Institute of Oceanology, Chinese Academy of Sciences
Article Information
- ZHANG Xiaojing(张晓静), SONG Xiaoling(宋晓玲), HUANG Jie(黄倢)
- Impact of Vibrio parahaemolyticus and white spot syndrome virus (WSSV) co-infection on survival of penaeid shrimp Litopenaeus vannamei
- Chinese Journal of Oceanology and Limnology, 34(6): 1278-1286
- http://dx.doi.org/10.1007/s00343-016-5165-3
Article History
- Received Jun. 8, 2015
- accepted for publication Aug. 3, 2015
- accepted in principle Aug. 29, 2015
2 Qingdao National Laboratory for Marine Science and Technology, Qingdao 266200, China
Since the rapid development of the worldwide shrimp farming industry in the 1970s, China has gradually become a large shrimp-producing country. Litopenaeus vannamei is an important farmed shrimp species in China and throughout the world. However, frequent disease outbreaks have occurred in farmed shrimp in recent years, particularly diseases caused by viruses and bacteria, and these have resulted in significant economic losses to the shrimp farming industry (Inouye et al., 1994 ; Park et al., 1998 ; Robertson et al., 1998 ; Aguirre-Guzmán et al., 2001). White spot syndrome virus (WSSV) is a pathogen that is seriously threatening shrimp farming globally (Sánchez-Paz, 2010) by causing 100% shrimp mortality in 5-7 d (Chou et al., 1995); hence, WSSV is drawing more and more attention from researchers (Huang et al., 2013 ; Ahanger et al., 2014 ; Sun et al., 2014).
Vibrio is part of the normal flora present in shrimp and shrimp culture water, but some species are opportunistic pathogens (Saulnier et al., 2000). Once a farming environment becomes severely damaged, Vibrio may multiply rapidly and cause massive shrimp death (Jiravanichpaisal et al., 1994, Vandenberghe et al., 1998). Particularly common Vibrio species, such as V. alginolyticus (Liu et al., 2004a), V. harveyi (Karunasagar et al., 1994 ; Zhou et al., 2012), V. parahaemolyticus, etc.(Vandenberghe et al., 1999) are more pathogenic. Acute hepatopancreatic necrosis disease (AHPND) was first discovered in southern China in 2010 and has significantly affected shrimp farming throughout Asia (Lightner et al., 2012). Many studies have reported that V. parahaemolyticus is the AHPND pathogen (Zhang et al., 2012a ; Joshi et al., 2014).
Farmed shrimp are very commonly infected by a variety of pathogens. Manivannan et al.(2002) simultaneously detected (Selvin and Lipton, 2003) monodon baculovirus virus, hepatopancreatic parvolike virus, and WSSV from dying Penaeus monodon on an Indian farm. V. alginolyticus has also been isolated from shrimp suffering from white spot syndrome on the Southwest coast of India. It was proven that WSSV had weakened immune resistance of the shrimp, which caused a superimposed infection of V. alginolyticus, leading to massive shrimp mortality. Jang et al.(2014) used artificial infection to prove that co-infection of WSSV and V. anguillarum promotes death of L. vannamei. Phuoc et al.(2008) also demonstrated that WSSV infection stimulates proliferation of V. campbellii in L. vannamei and, hence, promotes mortality of penaeid shrimp. Thus, infection with multiple pathogens can cause massive death of penaeid shrimp. Previous studies have demonstrated that a virus as the leading pathogen can stimulate a secondary bacterial infection and accelerate death. Jayasree et al.(2006) described symptoms of penaeid shrimp induced by six species of pathogenic Vibrio. V. parahaemolyticus is the major pathogen causing red body disease, and shrimp with red body disease often have a WSSV infection, which could cause massive mortality.
The Toll-like receptor/nuclear factor-kappaB signaling pathway plays an important role in innate immunity in invertebrates and vertebrates. The Toll signaling pathway transduces the pathogen invasion signal into cells, leading to a series of intracellular cascades that induce expression of immune-related genes, including antimicrobial peptides. LvMyD88 is an important molecule in the Toll signaling pathway of L. vannamei (Medzhitov, 2001 ; Zhang et al., 2012b). Phosphatidylinositol 3-kinase (PI3K)-Akt is another important intracellular signaling pathway involved in the invasion of some viruses as well as their replication in the host (Ehrhardt et al., 2006 ; Esfandiarei et al., 2006). Akt is a key molecule in the PI3K-Akt signaling pathway. In the study, we investigated LvMyD88 and LvAkt gene expression levels to further explain mortality caused by V. parahaemolyticus and WSSV.
Whether co-infection of WSSV and V. parahaemolyticus affects the survival rate of L. vannamei and the cause of such an impact are current concerns of many scholars and farm workers. In this study, we infected L. vannamei with WSSV and V. parahaemolyticus using a feeding method and low-dose injection, respectively. We performed contrasting experiments in which either WSSV or V. parahaemolyticus were used to infect shrimp first, followed by the other agent. We then compared the experimentally infected groups to determine whether co-infection with both pathogens affected L. vannamei survival and to identify the possible causes.
2 MATERIAL AND METHOD 2.1 Experimental shrimpL. vannamei (body length, 9.40±0.45 cm; weight, 9.84±1.18 g) were purchased from the penaeid shrimp farm of Qingdao Baorong Aquatic Product Technological Co., Ltd.(Qingdao, China). A polymerase chain reaction (PCR) analysis indicated that the shrimp were WSSV-negative, and no signs of V. parahaemolyticus infection were detected in hemolymph, hepatopancreas, muscle, gills, or intestines. The experimental shrimp were cultured in 60-L storage boxes with continuous aeration. The water was changed once daily, and the shrimp were fed three times daily. Water temperature, salinity, and pH were maintained at 25-28℃, 30-31, and 7.6-8.1, respectively.
2.2 Pathogenic bacteria and virusesV. parahaemolyticus 20100612001 was isolated from V. parahaemolyticus -infected L. vannamei in our laboratory (Disease Control and Molecular Pathology Laboratory for Mariculture Organisms, Yellow Sea Fisheries Research Institute, Qingdao, China). Pathogenic WSSV-containing feed 20120713001 was prepared from muscle of WSSVinfected Penaeus japonicus stored in our laboratory. The virulent strains were stored at-80℃.
2.3 Experimental groupsAfter a 1-week acclimation period in the aquatic research laboratory, apparently healthy L. vannamei of uniform body length were divided randomly into seven groups, with five replicates/group and 20 shrimp/replicate. Among the replicates, three were used to calculate mortality, and two were used as samples. The groups were:(1) the V. parahaemolyticus (Vp)+WSSV group: shrimp were injected initially with V. parahaemolyticus and then fed the WSSVcontaining feed 48 h later;(2) the WSSV+Vp group: shrimp were initially fed the WSSV- containing feed and then injected with V. parahaemolyticus 48 h later;(3) the WSSV+PBS group: shrimp were initially fed the WSSV-containing feed and then injected with PBS (pH 7.2-7.4)48 h later, as a control for the WSSV+Vp group;(4) the WSSV group: shrimp were fed the WSSV-containing feed only;(5) the Vp group: shrimp were injected with V. parahaemolyticus only;(6) the PBS group: shrimp were injected with PBS only, as an injection control; and (7) the C group (blank control group). All groups were managed during the experiment under the same conditions as those used during acclimation.
2.4 Artificial infection with V. parahaemolyticus and WSSVV. parahaemolyticus 20100612001 was reactivated from storage at -80℃ and cultured in 2216E broth with shaking at 37℃ overnight. The culture was centrifuged at 5 000 r/min for 10 min to remove the supernatant, and the pellet was resuspended in sterile PBS to a density of 4.0×104 CFU/mL (maximum dose to infect shrimp without causing mortality determined in a preliminary experiment). The shrimp were infected by injecting 50 μL of the bacterial suspension into the second abdominal segment; the corresponding control group was injected with an equal volume of sterile PBS.
The WSSV-containing feed 20120713001 was removed from storage at-80℃ and cut into uniform pieces under cold sterile conditions. The infectious dose was 0.05 g/shrimp (as determined in a preliminary experiment). Feeding was stopped 1 day before the WSSV infection to maintain a fasted state to facilitate equal consumption of the WSSV-containing feed.
2.5 Determining the number of Vibrio in shrimp hemolymphThree shrimp were collected randomly 24 and 72 h after infection in the Vp and WSSV groups and 24 h after the second infection, i.e., 72 h after the initial infection, in the Vp+WSSV and WSSV+Vp groups and washed three or four times with sterile water. Hemolymph was withdrawn from the pericardial cavity using a 1-mL sterile disposable syringe under sterile conditions and added to an equal volume of sterile anticoagulant. The hemolymph was serially diluted 10-fold with sterile PBS; each dilution was spread on three thiosulfate citrate bile salt sucrose agar plates placed upside down in a 37℃ incubator and cultured for 16-20 h. Plates containing 30-300 bacterial colonies were counted, and the numbers were recorded.
2.6 Determining WSSV copy number in shrimp gill tissuesGill tissue (10 mg) was cut and collected from three shrimp harvested randomly from each group 0, 24, 48, 72, 96, 144, 192, and 264 h after feeding the Vp+WSSV, WSSV, and WSSV+Vp groups with the WSSV-containing feed. Genomic DNA was extracted from gill tissue using the TIANamp Marine Animal DNA Kit (Tiangen Biotech, Beijing, China). The WSSV copy number was detected using a real-time quantitative PCR method with the WSSV-forward primer, 5'-CTCTTGTGGTTCATCAGGGGC-3' and the WSSV-reverse primer, 5'-CTGGATTTTCTCTCAGGGTCTTAGT- 3'.
The reaction system (25 µL) contained 12.5 µL of SYBR ® Premix Ex TaqII (2×Tli RNaseH Plus)(TaKaRa Bio, Shiga, Japan), 0.5 µL forward primer (10 µmol/L), 0.5 µL reverse primer (10 µmol/L), 10.5 µL RNase-free water, and 1 µL template DNA (200-400 ng/ µL). A plasmid containing the WSSV target fragment was used as a standard to prepare the standard curve. The amplification program was one cycle of initial denaturation for 5 min at 95℃, 30 s denaturation at 95℃, 30 s annealing at 59℃, and 40 30-s extension cycles at 72℃.
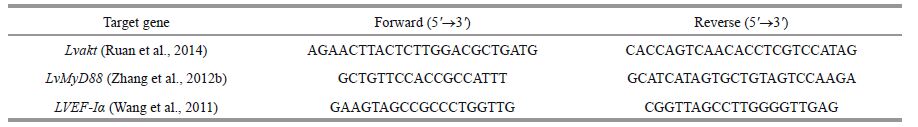
2.7 Determining LvMyD88 and Lvakt gene expression levelsThree shrimp were collected randomly from the Vp+WSSV, WSSV, and WSSV+Vp groups 0, 6, 12, 24, 48, and 96 h after the second infection. Gill tissue (50 mg) was harvested from each shrimp, frozen quickly in liquid nitrogen, and stored at -80℃.
2.7.1 RNA extraction and cDNA synthesisTotal RNA was isolated from gill tissues using TRIzol reagent (TaKaRa Bio., Dalian, China), according to the manufacturer's instructions. Total RNA optical density values were measured at 260 and 280 nm using the NanoDrop 2000c spectrophotometer (ThermoScientific, Waltham, MA, USA), and the samples were stored at -80℃ until further use. cDNA was synthesized from the stored total RNA using the PrimeScript ® RT Reagent Kit with the gDNAEraser (Perfect Real Time; TaKaRa, Dalian), according to the manufacturer's protocol.
2.7.2 Real-time PCR gene expression analysisTable 1 shows the primer sequences for the LvMyD88, Lvakt, and the internal EF- Ia control genes. The primers were synthesized by Shanghai Sangon Biological Engineering Co., Ltd.(Shanghai, China). Real-time PCR was performed using the SYBR ® Premix Ex Taq™ Kit (Tli RNaseHPlus; TaKaRa, Dalian) following the manufacturer's manual, on a Bio-rad Real-time CFX96 instrument (Bio-Rad, Hercules, CA, USA). The PCR reaction (20 µL) contained 10 µL 2× SYBR ® Premix Ex TaqII (Tli RNaseH Plus), 0.8 µL forward primer, 0.8 µL reverse primer, 6.8 µL RNase-free water, and 1.6 µL DNA template (500 ng/ µL). The amplification program was one 30-s cycle of initial denaturation at 95℃, 5-s denaturation at 95℃, and 40 30-s cycles of annealing at 60℃ extension. Relative gene expression levels were calculated using the 2 - ΔΔCt method (Livak and Schmittgen, 2001), as follows:
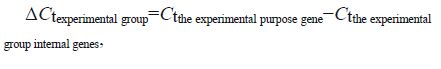
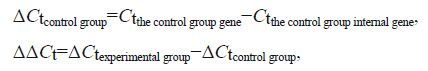
Gene expression levels=2 - ΔΔCt.
SPSS Statistics 17.0 software (SPSS Inc., Chicago, IL, USA) was used to perform the one-way analysis of variance. Duncan's method was used to conduct the multiple comparisons. A P-value<0.05 was considered significant.
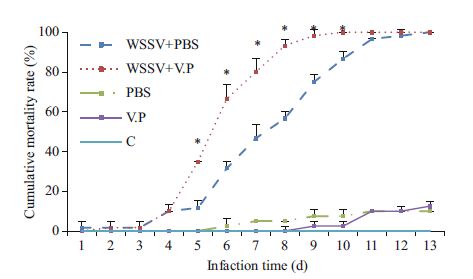
3 RESULT 3.1 Cumulative shrimp mortalityThe cumulative mortality rates of shrimp in the groups infected with WSSV first are shown in Fig. 1. At the end of the experiment, the cumulative mortality rates in the Vp, PBS, and C groups were 5.50%, 2.50%, and 0%, respectively, with no significant intergroup differences (P>0.05). The mortality rate of the WSSV+Vp group reached 100% on d 10 after WSSV infection, whereas that of the WSSV+PBS group approached 100% on d 13 after WSSV infection. No difference (P>0.05) in cumulative mortality rate was detected between the WSSV+Vp and WSSV+PBS groups during the first 4 d of the experiment. However, the cumulative mortality rate of the WSSV+Vp group was significantly higher than that of the WSSV+PBS group during d 5-10(P< 0.05).
|
| Figure 1 Cumulative mortality rate of Litopenaeus vannamei infected first with WSSV * indicates significant difference between groups at the same timepoint (P< 0.05). |
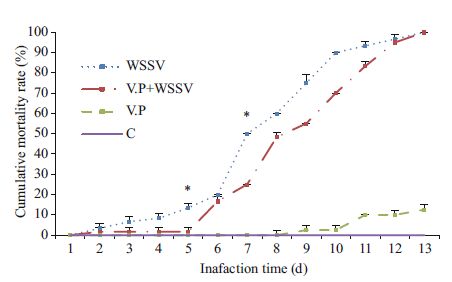
Figure 2 shows the cumulative mortality rate of each group of L. vannamei infected first with V. parahaemolyticus. Because the V. parahaemolyticus dose used in this experiment was the maximum dose that did not cause acute death, the beginning of the WSSV infection was recorded as d 1 in Fig. 2. The cumulative mortality of shrimp in the Vp+WSSV group reached 100% on d 11 after WSSV infection, whereas that of the WSSV group approached 100% on d 13 after WSSV infection. The mortality rate of the Vp+WSSV group was significantly higher than that of the WSSV group (P< 0.05) only on d 4 and 6 after WSSV infection.
|
| Figure 2 Cumulative mortality rates of Litopenaeus vannamei infected first with Vibrio parahaemolyticus * indicates significant difference between groups at same time-point (P< 0.05). |
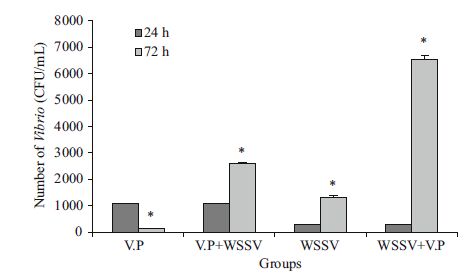
The numbers of Vibrio in the hemolymph of shrimp in the experimental groups are shown in Fig. 3. After infection with V. parahaemolyticus for 24 h, the number of Vibrio in L. vannamei hemolymph was as high as 107 CFU/mL. The number of Vibrio in the Vp group decreased significantly after 72 h (P< 0.05), whereas that in the Vp+WSSV group increased significantly (P< 0.05). The number of Vibrio in shrimp hemolymph was 107 CFU/mL 24 h after the WSSV infection. The numbers of Vibrio in the WSSV and WSSV+Vp groups increased significantly after 72 h, (P< 0.05), and that in the latter group was significantly higher than that in the former group (P< 0.05).
|
| Figure 3 Numbers of Vibrio in the hemolymph of shrimp in the Vibrio parahaemolyticus (Vp), white spot syndrome virus (WSSV), Vp+WSSV, and WSSV+Vp groups * indicates significant difference (P< 0.05). |
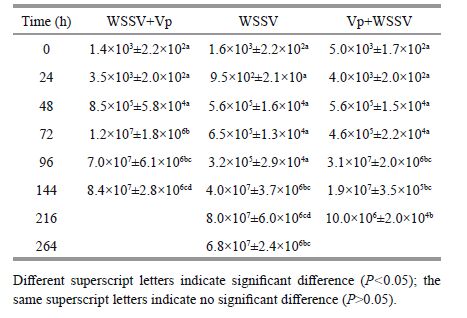
WSSV copy numbers in the gills of shrimp in the experimental groups are shown in Table 2. WSSV copy numbers in the gill tissues of L. vannamei 0, 24, and 48 h after WSSV infection were not different (P>0.05). WSSV copy number in the WSSV+Vp group increased rapidly from 105 to 107 copies/mg tissue at 72 h, which was significantly higher than that in the other two groups (P< 0.05). WSSV copy number in the Vp+WSSV group increased quickly from 105 to 107 copies/mg tissue at 96 h, which was significantly higher than that in the WSSV group.
|
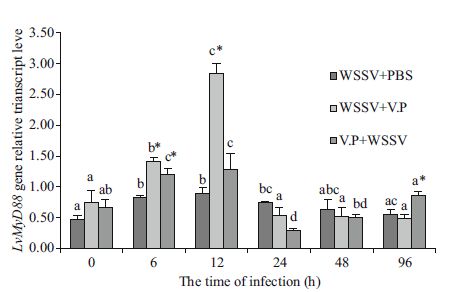
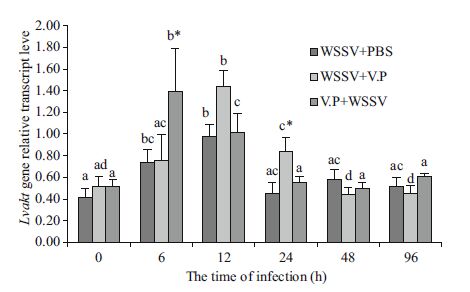
The LvMyD88 and Lvakt gene expression changes in L. vannamei gills were detected by real-time quantitative RT-PCR (Figs. 4 and 5). The LvMyD88 gene expression level in the WSSV+PBS group increase significantly beginning at 6 h (P< 0.05) and reached the maximum at 12 h (1.89 times baseline level at 0 h); it then started to decline and returned to the baseline level at 96 h. The Lvakt gene expression level increased significantly beginning at 6 h (P< 0.05) and reached the maximum at 12 h (2.33 times baseline level at 0 h) and then returned to baseline at 24 h and did not change thereafter (P> 0.05). The LvMyD88 gene expression level in the WSSV+Vp group increased significantly at 6 h (P< 0.05), reached the maximum at 12 h (3.79 times baseline level at 0 h) and then returned to baseline at 24 h, without any changes thereafter (P> 0.05). The Lvakt gene expression level started to rise gradually after the V. parahaemolyticus injection, increased significantly to the maximum at 12 h (2.40 times baseline level at 0 h), and returned to baseline at 24 h, without any changes thereafter (P> 0.05). The LvMyD88 gene expression level in the Vp+WSSV group increased significantly at 6 h (P< 0.05), reached the maximum at 12 h (1.91 times baseline level at 0 h), and then declined to baseline at 24 h, exhibiting no changes thereafter (P> 0.05). Similarly, Lvakt gene expression level increased significantly at 6 h (P< 0.05), reached the maximum rapidly (2.73 times baseline level at 0 h), and then started to decline and returned to baseline at 24 h, exhibiting no changes thereafter (P> 0.05).
|
| Figure 4 LvMD88 gene expression levels in the gill tissues of L. vannamei from the different experimental groups Different superscript letters indicate a significant difference (P< 0.05), whereas the same superscript letters indicate no significant difference (P> 0.05). * indicates significant difference at the same time-point (P< 0.05). |
|
| Figure 5 Lvakt gene expression levels in the gill tissues of L. vannamei from the different experimental groups Different superscript letters indicate significant difference (P< 0.05), whereas the same superscript letters indicate no significant difference (P> 0.05). * indicates significant difference at the same time-point (P< 0.05). |
Vibrio species are opportunistic pathogens and are predominant bacteria in farmed shrimp and culture water under normal conditions (Oxley et al., 2002 ; Hatha et al., 2005). Viruses are usually the main pathogens infecting shrimp and decrease shrimp immunity, but superimposed bacterial infections accelerate shrimp mortality (Lightner et al., 1983). The V. parahaemolyticus 20100612001 used in this study was isolated from V. parahaemolyticus -infected L. vannamei, and a preliminary experiment determined that 107 CFU/mL was the maximal dose of this V. parahaemolyticus strain that did not result in acute death of infected L. vannamei. WSSV 20120713001 was obtained from preserved muscle of WSSVinfected P. japonicus, which was proven highly pathogenic to L. vannamei in a preliminary experiment. We used the highly pathogenic V. parahaemolyticus to conduct the low-dose infection, and WSSV was employed as the main causative viral pathogen. The number of Vibrio in the hemolymph of shrimp in the Vp group decreased gradually, whereas the numbers of Vibrio in the hemolymph of shrimp in the Vp+WSSV and WSSV+Vp groups increased significantly. Healthy penaeid shrimp can clear Vibrio (Martin et al., 1993 ; Van de Braak et al., 2002 ; Liu et al., 2004b), but these bacteria may take advantage of shrimp with a damaged immune system to proliferate and cause massive death. Infection with a low dose of Vibrio does not destroy the shrimp's immune system; therefore, the number of V. parahaemolyticus in shrimp hemolymph decreased gradually. However, the subsequent WSSV infection damaged the immune system, resulting in rapid proliferation of the residual Vibrio in the shrimp, thereby accelerating mortality. The WSSV copy numbers in the gill tissues of shrimp in the WSSV+Vp, Vp+WSSV, and WSSV groups increased from 107 to 107 copies/mg tissue at 72, 96, and 144 h, respectively. The number of Vibrio in the hemolymph of the Vp+WSSV group was significantly higher at 72 h than that in shrimp infected with WSSV alone. A latent WSSV infection does not cause acute mortality of shrimp, but massive death ensues when the WSSV copy number reaches a particular level (depending on shrimp species, feeding environment, etc.)(Meng et al., 2010 ; Sun et al., 2013), possibly due to the reciprocally accelerated proliferation of both WSSV and V. parahaemolyticus. Jang et al.(2014) reported that co-infection with V. anguillarum and WSSV accelerates death of penaeid shrimp because of the rapid proliferation of WSSV in vivo. Phuoc et al.(2009) and Oxley et al.(2002) reported that infecting shrimp with WSSV first paralyzes the defense system, allowing V. campbellii to proliferate, leading to accelerated death, and that the synergy between Vibrio and WSSV is associated with the number, species, and method of Vibrio infection.
Zhang et al.(2012b) first described the LvMyD88 gene in L. vannamei and reported that LvMyD88 is expressed ubiquitously in all shrimp tissues, but at a higher level in gill. They also showed that LvMyD88 expression decreases significantly 4 and 8 h after V. parahaemolyticus infection, but then increases significantly after 12 h and slowly returns to normal level thereafter; however, the LvMyD88 expression level was consistently higher after infection with WSSV than that observed normally. In our study, LvMyD88 expression levels in the WSSV+Vp and WSSV+PBS groups increased gradually at 0-12 h and peaked at 12 h, which was significantly higher than that in the control. LvMyD88 expression level in the WSSV+Vp group continued to increase after infection with V. parahaemolyticus and did not decrease before 12 h, which was inconsistent with the results of Zhang et al.(2012b), probably due to the effect of the pre-established WSSV infection on host gene expression in our study. Although the LvMyD88 expression level in the Vp+WSSV group also peaked at 12 h, the level was significantly lower than that in the WSSV+Vp group. Li et al.(2014) reported that activating the NF-κB pathway promotes replication of WSSV and host immunity against the viral infection. However, excess activation of the immune system can damage the host (Mogensen, 2009). The high expression of LvMyD88, rapid replication of WSSV, and high mortality rate of L. vannamei in the WSSV+Vp group of our study agree with these views.
Ruan et al.(2014) first reported the Lvakt gene in L. vannamei and showed that this gene is expressed ubiquitously in all shrimp tissues, with the gill displaying the highest level. In our study, Lvakt gene expression level was upregulated early after WSSV infection, reached the maximum at 12 h, and returned to a normal level thereafter. We also showed that Lvakt gene expression levels in the WSSV+Vp and WSSV+PBS groups increased gradually at 0-12 h and peaked at 12 h, which may have been due to WSSV replication promoted by V. parahaemolyticus or an effect of V. parahaemolyticus. Lvakt gene expression was upregulated rapidly in the Vp+WSSV group, reached the maximum at 6 h, and then declined to the normal level, which was probably caused by the promoting effect of the preexisting V. parahaemolyticus infection on the WSSV infection, resulting in earlier expression of the Lvakt gene.
The following conclusions can be drawn from our results:(1) when L. vannamei was infected by WSSV, the superimposed V. parahaemolyticus infection significantly accelerated their death;(2) the superimposed WSSV infection of L. vannamei infected first with low dose V. parahaemolyticus did not significantly accelerate death of the shrimp;(3) when L. vannamei was infected with a low dose of V. parahaemolyticus under a well-maintained feeding environment, the number of Vibrio in the shrimp decreased gradually. However, once infected with WSSV, the number of Vibrio increased significantly, and the secondary V. parahaemolyticus infection increased the number of Vibrio dramatically;(4) the secondary V. parahaemolyticus infection of WSSVinfected L. vannamei accelerated proliferation of WSSV in vivo, whereas the superimposed infection of V. parahaemolyticus -infected L. vannamei with WSSV also accelerated proliferation of WSSV in L. vannamei ;(5) infecting V. parahaemolyticus -infected shrimp with WSSV or infecting WSSV-infected shrimp with V. parahaemolyticus promoted expression of the LvMyD88 and Lvakt genes, suggesting a possible mutual promoting effect of these pathogens during infection of L. vannamei.
| Aguirre-Guzmán G, Vázquez-Juárez R, Ascencio F, 2001. Differences in the susceptibility of American white shrimp larval substages (Litopenaeus vannamei) to Four Vibrio Species. Journal of Invertebrate Pathology, 78 (4) : 215 –219. Doi: 10.1006/jipa.2001.5073 |
| Ahanger S, Sandaka S, Ananad D, Mani M K, Kondadhasula R, Reddy C S, Marappan M, Valappil R K, Majumdar K C, Mishra R K, 2014. Protection of shrimp Penaeus monodon from WSSV infection using antisense constructs. Marine Biotechnology, 16 (1) : 63 –73. Doi: 10.1007/s10126-013-9529-9 |
| Chou H Y, Huang C Y, Wang C H, Chiang H C, Lo C F, 1995. Pathogenicity of a baculovirus infection causing white spot syndrome in cultured penaeid shrimp in Taiwan. Diseases of Aquatic Organisms, 23 (3) : 165 –173. |
| Ehrhardt C, Marjuki H, Wolff T, Nürnberg B, Planz O, Pleschka S, Ludwig S, 2006. Bivalent role of the phosphatidylinositol-3-kinase (PI3K) during influenza virus infection and host cell defenscs. Cellular Microbiology, 8 (8) : 1 336 –1 348. Doi: 10.1111/cmi.2006.8.issue-8 |
| Esfandiarei M, Suarez A, Amaral A, Si X N, Rahmani M, Dedhar S, McManus B M, 2006. Novel role for integrinlinked kinase in modulation of coxsackievirus B3 replication and virus-induced cardiomyocyte injury. Circulation Research, 99 (4) : 354 –361. Doi: 10.1161/01.RES.0000237022.72726.01 |
| Hatha A A M, Suluja T, Rahiman K M M, Thomas A P, 2005. Bacterial flora associated with the larval rearing environment and larvae of giant freshwater prawn Macrobrachium rosenbergii. Fishery Technology, 42 (2) : 16 931 . |
| Huang Z J, Kang S T, Leu J H, Chen L L, 2013. Endocytic pathway is indicated for white spot syndrome virus(WSSV) entry in shrimp. Fish & Shellfish Immunology, 35 (3) : 707 –715. |
| Inouye K, Miwa S, Oseko N, Nakano H, Kimura T, Momoyama K, Hiraoka M, 1994. Mass mortalities of cultured kuruma shrimp Penaeus japonicus in Japan in 1993:electron microscopic evidence of the causative virus. Fish Pathology, 29 (2) : 149 –158. Doi: 10.3147/jsfp.29.149 |
| Jang I K, Qiao G, Kim S K, 2014. Effect of multiple infections with white spot syndrome virus and Vibrio anguillarum on Pacific white shrimp Litopenaeus vannamei (L. ):mortality and viral replication. Journal of Fish Diseases, 37 (10) : 911 –920. |
| Jayasree L, Janakiram P, Madhavi R, 2006. Characterization of Vibrio spp. associated with diseased shrimp from culture ponds of Andhra Pradesh (India). Journal of the World Aquaculture Society, 37 (4) : 523 –532. |
| Jiravanichpaisal P, Miyazaki T, Limsuwan C, 1994. Histopathology, biochemistry, and pathogenicity of Vibrio harveyi infecting black tiger prawn Penaeus monodon. Journal of Aquatic Animal Health, 6 (1) : 27 –35. Doi: 10.1577/1548-8667(1994)006<0027:HBAPOV>2.3.CO;2 |
| Joshi J, Srisala J, Truong V H, Chen I T, Nuangsaeng B, Suthienkul O, Lo C F, Flegel T W, Sritunyalucksana K, Thitamadee S, 2014. Variation in Vibrio parahaemolyticus isolates from a single Thai shrimp farm experiencing an outbreak of acute hepatopancreatic necrosis disease(AHPND). Aquaculture, 428-429 : 297 –302. Doi: 10.1016/j.aquaculture.2014.03.030 |
| Karunasagar I, Pai R, Malathi G R, Karunasagar I, 1994. Mass mortality of Penaeus monodon larvae due to antibioticresistant Vibrio harveyi infection. Aquaculture, 128 (3-4) : 203 –209. Doi: 10.1016/0044-8486(94)90309-3 |
| Li C Z, Chen Y X, Weng S P, Li S D, Zuo H L, Yu X Q, Li H Y, He J G, Xu X P, 2014. Presence of Tube isoforms in Litopenaeus vannamei suggests various regulatory patterns of signal transduction in invertebrate NF-κB pathway. Developmental & Comparative Immunology, 42 (2) : 174 –185. |
| Lightner D V, Redman R M, Bell T A, 1983. Observations on the geographic distribution, pathogenesis and morphology of the baculovirus from Penaeus monodon Fabricius. Aquaculture, 32 (3-4) : 209 –233. Doi: 10.1016/0044-8486(83)90220-X |
| Lightner D V, Redman R M, Pantoja C R, Noble B L, Tran L, 2012. Early mortality syndrome affects shrimp in Asia. Global Aquaculture Advocate, 1 (15) : 40 . |
| Liu C H, Cheng W, Hsu J P, Chen J C, 2004a. Vibrio alginolyticus infection in the white shrimp Litopenaeus vannamei confirmed by polymerase chain reaction and 16S rDNA sequencing. Diseases of Aquatic Organisms, 61 (1-2) : 169 –174. |
| Liu C H, Yeh S T, Cheng S Y, Chen J C, 2004b. The immune response of the white shrimp Litopenaeus vannamei and its susceptibility to Vibrio infection in relation with the moult cycle. Fish & Shellfish Immunology, 16 (2) : 151 –161. |
| Livak K J, Schmittgen T D, 2001. Analysis of relative gene expression data using real-time quantitative PCR and the 2t-ΔΔC method. Methods, 25 (4) : 402 –408. Doi: 10.1006/meth.2001.1262 |
| Manivannan S, Otta S K, Karunasagar I, Karunasagar I, 2002. Multiple viral infection in Penaeus monodon shrimp postlarvae in an Indian hatchery. Diseases of Aquatic Organisms, 48 (3) : 233 –236. |
| Martin G G, Poole D, Poole C, Hose J E, Arias M, Reynolds L, Mckrell N, Whang A, 1993. Clearance of bacteria injected into the hemolymph of the penaeid shrimp, Sicyonia ingentis. Journal of Invertebrate Pathology, 62 (3) : 308 –315. Doi: 10.1006/jipa.1993.1118 |
| Medzhitov R, 2001. Toll-like receptors and innate immunity. Nature Reviews Immunology, 1 (2) : 135 –145. Doi: 10.1038/35100529 |
| Meng X H, Jang I K, Seo H C, Cho Y R, 2010. A TaqMan realtime PCR assay for survey of white spot syndrome virus(WSSV) infections in Litopenaeus vannamei postlarvae and shrimp of farms in different grow-out seasons. Aquaculture, 310 (1-2) : 32 –37. Doi: 10.1016/j.aquaculture.2010.10.010 |
| Mogensen T H, 2009. Pathogen recognition and inflammatory signaling in innate immune defenses. Clinical Microbiology Reviews, 22 (3) : 240 –273. |
| Oxley A A, Shipton W, Owens L, McKay D, 2002. Bacterial flora from the gut of the wild and cultured banana prawn, Penaeus merguiensis. Journal of Applied Microbiology, 93 (2) : 214 –223. Doi: 10.1046/j.1365-2672.2002.01673.x |
| Park J H, Lee Y S, Lee S, Lee Y, 1998. An infectious viral disease of penaeid shrimp newly found in Korea. Diseases of Aquatic Organisms, 34 (1) : 71 –75. |
| Phuoc L H, Corteel M, Nauwynck H J, Pensaert M B, AldaySanz V, Van den Broeck W, Sorgeloos P, Bossier P, 2008. Increased susceptibility of white spot syndrome virusinfected Litopenaeus vannamei to Vibrio campbellii. Environmental Microbiology, 10 (10) : 2 718 –2 727. Doi: 10.1111/j.1462-2920.2008.01692.x |
| Phuoc L H, Corteel M, Thanh N C, Nauwynck H, Pensaert M, Alday-Sanz V, Van den Broeck W, Sorgeloos P, Bossier P, 2009. Effect of dose and challenge routes of Vibrio spp. on co-infection with white spot syndrome virus in Penaeus vannamei. Aquaculture, 290 (1-2) : 61 –68. |
| Robertson P A W, Calderon J, Carrera L, Stark J R, Zherdmant M, Austin B, 1998. Experimental Vibrio harveyi infections in Penaeus vannamei larvae. Diseases of Aquatic Organisms, 32 (2) : 151 –155. |
| Ruan L W, Liu R D, Xu X, Shi H, 2014. Molecular cloning and characterization of a threonine/serine protein kinase lvakt from Litopenaeus vannamei. Chinese Journal of Oceanology and Limnology, 32 (4) : 792 –798. Doi: 10.1007/s00343-014-3295-z |
| Sánchez-Paz A, 2010. White spot syndrome virus:an overview on an emergent concern. Veterinary Research, 41 (6) : 43 . Doi: 10.1051/vetres/2010015 |
| Saulnier D, Haffner P, Goarant C, Levy P, Ansquer D, 2000. Experimental infection models for shrimp vibriosis studies:a review. Aquaculture, 191 (1-3) : 133 –144. Doi: 10.1016/S0044-8486(00)00423-3 |
| Selvin J, Lipton A P, 2003. Vibrio alginolyticus associated with white spot disease of Penaeus monodon. Diseases of Aquatic Organisms, 57 (1-2) : 147 –150. |
| Sun Y M, Li F H, Sun Z, Zhang X J, Li S H, Zhang C S, Xiang J H, 2014. Transcriptome analysis of the initial stage of acute WSSV infection caused by temperature change. PloS One, 9 (3) : e90732 . Doi: 10.1371/journal.pone.0090732 |
| Sun Y M, Li F H, Xiang J H, 2013. Analysis on the dynamic changes of the amount of WSSV in Chinese shrimp Fenneropenaeus chinensis during infection. Aquaculture, 376 (376-379) : 124 –132. |
| Van de Braak C B T, Botterblom M H A, Taverne N, van Muiswinkel W B, Rombout J H W M, van der Knaapa W W, 2002. The roles of haemocytes and the lymphoid organ in the clearance of injected Vibrio bacteria in Penaeus monodon shrimp. Fish & Shellfish Immunology, 13 (4) : 293 –309. |
| Vandenberghe J, Li Y, Verdonck L, Li J, Sorgeloos P, Xu H S, Swings J, 1998. Vibrios associated with Penaeus chinensis(Crustacea:Decapoda) larvae in Chinese shrimp hatcheries. Aquaculture, 169 (1-2) : 121 –132. Doi: 10.1016/S0044-8486(98)00319-6 |
| Vandenberghe J, Verdonck L, Robles-Arozarena R, Rivera G, Bolland A, Balladares M, Gomez-Gil B, Calderon J, Sorgeloos P, Swings J, 1999. Vibrios associated with Litopenaeus vannamei larvae, postlarvae, broodstock, and hatchery probionts. Applied and Environmental Microbiology, 65 (6) : 2 592 –2 597. |
| Wang P H, Wan D H, Gu Z H, Deng X X, Weng S P, Yu X Q, He J G, 2011. Litopenaeus vannamei tumor necrosis factor receptor-associated factor 6 (TRAF6) responds to Vibrio alginolyticus and white spot syndrome virus(WSSV) infection and activates antimicrobial peptide genes. Developmental & Comparative Immunology, 35 (1) : 105 –114. |
| Zhang B C, Liu F, Bian H H, Liu J, Pan L Q, Huang J, 2012a. Isolation, identification, and pathogenicity analysis of a Vibrio parahaemolyticus strain from Litopenaeus vannamei. Progress in Fishery Sciences, 33 (2) : 56 –62. |
| Zhang S, Li C Z, Yan H, Qiu W, Chen Y G, Wang P H, Weng S P, He J G, 2012b. Identification and function of myeloid differentiation factor 88 (MyD88) in Litopenaeus vannamei. PLoS One, 7 (10) : e47038 . Doi: 10.1371/journal.pone.0047038 |
| Zhou J F, Fang W H, Yang X L, Zhou S, Hu L L, Li X C, Qi X Y, Su H, Xie L Y, 2012. A nonluminescent and highly virulent Vibrio harveyi strain is associated with "bacterial white tail disease" of Litopenaeus vannamei shrimp. PloS One, 7 (2) : e29961 . Doi: 10.1371/journal.pone.0029961 |
 2016, Vol. 34
2016, Vol. 34