novel enzymes, which are a great source for industrial enzymes and await further exploitation. In our study, we report the identification and characterization of an alkaline microbial esterase BSE04211 from the Indian Ocean. BSE04211, an esterase with esterification activities, can hydrolyze racemic methyl mandelate and generate optically pure (R)-methyl mandelate rather than (S)-methyl mandelate, as per previous studies (Scheme 1).
2 MATERIAL AND METHOD 2.1 Microorganisms and enzymesBacillus sp. SCSIO 15121 was obtained from the -3400 sediments from the Indian Ocean (89°29.22′E, 10°00.12′N). The plasmid pET-28a (+)(Novagen, George Town, KY, USA) was used as the expression vector of BSE04211. Escherichia coli DH5a was used for routine cloning and Escherichia coli BL21(DE3) was used for gene expression. The E. coli and recombinant E. coli strains were routinely grown at 37℃ and 200 r/min in Luria-Bertani medium both with and without 50 μg/mL kanamycin. Restriction enzymes, ligase, and TransStart® FastPfu DNA PolyMerase were all obtained from TransGen Biotech (Beijing, China). P-nitrophenyl (p-NP) esters were purchased from Sigma (St. Louis, MO, USA). Racemic methyl mandelate and corresponding chiral enantiomers were purchased from Aladdin Industrial Corporation (Shanghai, China). Other chemicals were of analytical grade.
2.2 Gene cloning and expression vector constructionBased on the coding DNA sequence of a paranitrobenzyl esterase (defined as BSE04211) from the genome of Bacillus sp. SCSIO 15121(genome sequence was completed by Majorbio LLC), primers for the cloning of this esterase gene with corresponding restriction recognition sites were designed as follows.
The forward primer was 5′-TGCTAGCCATATGTATGAAACGACTGTCCAAACGTG- 3′, and the reverse primer was 5′-CGAATTCCTAAACCTGCAO GGTTTGAGGCTG-3′(Nde I and Eco RI restriction sites are underlined). PCR products were analyzed and purified using 0.8% agarose gel electrophoresis and cloned into pET-28a (+) vector. Constructed plasmids were transformed into E. coli BL21(DE3) competent cells for further protein expression.
2.3 Sequence analysisThe BLASTP program was used to blast against the NCBI protein database (http://www.ncbi.nlm.nih.gov/blast/). Multiple sequence alignments were constructed using the Vector NTI program (Lu and Moriyama, 2004). A three-dimensional enzyme model was built and analyzed through an online Automatic Modeling Mode server at http://www.sbg.bio.ic.ac.uk/phyre2/html/page.cgi?id=index (Kelley and Sternberg, 2009).
2.4 Expression and purification of BSE04211When the cell density of the incubated recombinant cells reached approximately 0.8 at 600 nm, isopropylbeta- D-thiogalactopyranoside (IPTG) was added up to a final concentration of 0.5 mmol/L for protein induction. After 18 h induction at 20℃, the cells were harvested by centrifugation at 4 500× g for 15 min, washed twice with 100 mmol/L potassium phosphate buffer (pH 7.0), resuspended in the same buffer, and then disrupted by sonication on ice for 10 min. Cell debris was removed through centrifugation at 10 000× g for 30 min. The recombinant proteins were purified and desalted using nickel-nitrilotriacetic acid agarose resin (Qiagen, Hilden, Germany) and PD-10 desalting columns (GE Healthcare Life Sciences, UK) according to the manufacturer's instructions. Protein concentration was determined using the Bradford method, with bovine serum albumin as a standard. Purified BSE04211 was analyzed by SDSPAGE and freeze-dried. The result of SDS-PAGE analysis showed a single protein band of approximately 54 kDa (Supplementary Fig. 1, lane 3).
2.5 Enzyme assayStandard reactions were conducted by adding 0.5 μg purified enzyme to a 1-mL reaction mixture containing 100 mmol/L phosphate buffer (pH 7.0) and 2 mmol/L substrate (p-NP esters) followed by incubation at 30℃ for 10 min. One volume of distilled water equal to that of the purified enzyme was added to the reaction mixture as a control. Reactions were terminated by the addition of three volumes of isopropanol. Esterase activities were determined by measuring the release (absorbance) of p-NP from p- NP esters at 405 nm. One unit of enzyme activity was defined as the amount of esterase liberating 1 μmol of p-NP per minute. Next, a series of biochemical analyses, including optimal pH, optimal temperature, substrate specificities, enzymatic activities, and esterification reactions, were carried out using the above methods. All experiments were performed in triplicate and repeated at least three times. Kinetic parameters were obtained using a Lineweaver-Burk plot under the optimal reaction conditions.
2.6 Substrate specificity of BSE04211Different substrates (p-NP C2 to C12) were used to determine the hydrolytic activities of BSE04211 under standard reaction conditions. Control experiments without enzymes were also performed in parallel. The relative hydrolytic activities of BSE04211 were determined by monitoring the absorbance of products (p-NP) at 405 nm.
2.7 Effects of pH, temperature, and metal ions on BSE04211 activitiesP-nitrophenyl butyrate (C 4) was used as the substrate for the functional characterization of BSE04211. The enzyme activities were determined at 30℃ in the following buffers for different pH ranges: 100 mmol/L Na2 HPO4 -NaH2 PO4(pH 6.0-8.0), 100 mmol/L Tris-HCl (pH 8.0-9.5), and 100 mmol/L glycine-NaOH (pH 9.5-10.0). Reactions were set at different temperatures, ranging from 25 to 60℃ to detect the effect of temperature on enzyme activity at pH 8.0(phosphate buffer). The effects of various metal ions (K+, Fe3+ etc.) toward the hydrolytic activities of BSE04211 were determined by measuring enzymatic activities in the presence of 5 mmol/L and 10 mmol/L of metal ions under optimal reaction conditions.
2.8 Effect of organic solvents on hydrolytic activitiesThe effect of various organic solvents was determined by measuring enzyme activities in the presence of 5 to 30%(v / v) organic solvents under optimal reaction conditions.
2.9 Esterification reactionsThe standard esterification system was carried out in n-hexane media in a 100-mL capped flask (working volume of 10 mL) in an orbital shaker (200 r/min) at 40℃. 30 g/L BSE04211 and 0.2 mol/L of carboxylic acids and equal molar ratio of alcohols were used as substrates. After the termination of enzymatic reactions, product characterization was performed by a Gas Chromatograph-Mass Spectrometer (Shimadzu, GCMS-QP2010 Ultra, Japan) equipped with flame ionization detector and a Rxi ® -5Sil MS capillary column (30 m×0.25 mm ID, 0.25 μm df). The esterification yields were calculated by measuring the amount of residual acids using titration as per Graebin et al.(2012) and Friedrich et al.(2013). All experiments were performed in triplicate.
2.10 Kinetic resolution of racemic methylmandelate by enzymatic hydrolysis reactions Standard hydrolytic reactions were carried out by adding 10 μg purified enzyme to a 500-μL reaction mixture containing 100 mmol/L phosphate buffer (pH 8.0) and 10 mmol/L (±)-methyl mandelate followed by incubating at 40℃ for 2 h. Equal amounts of distilled water and the purified enzyme were added to the reaction mixture as a control. After the termination of the enzymatic reactions, a final concentration of 5 mmol/L dodecane was added to the reaction samples as an internal standard. The samples were extracted with an equal volume of ethyl acetate and the organic phase was analyzed by a Gas Chromatograph (FULI GC-9790 II) equipped with H2 flame ionization detector and 112-6632 CYCLOSIL-B chiral capillary column (30 m×0.25 mm ID, 0.25 μm df). Nitrogen served as the carrier gas at a split flow rate of 1.20 mL/ min and column temperature ranging from 60 to 200℃. The injector temperature was 250℃, and the detector temperature 280℃. The yield (YR) and enantiomeric excess (e. e. R) of (R)-methyl mandelate were calculated using the equation of Chen et al.(1982) :
Enantiomeric excess (e. e. R)=[(R-methyl mandelate)(S-methyl mandelate)]/[(R-methyl mandelate)+(S-methyl mandelate)],
Yield of R-methyl mandelate (YR)=(A)/(A0).
A and A0 represent the content of (R)-methyl mandelate after and before the reaction, respectively. The working conditions, including temperature, buffer pH, and substrate concentrations, for the kinetic resolution of (±)-methyl mandelate were further optimized to obtain chiral products with higher optical purity and yield.
2.11 Enzymatic reactions of racemic methyl mandelate with methanol, vinyl acetate and butanolThe kinetic resolution of racemic methyl mandelate was carried out by the esterification reaction of mandelic acid with methanol. The initial esterification reaction was carried out by adding 0.3 g BSE04211 to 10 mL hexane containing 10 mmol/L racemic mandelic acid and 20 mmol/L methanol. The kinetic resolution of racemic methyl mandelate was performed by the transesterification reaction of methyl mandelate with vinyl acetate or butanol. Transesterification was conducted by adding 0.3 g BSE04211 in 10 mL hexane containing 10 mmol/L racemic methyl mandelate and 20 mmol/L vinyl acetate or butanol. Twenty-four hours after the enzymatic reactions, the final products were characterized using the gas chromatography methods described above.
3 RESULT 3.1 Sequence analysis of BSE04211The gene BSE04211 contains 1 476 bp encoding a protein that contains 492 amino acids, and the GC content of DNA is 49.66%. The protein sequence of BSE04211 was used to blast against the NCBI protein sequence database, which revealed similarities between BSE04211 and six para-nitrobenzyl esterases in the database, including a para-nitrobenzyl esterase from Bacillus licheniformis 9945A (99%)(accession No: YP_008076860.1), a para-nitrobenzyl esterase from Bacillus sp. CPSM8(99%)(accession No: WP_023855546.1), and four para-nitrobenzyl esterases from Bacillus licheniformis (90%)(GenBank No: EWH20528.1 and accession No: WP_021837169.1, YP_077866.1, and WP_003179394.1). However, the functions of all six para-nitrobenzyl esterases have not been well characterized. BSE04211 exhibited much lower sequence identities than other esterases reported so far (≤65%). Using a para-nitrobenzyl esterase as a template (PDB ID: d1qe3a), a three-dimensional model of BSE04211 was built and analyzed through a Phyre2 server. The model confidence was 100% and identity was 61%. Based on structural homology and predicted catalytic sites, BSE04211 belongs to the α/β hydrolase family (Ollis et al., 1992 ; Kim et al., 1997) and contains a catalytic triad formed by A106, S188, A189, E309, and H400(Supplementary Fig. 1). The protein sequences at the active sites of paranitrobenzyl esterases are similar, but dramatically different from those of lipases and other esterases (Supplementary Fig. 2). BSE04211 contains a Glu instead of an Asp at its active site, which is rare among esterases (Sussman et al., 1991).

|
| Figure 1 Substrate specificities of BSE04211 toward various p- nitrophenyl esters The reactions were conducted according to the standard reaction system mentioned above. 100% relative activity was denoted as the highest absorbance of product p-NP. |

|
| Figure 2 Effects of pH and temperature on the hydrolytic activities of BSE04211 a. the enzyme activities were determined at 30℃ using p-NP C4 as the test substrate in the following buffers for different pH ranges: 100 mmol/L Na2 HPO4 -NaH2 PO4(pH 6.0-8.0), 100 mmol/L Tris- HCl (pH 8.0-9.5), and 100 mmol/L glycine-NaOH (pH 9.5-10.0); b. reactions were set at different temperatures, ranging from 25℃ to 60℃ to detect the effect of temperature on enzyme activity at pH 8.0(phosphate buffer). |
The highest hydrolytic activity of BSE04211 was toward substrate p-NP C4, and was classified as 100% relative activity (Fig. 1). The relative hydrolytic activity of BSE04211 toward p-NP C6 was ~60%, while the relative activities toward p-NP C2, p-NP C8, and p-NP C12 were much lower (<10%). These results indicate that short-chain p-NP esters, such as p-NP C4 and p-NP C6, rather than long-chain p-NP esters were the preferred substrates of BSE04211. BSE04211 was therefore characterized as a true esterase instead of a lipase (Arpigny and Jaeger, 1999).
3.3 Effects of pH, temperature, and metal ions on BSE04211 activitiesThe optimal pH for the hydrolytic activity of BSE04211 was 8.0(PB). BSE04211 exhibited high hydrolytic activities from pH 8.0 to 9.5, indicating that BSE04211 is an alkaline esterase (Fig. 2a). BSE04211 exhibited its high activities between 35 and 55℃, with the highest hydrolytic activity at 40℃(Fig. 2b). The hydrolytic activity of BSE04211 was obviously inhibited by Fe2+(with ~35% relative activity in the presence of 10 mmol/L of FeCl2), whereas its hydrolytic activities were only partially inhibited by Zn2+, Cu2+, Fe3+, Al3+, Mn2+, Ca2+, and EDTA. Moreover, Mg2+, K+, and Ba2+ ions had no significant effects on its hydrolytic activities (Supplementary Table 1).

|
Many esterases or lipases show very good stability and even improved activities in the presence of high concentrations of various organic solvents (Cherif and Gargouri, 2010 ; Jin et al., 2012). However, esterase BSE04211 proved to be sensitive to many organic solvents. It exhibited relatively low hydrolytic activities in the presence of multiple organic solvents, especially some water-immiscible solvents with low polarity (high log P), such as butanol, n-hexane, n-heptane, and isooctane (Supplementary Table 2), indicating that organic solvents can greatly affect the hydrolytic activities of BSE04211.
3.5 Kinetic analysis of BSE04211Kinetic analysis of BSE04211 was performed using p-NP esters as the substrates (see Supplementary Table 3). The Km value for p-NP C4 is lower than those of p-NP C2 and p-NP C6, indicating that BSE04211 preferred p-NP C4 rather than p-NP C2 or p-NP C6 as its optimal substrate. Furthermore, under optimal reaction conditions, BSE04211 exhibited its highest catalytic activity also using p-NP C4 as the substrate.
3.6 Synthesis of short-chain flavor estersEsterase BSE04211 was able to synthesize many industrially important short-chain flavor esters, with high yields of esterification products after 24 h. Notably, flavor esters such as ethyl valerate (yield 90.15%), propyl valerate (yield 91.64%) and butyl valerate (yield 97.67%) were enzymatically synthesized by esterase BSE04211(Supplementary Table 4).
3.7 Kinetic resolution of (±)-methyl mandelate through hydrolysis reactionsHydrolases, represented by esterases, can resolve racemic chemicals by direct hydrolysis of their ester derivatives. This is because of the ability of hydrolases to recognize chirality in chemicals. Esterase BES04211 is able to hydrolyze racemic methyl mandelate in an aqueous system, generating (S)- mandelic acid and (R)-methyl mandelate after the analysis of enzymatic products using chiral GC (Fig. 3). The effects of reaction temperature, pH and concentrations of substrate were further investigated to determine the optimal reaction conditions for the kinetic resolution of racemic methyl mandelate catalyzed by BSE04211.
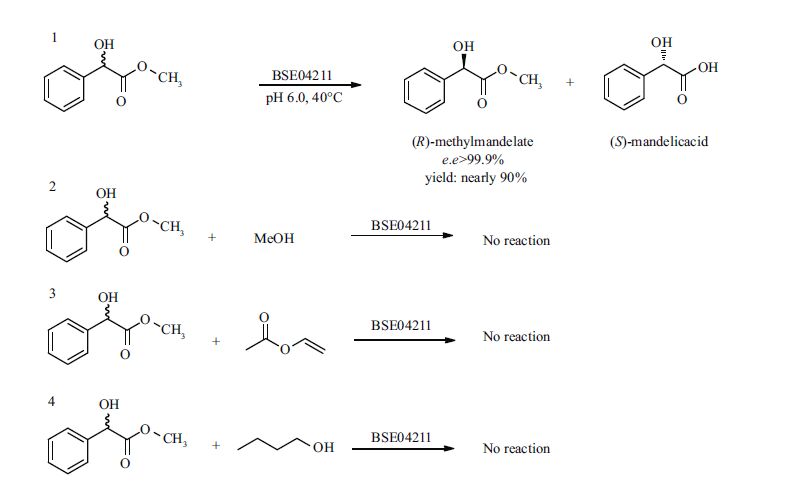
|
| Figure 3 Enzymatic reactions catalyzed by BSE04211 for the generation of chiral (R)-methyl mandelate 1. hydrolytic reaction catalyzed BSE04211 to produce optically pure (R)-methyl mandelate; 2. esterification reaction of racemic mandelic acid with methanol catalyzed by BSE04211; 3. transesterification reaction of racemic methyl mandelate with vinyl acetate catalyzed by BSE04211; 4. transesterification reaction of racemic methyl mandelate with butanol catalyzed by BSE04211. |
Temperature is a key factor affecting the stereoselectivity, activity and stability of biocatalysts. The resolution of (±)-methyl mandelate catalyzed by BSE04211 was monitored after incubating enzymatic reactions at temperatures ranging from 10 to 50℃. The yield and enantiomeric excess of (R)-methyl mandelate from enzymatic reactions under different temperatures are shown in Table 1. The optimal temperature for the highest e. e. R was found to be 40℃ after 2 h of enzymatic hydrolysis reactions, with the e. e. R as high as 99.9% and a yield of 62.9%. However, the e. e. R quickly decreased when the reaction temperature was above 40℃ or below 30℃, indicating that reaction temperature can greatly affect the optical purity and yield of final product (R)-methyl mandelate.
3.9 Effect of pH on the optical purity and yield of (R)-methyl mandelateHigh e. e. R of (R)-methyl mandelate (>99%) could be obtained under different pH values (from 6.0 to 10.0) at the optimal reaction temperature of 40℃(Table 1). However, the yields of (R)-methyl mandelate under different pH varied dramatically. The highest yield from the enzymatic reaction was achieved at pH 6.0, indicating this is the optimal pH for the generation of chiral (R)-methyl mandelate with high optical purity and yield.
3.10 Effect of substrate concentrations on the optical purity and yield of (R)-methyl mandelateUnder optimal reaction temperature (40℃) and pH (6.0), different concentrations of substrate were tested to determine the ideal concentration of substrate methyl mandelate for the kinetic resolution of racemic methyl mandelate. High e. e. R of (R)-methyl mandelate was obtained when substrate (±)-methyl mandelate was added at a concentration of 5 mmol/L or 10 mmol/L (Table 1). A high yield of (R)-methyl mandelate was only achieved when 10 mmol/L substrate was added.
Therefore, the optimal working conditions for the production of chiral (R)-methyl mandelate with the highest optical purity and yield were found to be: 10 mmol/L (±)-methyl mandelate, pH 6.0 at 40℃ for 2 h.
3.11 Kinetic resolution of (±)-methyl mandelate through esterification and transesterification reactionsAlthough esterase BSE04211 can synthesize many short-chain ester products in organic solvents, we did not detect any methyl mandelate in the esterification reaction of racemic mandelic acid and methanol catalyzed by BSE04211. Additionally, BSE04211 was unable to catalyze the transesterification reactions of racemic methyl mandelate and vinyl acetate or butanol. Thus, chiral methyl mandelate could not be enzymatically prepared through esterification or transesterification reactions catalyzed by esterase BSE04211(Fig. 3).
4 DISCUSSIONIn our study, a novel microbial alkaline esterase, BES04211, was identified and further functionally characterized. Esterase BSE04211 can catalyze the formation of many short-chain esters in organic mediums such as hexane. However, BSE04211 could not catalyze the formation of methyl mandelate using mandelic acid and methanol as the substrates in organic solvents, possibly because the chemical structure of mandelic acid was too large to be accepted by BSE04211. We also did not detect the formation of expected products in the transesterification reaction of methyl mandelate and vinyl acetate or in the transesterification reaction of methyl methelate and butanol catalyzed by BSE04211, possibly because esterase BSE04211 could not catalyze transesterification reactions based upon methyl mandelate.
However, esterase BSE04211 can stereoselectively hydrolyze racemic methyl mandelate and generate (R)-methyl mandelate in aqueous reactions. The chiral product, (R)-methyl mandelate, was enzymatically generated from the hydrolysis of racemic methyl mandelate with an optical purity of 99.9% and a yield of nearly 90% under optimum temperature (40℃) and pH (6.0). No other organic solvents or additives were required to further improve the optical purity of chiral (R)-methyl mandelate.
A number of other studies have examined the kinetic resolution of racemic methyl mandelate using microbial lipases (Ma et al., 2013 ; Yao et al., 2013 ; Cao et al., 2014 ; Chen and Yang, 2014). However, the lipases used in these cases, Novezym 435 and one extracellular lipase, could only hydrolyze racemic methyl mandelate and generated (S)-methyl mandelate rather than (R)-methyl mandelate (Kim et al., 2000 ; Yadav and Sivakumar, 2004 ; Wei and Wu, 2008). The stereoselectivity of esterase BSE04211 in our study is opposite to that of the two lipases mentioned above in the hydrolysis of racemic methyl mandelate. The protein sequence of BSE04211 does not show high similarity to the reported protein sequence of lipase Novezym 435. Notably, the optical purity of (R)- methyl mandelate prepared by esterase BSE04211 was as high as 99.9%.
5 CONCLUSIONA novel microbial esterase, BSE04211, was heterologously expressed and further functionally characterized. BSE04211 can efficiently catalyze the hydrolysis of racemic methyl mendelate and generate (R)-methyl mandelate with an optical purity of 99.9% and a yield of nearly 90% under optimal reaction conditions. The direct hydrolysis of inexpensive racemic methyl mendelate by esterase BSE04211 in aqueous systems can potentially simplify the industrial production procedure of optically pure (R)- methyl mandelate.
6 ACKNOWLEDGEMENTWe are grateful for the help from the research groups of Professor JU Jianhua and Professor ZHANG Changsheng from South China Sea Institute of Oceanology of CAS.
| Arpigny J L, Jaeger K E, 1999. Bacterial lipolytic enzymes:classification and properties. Biochem. J., 343 (1) : 177 –183. Doi: 10.1042/bj3430177 |
| Banerjee A, Kaul P, Banerjee U C, 2006. Purification and characterization of an enantioselective arylacetonitrilase from Pseudomonas putida. Arch. Microbiol., 184 (6) : 407 –418. Doi: 10.1007/s00203-005-0061-9 |
| Cao Y, Wu S S, Li J H, Wu B, He B F, 2014. Highly efficient resolution of mandelic acid using lipase from Pseudomonas stutzeri LC2-8 and a molecular modeling approach to rationalize its enantioselectivity. J. Mol.Catal. B-Enzym., 99 : 108 –113. Doi: 10.1016/j.molcatb.2013.10.026 |
| Chen C S, Fujimoto Y, Girdaukas G, Sih C J, 1982. Quantitative analyses of biochemical kinetic resolutions of enantiomers. J. Am. Chem. Soc., 104 (25) : 7 294 –7 299. Doi: 10.1021/ja00389a064 |
| Chen P R, Yang W H, 2014. Kinetic resolution of mandelate esters via stereoselective acylation catalyzed by lipase PS-30. Tetrahedron Letters, 55 (14) : 2 290 –2 294. Doi: 10.1016/j.tetlet.2014.02.095 |
| Cherif S, Gargouri Y, 2010. An organic-solvent-tolerant esterase from turkey pharyngeal tissue. Bioresour.Technol., 101 (10) : 3 732 –3 736. Doi: 10.1016/j.biortech.2009.12.106 |
| Friedrich J L R, Peña F P, Garcia-Galan C, Fernandez-Lafuente R, Ayub M A Z, Rodrigues R C, 2013. Effect of immobilization protocol on optimal conditions of ethyl butyrate synthesis catalyzed by lipase B from Candida antarctica. J. Chem. Technol. Biotechnol.., 88 (6) : 1 089 –1 095. Doi: 10.1002/jctb.2013.88.issue-6 |
| Graebin N G, Martins A B, Lorenzoni A S G, Garcia-Galan C, Fernandez-Lafuente R, Ayub M A Z, Rodrigues R C, 2012. Immobilization of lipase B from Candida antarctica on porous styrene-divinylbenzene beads improves butyl acetate synthesis. Biotechnology Progress, 28 (2) : 406 –412. Doi: 10.1002/btpr.1508 |
| Gröger H, 2001. Enzymatic routes to enantiomerically pure aromatic α-hydroxy carboxylic acids:A further example for the diversity of biocatalysis. Adv. Synth. Catal., 343 (6-7) : 547 –558. Doi: 10.1002/1615-4169(200108)343:6/7<>1.0.CO;2-5 |
| Jin P, Pei X L, Du P F, Yin X P, Xiong X L, Wu H L, Zhou X L, Wang Q Y, 2012. Overexpression and characterization of a new organic solvent-tolerant esterase derived from soil metagenomic DNA. Bioresour. Technol., 116 : 234 –240. Doi: 10.1016/j.biortech.2011.10.087 |
| Kelley L A, Sternberg M J E, 2009. Protein structure prediction on the Web:a case study using the Phyre server. Nat.Protoc., 4 (3) : 363 –371. Doi: 10.1038/nprot.2009.2 |
| Kim B Y, Hwang K C, Song H S, Chung N, Bang W G, 2000. Optical resolution of RS-(±)-mandelic acid by Pseudomonas sp. Biotechnol. Lett., 22 (23) : 1 871 –1 875. Doi: 10.1023/A:1005649908991 |
| Kim K K, Song H K, Shin D H, Hwang K Y, Choe S, Yoo O J, Suh S W, 1997. Crystal structure of carboxylesterase from Pseudomonas fluorescens, an α/β hydrolase with broad substrate specificity. Structure, 5 (12) : 1 571 –1 584. Doi: 10.1016/S0969-2126(97)00306-7 |
| Kinbara K, Sakai K, Hashimoto Y, Nohira H, Saigo K, 1996. Design of resolving reagents:p-substituted mandelic acids as resolving reagents for 1-arylalkylamines. Tetrahedron:Asymmetr, 7 (6) : 1 539 –1 542. Doi: 10.1016/0957-4166(96)00175-9 |
| Lu G Q, Moriyama E N, 2004. Vector NTI, a balanced all-inone sequence analysis suite. Brief. Bioinform., 5 (4) : 378 –388. Doi: 10.1093/bib/5.4.378 |
| Ma J B, Wu L, Guo F, Gu J L, Tang X L, Jiang L, Liu J, Zhou J H, Yu H W, 2013. Enhanced enantioselectivity of a carboxyl esterase from Rhodobacter sphaeroides by directed evolution. Appl. Microbiol. Biotechnol., 97 (11) : 4 897 –4 906. Doi: 10.1007/s00253-012-4396-2 |
| Ollis D L, Cheah E, Cygler M, Dijkstra B, Frolow F, Franken S M, Harel M, Remington S J, Silman I, Schrag J, Sussman J L, Verschueren K H G, Goldman A, 1992. The α/β hydrolase fold. Protein Eng., 5 (3) : 197 –211. Doi: 10.1093/protein/5.3.197 |
| Sussman J L, Harel M, Frolow F, Oefner C, Goldman A, Toker L, Silman I, 1991. Atomic structure of acetylcholinesterase from Torpedo californica:a prototypic acetylcholinebinding protein. Science, 253 (5022) : 872 –879. Doi: 10.1126/science.1678899 |
| Tsuchiya S, Miyamoto K, Ohta H, 1992. Highly efficient conversion of (±)-mandelic acid to its (R)-(-)-enantiomer by combination of enzyme-mediated oxidation and reduction. Biotechnol. Lett., 14 (12) : 1 137 –1 142. Doi: 10.1007/BF01027017 |
| Wei H N, Wu B, 2008. Screening and immobilization Burkholderia sp. GXU56 lipase for enantioselective resolution of (R,S)-methyl mandelate. Appl. Biochem.Biotechnol., 149 (1) : 79 –88. |
| Xiao M T, Huang Y Y, Ye J, Guo Y H, 2008. Study on the kinetic characteristics of the asymmetric production of R-(-)-mandelic acid with immobilized Saccharomyces cerevisiae FD11b. Biochem. Eng. J., 39 (2) : 311 –318. Doi: 10.1016/j.bej.2007.10.002 |
| Yadav G D, Sivakumar P, 2004. Enzyme-catalysed optical resolution of mandelic acid via RS(∓)-methyl mandelate in non-aqueous media. Biochem. Eng. J., 19 (2) : 101 –107. Doi: 10.1016/j.bej.2003.12.004 |
| Yao C J, Cao Y, Wu S S, Li S, He B F, 2013. An organic solvent and thermally stable lipase from Burkholderia ambifaria YCJ01:Purification, characteristics and application for chiral resolution of mandelic acid. J. Mol. Catal. BEnzym, 85-86 : 105 –110. Doi: 10.1016/j.molcatb.2012.08.016 |
| Zhang Z J, Xu J H, He Y C, Ouyang L M, Liu Y Y, Imanaka T, 2010. Efficient production of (R)-(-)-mandelic acid with highly substrate/product tolerant and enantioselective nitrilase of recombinant Alcaligenes sp. Process Biochemistry, 45 (6) : 887 –891. Doi: 10.1016/j.procbio.2010.02.011 |
 2016, Vol. 34
2016, Vol. 34


