Institute of Oceanology, Chinese Academy of Sciences
Article Information
- LIU Liming(刘立明), DU Rongbin(杜荣斌), ZHANG Xiaoling(张晓凌), DONG Shuanglin(董双林), SUN Shichun(孙世春)
- Succession and seasonal variation in epilithic biofilms on artificial reefs in culture waters of the sea cucumber Apostichopus japonicus
- Chinese Journal of Oceanology and Limnology, 35(1): 132-152
- http://dx.doi.org/10.1007/s00343-016-5205-z
Article History
- Received Jul. 21, 2015
- accepted for publication Sep. 28, 2015
- accepted in principle Nov. 18, 2015
2 Ocean School, Yantai University, Yantai 264005, China
In natural aquatic environments, submerged surfaces of substrata are readily colonized by periphyton (Callow, 1986 ; Cooksey and Wigglesworth-Cooksey, 1995), a mixture of microbes, fungi, algae, and nonliving detrital materials. Periphyton attaches to various substrata in the photic zones of aquatic systems (Wetzel, 1983). The numerous habitat types comprised of periphyton, such as epipelon, epilithon and epiphyton (Lane et al., 2003), are of both ecological and industrial significance (Ford et al., 1989). Epilithic biofilms are an important component of various aquatic habitats, including streams, rivers, lakeshores and the littoral zone of oceans. Many studies have examined the succession of taxonomical composition in epilithon communities, including microorganisms and benthic macroinvertebrates. Microalgae, mostly benthic diatoms, are generally regarded as the principal constituent of the primary colonizers in epilithon communities, and thus the colonization and succession of microalgae have been well studied (Hoagland et al., 1982 ; Korte and Blinn, 1983 ; Liess and Hillebrand, 2004). Three dimensional microalgal succession was also observed in biofilms on artificial substrate using scanning electron microscopy (Hudon and Bourget, 1981 ; Korte and Blinn, 1983 ; Wu et al., 1999). Since variations in aquatic environmental conditions affect species composition and quantity, epilithic diatoms are usually the favoured community for monitoring water quality (Kelly et al., 1998).
Studies on succession in freshwater epilithic biofilms have been conducted in lotic systems, where water movement is likely to influence colonization, as well as in undisturbed lentic freshwater systems (Sekar et al., 2004). Studies performed in brackish water have frequently focused on the natural marine intertidal zone, which is affected by tide scour. However, few studies have been performed on the succession of epilithic communities in mariculture waters.
Epilithic algae and phytoplankton are principal contributors to algal productivity in the natural aquatic environment. It has been reported that the productivity of attached algae in the littoral zone of freshwater habitats may equal or exceed that of planktonic algae (Wetzel, 1964 ; Hoagland et al., 1993). Although many studies have addressed the productivity and distribution of phytoplankton, few studies have focused on epilithic algae (Ishida et al., 2006). Periphytic biofilms on substrates could be used for improving water quality in enriched brackish aquaculture shrimp ponds (Khatoon et al., 2007). It was also reported that shrimp reared in the presence of artificial substrates achieved significantly higher survival, growth and total biomass, and thus biofilms were suggested to be an additional food source for the reared animals (Ballester et al., 2007). However, studies on the biomass variations of epilithic biofilms on artificial reefs are still very limited. This is particularly important, in light of their essential role for some cultured aquatic animals, such as sea cucumbers.
Commercial farming of sea cucumbers in the Asia- Pacific region, particularly in China, has developed rapidly over the past two decades (Battaglene et al., 1999 ; Hamel et al., 2003 ; Chang et al., 2006 ; Sui et al., 2010). Artificial reefs in sea cucumber culture waters not only provide shelters (Dong et al., 2010) but also biofilms containing various kinds of algae, other microorganisms and nonliving detritus that can be the food resource for the animals. Sea cucumbers exhibit both benthos- and deposit-feeding habits, as well as seasonal growth characteristics (Yang et al., 2005 ; Slater and Jeffs, 2010). It is thus important to study temporal variations in epilithic biofilms in culture waters. The Homey Company, Rongcheng, Shandong Province, China, is one of the biggest sea cucumber farms for Apostichopus japonicus(Selenka), in China. In just one autumn harvest season, the yield of sea cucumbers in this farm can reach (2.0-2.5)×106 kg in 2 500-3 000 ha. culture waters. In this farm, sea cucumbers only feed on benthos and particulate matter sinking through the water column. The benthos taken by sea cucumbers is mostly composed of periphyton and the sedimentation of phytoplankton. The latter could provide an important natural food source for the animal (Ren et al., 2012), and is a major source of organic deposit. Therefore, studies on the growth and development of phytoplankton and periphyton, and their relationships to environmental factors are important for understanding the biological processes in sea cucumber culture waters and managing such aquaculture ecosystems.
In sea cucumber culture waters at this farm, seasonal fluctuations in the phytoplankton flora have been reported (Ren et al., 2010), however such variation in the periphyton community remains unknown. In 2008-2009, we conducted experiments at the farm to investigate the succession and seasonal variation of epilithon communities on artificial substrata. The aims were (1) to gain basic knowledge of the development and succession of epilithic periphyton in sea cucumber culture waters, including community composition, production in terms of biomass and succession process, and it's relationships to environmental conditions, and (2) to provide primary reference data for the management of sea cucumber culture systems, particularly for optimizing the natural food composition in A. japonicus farm waters.
2 MATERIAL AND METHOD 2.1 Description of study siteThe study was conducted in a sea cucumber culture pond (36°55′N, 122°10′E) at the aforementioned Homey Company from May 2008 to May 2009. The pond is about 2 hectares (100 m×200 m) in area and 2 m in depth, with an average water depth of 1.5 m. The seawater within the pond was exchanged regularly by floodtides, about 40%-60% on each occasion. Sea cucumbers and other co-cultured animals were reared in 0.5-cm net enclosures (Ren et al., 2012). To avoid potential grazing and disturbance on the biofilms by the cultured animals, the present experiments were conducted outside the enclosures.
2.2 Year round succession of biofilmThe colonization and succession of biofilms were studied using granite (a commonly used material for artificial reefs in sea cucumber culture) blocks (5 cm×3 cm×1.2 cm) as test panels. The panels were attached vertically onto three 45 cm×35 cm×0.5 cm PVC plates, each fixed horizontally on a polyethylene (PE) plastic basket, and settled on the bottom of the pond. The panels were about 20 cm above the bottom of the pond with the test sides oriented south. In May 2008, 126 test panels were immersed, with the experiment conducted until May 2009.
During the experimental period, panels were randomly sampled on a monthly basis. On each sampling date, nine panels (three from each basket) were usually retrieved, among which three (replicated) panels were used for identifying species and determining the densities of each species, three for measuring the dry weight (DW) and ash free dry weight (AFDW), and the other three for analysing Chlorophyll- a and phaeophytin. In view of the lower biomass, eighteen panels (double quantity) were retrieved for the initial two sampling dates, and twice the number of panels were adopted for each replicate. The panels were rinsed with filtered pond water to remove loosely attached planktonic forms. The biofilm on the sun-facing side (5 cm×3 cm) of the panel was gently scraped with a surgical knife and a soft nylon brush and made up to a 50-mL volume using granule free seawater.
The samples used for species identification and density determination were preserved in 1.5% Lugol's solution, and then observed in a Nanoplankton counting chamber (2 cm×2 cm in area and 0.1 cm 3 in volume) under a light microscope (Nikon ® E100, Nikon Corporation, Tokyo, Japan). Only diatom density was determined, and the relative abundances for other organisms were roughly estimated due to difficulties in separating and counting. When necessary, diatom samples were cleaned of organic matter by boiling samples in concentrated nitric and sulphuric acids, followed by rinsing with distilled water. The algal density and species composition were analysed, following the method of Sekar et al.(2004). Identification of algae was performed, mainly according to the description by Jin et al.(1982, 1992), Tseng (1983), Williams and Round (1986) and Cheng and Gao (2012, 2013). The main algal species were photographed using a light microscope (Olympus BX51) adapted with an Olympus DP72 CCD, in order that the species identification could be referred by later studies.
The samples for DW and AFDW measurements were filtered through pre-weighed 0.45-μm Whatman ® GF/C filters treated by ignition at 500℃ for 4 h. The filters were then dried at 65℃ in a drying oven until constant weight (i.e. the DW, which was determined by a METTLER TOLEDO ® XP6U ultra-microbalance, Mettler-Toledo International Inc., Zurich, Switzerland). After being treated in a furnace at 500℃ for a minimum of 6 h, the filters were weighed again and the ash free dry weight (AFDW) was obtained (American Public Health Association, 1992).
The samples for Chlorophyll- a and phaeophytin analysis were filtered through 0.45-μm Whatman® GF/C filters and the Chlorophyll- a was extracted overnight in 90% acetone in a dark refrigerator (4℃). The quantities of Chlorophyll- a and phaeophytin in the acetone extracts were measured with a Trilogy® Laboratory Fluorometer (Tuner Designs Company, Sunnyvale, CA, USA).
2.3 Seasonal variation in biofilm developmentSeasonal changes in biofilm colonization and growth were studied by the immersion of granite blocks as test panels in July (summer) and October (autumn)2008, and January (winter) and April (spring)2009. The panels were set in the pond, as previously described. For each season, 18 panels (5 cm×3 cm×1.2 cm) were immersed to identify species and determine species density, measure the DW and AFDW of biofilms, and analyse the Chlorophyll- a and phaeophytin (3 replicates, 2 panels for 1 sample), as described in the above experiment. Considering the recommended incubation period of one to two months for diatoms on artificial substrata (Tuchman and Blinn, 1979 ; Chessman, 1985 ; Robinson and Rushforth, 1987 ; Fairchild and Sherman, 1992 ; Kutka and Richards, 1996), and the minimum period of 4 weeks' incubation recommended by Kelly et al.(1998), an incubation time of 6 weeks was adopted. In this experiment, panel immersion was conducted at the beginning of the abovementioned months, and the test panels were retrieved in the middle of the following months. The biofilms on the test panels were treated and analysed following the same methods as described for the above experiment.
2.4 Physico-chemical analysis for pond waterthe test panels using a 2.5-L depth locating organic glass water sampler (WB-PM). Samples were taken approximately 1 hour after water exchange. Water temperature, salinity, and pH values were measured in-situ using a portable multi-parameter measurement instrument (HACH ® sension TM 156, Hach Company, Loveland, CO, USA). Dissolved oxygen was determined using the Winkler's method, and Secchi disc transparency was measured at each sample time (American Public Health Association, 1992). One litre of seawater from each sample was filtered through 0.45 μm glass-fiber filters (Whatman ® GF/C) previously combusted at 500℃ for 4 h and preweighed (American Public Health Association, 1992). After filtration, filters were rinsed with Milli-Q water to remove sea salts and then dried at 65℃ in a drying oven until constant weight (determined using a METTLER TOLEDO ® XP6U ultra-microbalance), based on which the concentration of suspended particulate matter (SPM) could be obtained. Filters with retained material were combusted at 500℃ for 6 h and reweighed to obtain the concentration of particulate organic matter (POM) of pond water. Onelitre of seawater was filtered for Chlorophyll- a measurement, using the method previously described. Total ammonia and phosphorus concentrations were determined according to the methods described by Strickland and Parsons (1977).
2.5 Data analysisData analysis of ecological indices of diatom communities, including the total number of diatom species, density of diatoms, dominance index (Berger and Parker, 1970), Margalef's index (Margalef, 1958), Shannon-Wiener diversity index (Shannon and Weaver, 1963), and evenness index (Pielou, 1966), as well as the analysis of the similarities of diatom communities, was undertaken using the statistical program ‘Primer Version 5.0'(Primer-E Ltd., Plymouth, UK)(Clarke and Warwick, 2001).
Dominance index: D= 100×(N 1 + N 2)/ N, where N 1 is the largest abundance of the species, N 2 is the secondary largest abundance of the species, and N is total abundance of all species.
Margalef's richness index: d =(S -1)/ln N, where S is the species richness, and N is total number of the individuals of all species.
Among the diversity indices, species diversity was calculated using the Shannon index H ', which was used together with the species richness S and evenness index J.
Shannon-Wiener index:
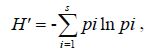
Evenness index: J = H' / H' max= H' /ln S,
where S is the species richness, and pi is the ratio of the individuals of the species i to the total number of individuals of all species in a sample.
The similarities of diatom communities between sampling months as well as between seasons were calculated using the Bray-Curtis coefficient (Bray and Curtis, 1957):
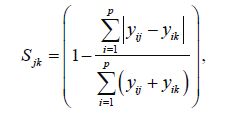
where S jk =similarity between the j th and k th samples, p =total number of species, i = i th species, ranging from 1 to p, y ij =number of individuals for the i th species in j th sample, and y ik =number of individuals for the i th species in k th sample. Differences in the diatom community structure were compared using multidimensional scaling (MDS) ordination and Analysis of Similarity (ANOSIM) test (Clarke and Warwick, 2001).
The algal density was expressed as individuals/cm2. Both DW and AFDW were expressed as mg/cm2. Values of chlorophyll- a were expressed in μg/cm2 for epilithon. The ratio of AFDW vs. DW was calculated as AFDW/DW, and the autotrophic index (AI) was calculated as: AFDW (mg/cm2)/Chlorophyll- a (μg/cm2)×1 000 μg/mg (American Public Health Association, 1992).
Statistical analysis was conducted using SPSS 16.0 for Windows (SPSS Inc., Chicago, IL, USA). The variation coefficients of water quality parameters examined in each month were calculated. Significant differences in biomass, and the ecological indices of diatom community in the biofilms were analysed separately for each month and season using one-way ANOVAs followed by Duncan's tests. The assumptions of normality and homoscedasticity were evaluated using Shapiro-Wilk (Shapiro and Wilk, 1965) and Levene's tests, respectively. Logarithmic transformations of data were adopted to satisfy both assumptions when required. Differences were considered significant at P<0.05.
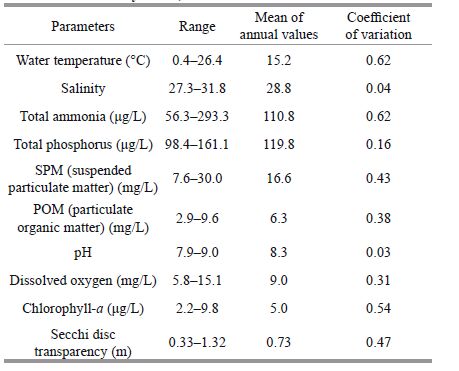
3 RESULT 3.1 Physical and chemical variables of pond waterAs shown in Fig. 1 and Table 1, salinity and pH values of the pond water showed little variation and had the minimum variation coefficients, while other parameters showed remarkable seasonal variations. For instance, the minimum temperature of 0.4℃ and maximum dissolved oxygen (DO) of 15.1 mg/L were recorded in December, and the maximum values of 28℃ and minimum DO of 5.8 mg/L were found in August. For SPM and POM, the lowest densities of 7.6 mg/L and 2.9 mg/L were observed in December, respectively, coinciding with the largest Secchi disc transparency (1.3 m) and minimum Chlorophyll- a concentration (2.2 μg/L) recorded. In contrast, the highest values of SPM and POM (30 mg/L and 9.6 mg/L, respectively), the smallest transparency value (0.3 m) and the maximum Chlorophyll- a concentration (9.8 μg/L) were all determined in August. For total ammonia, a peak (293.3 μg/L) was detected in August and the lowest value (56.3 μg/L) in December, while a smaller annual variation was observed for total phosphorus.

|
| Figure 1 Variations in physical and chemical parameters recorded in the experimental waters during the study period (May 2008-May 2009) |
|
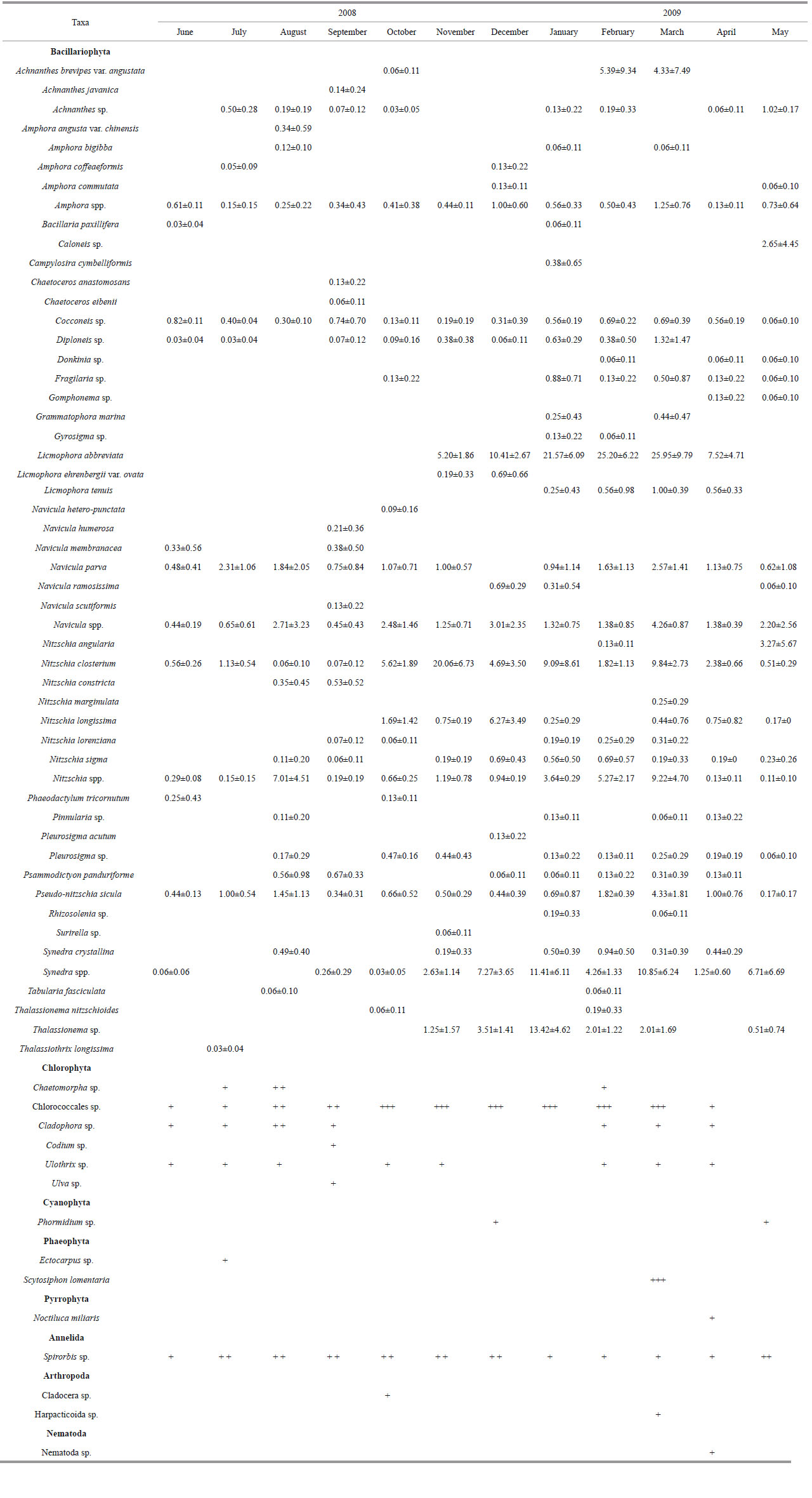
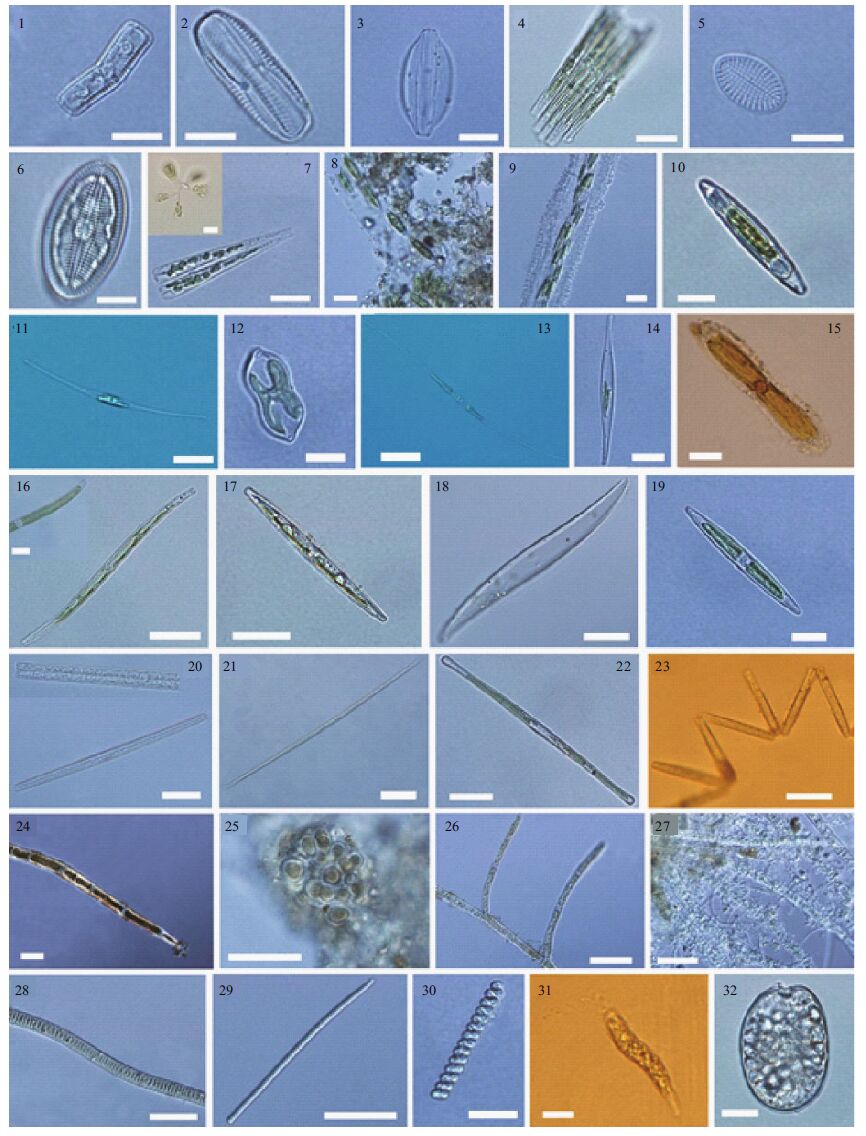
The species observed in biofilms during the experimental period are given in Table 2. More than 67 species were identified from the biofilm samples. Most of the species identified were algae of the phyla Bacillariophyta (27 genera and >53 species) and Chlorophyta (6 genera and 6 species). The others belonged to Cyanophyta, Phaeophyta, Pyrrophyta, Annelida, Arthropoda and Nematoda. Photos for the main algal species are shown in Plate I.
|
|
| Plate I The main algae species in biofilms on artificial reefs 1. Achnanthes sp.; 2. Amphora commutata ; 3. Amphora sp.; 4. Bacillaria paxillifera ; 5. Cocconeis sp.; 6. Diploneis sp.; 7. Licmophora abbreviata ; 8. Navicula parva ; 9. Navicula ramosissima ; 10. Navicula sp.; 11. Nitzschia closterium ; 12. Nitzschia constricta ; 13. Nitzschia longissima ; 14. Nitzschia lorenziana ; 15. Nitzschia marginulata ; 16. Nitzschia sigma ; 17. Nitzschia sp.; 18. Pleurosigma sp.; 19. Pseudo - nitzschia sicula ; 20. Synedra sp.; 21. Synedra sp.; 22. Tabularia fasciculata ; 23. Thalassionema sp.; 24. Chaetomorpha sp.; 25. Chlorococcales sp.; 26. Cladophora sp.; 27. Ulothrix sp.; 28. Oscillatoria sp.; 29. Phormidium sp.; 30. Spirulina sp.; 31. Euglena sp.; 32. Prorocentrum compressum. Scale bars: 1-3, 5-7, 10, 12, 14, 19, 30-32=10 μm; 4, 8, 9, 11, 13, 15-17, 22, 23=20 μm; 18, 20, 21, 25, 27-29=20 μm; 26=100 μm. |
Cocconeis sp., Amphora spp. and Nitzschia closterium were the dominant diatom species observed in June, just one month after the immersion of test panels. The dominant species were Navicula parva, Nitzschia closterium and Pseudo-nitzschia sicula in July, and Nitzschia spp., Navicula parva and Navicula spp. in August. Navicula parva, Cocconeis sp. and Psammodictyon panduriforme were relatively more abundant in September, and Nitzschia closterium, Navicula spp. and Nitzschia longissima were the most frequently observed diatom species in October. From November 2008 to April 2009, the diatom community was dominated by stalked diatoms of genus Licmophora, especially Licmophora abbreviata, also Synedra spp. and Nitzschia closterium. Other species were also frequently found, such as Nitzschia longissima in December, Thalassionema sp. in January, Achnanthes brevipes var. angustata and Nitzschia spp. in February and Navicula spp. in April. Nitzschia angularia, Synedra spp. and Caloneis sp. then emerged as the dominant diatom species in May 2009. In addition, Navicula ramosissima, a tube-forming diatom species, formed algal colonies on panels in December and January.
Filamentous species of Chlorophyta, including Chaetomorpha sp., Cladophora sp. and Ulothrix sp., first began to appear in June and July, about 1-2 months after the immersion of panels. They became abundant in August, and then decayed with the reduction in water temperature between September and November. Chlorococcales sp.(Chlorophyta) emerged as a membranous assemblage 2 months after panel immersion, forming a cohesive layer on the panels, and decaying completely in May 2009. Scytosiphon lomentaria(Phaeophyta) flourished on the surface of the panels and the bottom of pond in March 2009, then rotted away in April.
The polychaete Spirorbis sp., which was the most dominant invertebrate species found in the biofilms, settled on the surface of the panels just one week after immersion. The polychaetes gradually grew and increased in density, reached a relatively stable status 2-3 months later, and died in the winter with only empty shell left. A new attachment of Spirorbis sp. occurred just after the decay of the filamentous and membranous green algae in May 2009.
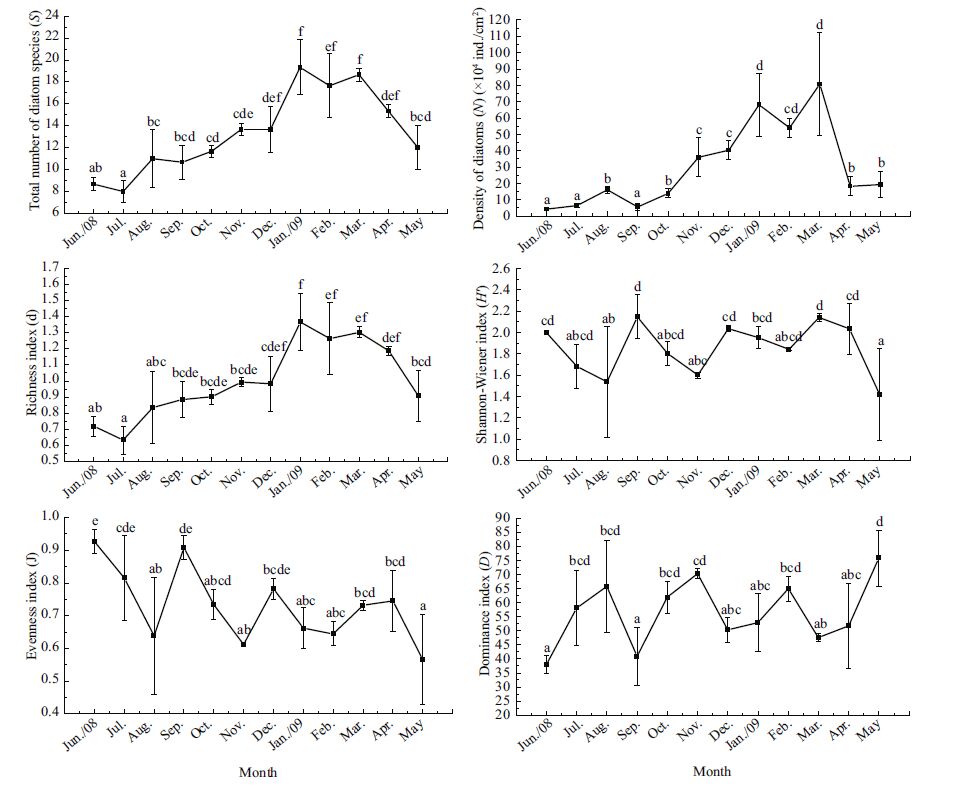
As shown in Fig. 2, there were significant differences in the six ecological indices among months. The number of diatom species (S) and the richness index (d) continually rose after the immersion of test panels, reached their highest values of 19.33±2.52 and 1.37±0.18 in January 2009, and then declined with the decay of the filamentous algae in the following months. However, the density of benthic diatoms (N) increased gradually from (4.33±0.69)× 104 ind./cm2 in June to (16.12±2.02)×104 ind./cm2 in August, and dropped sharply to (5.64±2.06)×104 ind./ cm2 in September, with the decline of the summer thriving species such as Nitzschia spp., Navicula spp. and Navicula parva. The density began to increase again from October to March, achieved the highest value of (80.74±31.65)×104 ind./cm2 in March, and then decreased to (18.24±5.94)×104 ind./cm2 in April with the decay of the membranous Chlorococcales sp. and the filamentous Scytosiphon lomentaria.
|
| Figure 2 Variations in the total number of diatom species (S), the total density of diatoms (N), richness (d), Shannon-Wiener (H′), evenness (J) and dominance (D) indices of diatoms in the succession experiment Values are given as means (±S.D.)(n =3). Bars with different letters indicate significant differences among months (ANOVA for lg S, F 11, 24 =9.061, P<0.001; ANOVA for lg N, F 11, 24 =37.291, P<0.001; ANOVA for lg (d +1), F 11, 24 =5.730, P<0.001; ANOVA for lg (H′ +1), F 11, 24 =2.863, P =0.015; ANOVA for lg (J +1), F 11, 24 =4.486, P =0.001; ANOVA for lg D, F 11, 24 =4.122, P =0.002). |
The Shannon-Wiener (H′), Evenness (J) and Dominance (D) indices showed vast fluctuations during an annual cycle. Variations in the two former indices showed similar tendencies, having four peaks (in June, September and December 2008, March 2009) and four lower values (in August and November 2008, February and May 2009), which were antithetic to the dominance index (D). Evenness index (J) reached its highest value (0.93±0.04) in June, just one month after immersion, and the Shannon-Wiener index (H′) reached the highest value (2.15±0.21) in September. Variations in these two indices had a similar tendency; both fell to their lowest values in May 2009. As for the dominance index (D), two troughs of 38.00±3.22 and 40.85±10.23 occurred in June and September, and two peaks of 70.28±1.66 and 75.78±9.92 were found in November 2008 and May 2009, respectively.
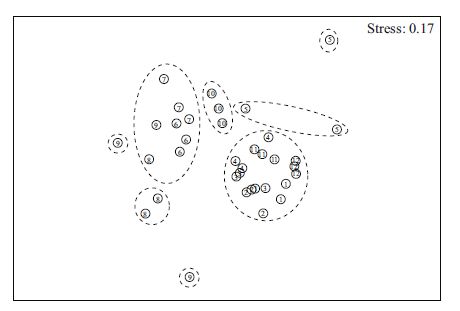
Non-metric multidimensional scaling (MDS) ordination of differences in diatom communities, which examines similarities in community structure, is illustrated in Fig. 3. The MDS plot shows that sampling months were classified into eight groups based on diatom abundance. The ANOSIM test, which was used to compare the diatom community between the months, produced a global R value of 0.716(P<0.001). Results indicated that the diatom community structure varied significantly among months, at the P =0.05 significance level.
|
| Figure 3 MDS ordination of the 12 months, based on square root transformed diatom abundance and Bray-Curtis similarities, with eight groups from the cluster analysis defined at similarity levels of 51.4%(stress=0.17) The numbers 1-12 represent the calendar months, namely, 6-12 represent June to December 2008, 1-5 represent January to May 2009. |
As presented in Fig. 4, DW and AFDW rose for the first 5 months after immersion and reached their first peak of 12.86±4.73 mg/cm2 and 4.16±1.09 mg/cm2 in September, respectively, due to the abundance of several filamentous species of Chlorophyta in August. They both then declined when the algae rotted in October. From November to February, there were no significant differences in biomass. The peak values of biomass DW (27.30±4.26 mg/cm2) and AFDW (6.61±0.85 mg/cm2) observed in March, which were significantly higher than other months, could be ascribed to the thriving of Scytosiphon lomentaria(Phaeophyta) and diatoms. Both DW and AFDW dropped sharply in the following two months, apparently because of the decay of S. lomentaria, Chlorococcales sp.(Chlorophyta) and some diatoms in April and May. The means of AFDW/DW were usually higher than 0.3, but two significant decreases were observed in July (0.296±0.032) and March (0.243±0.014).

|
| Figure 4 Variations in DW (dry weight), AFDW (ash free dry weight), and AFDW/DW (ratio of AFDW vs. DW) in biofilms in the succession experiment Values are given as means (±S.D.)(n =3). Different letters indicate statistically significant differences among months. Capital letters are linked to DW (ANOVA for lg (DW+1), F 11, 24 =74.791, P<0.001). Lowercase letters are linked to AFDW (ANOVA for lg (AFDW+1), F 11, 24 =66.754, P<0.001). Lowercase letters with an asterisk are associated with AFDW/DW (ANOVA for lg (AFDW/DW+1), F 11, 24 = 1.895, P =0.092). |
Chlorophyll- a reached a peak concentration of 1.91±0.06 μg/cm2 in August, which was significantly higher than in other months, due to the thriving of filamentous species of Chlorophyta. Subsequently, Chlorophyll- a and pheophytin changed in opposing tendencies. The decay of green algae is probably responsible for the peak of pheophytin in September. Chlorophyll- a concentration rose in October and November due to the growth of diatoms, and decreased with the rotting of Ulothrix sp. in December. The increased concentration of Chlorophyll- a in April might be related to the incrassation of the membranous green algae Chlorococcales sp., which coincided with the decline in pheophytin. Both declined in concentration in May 2009, which was a result of the decline in biofilm biomass (Fig. 4)(algae rotted offthe panels). The mean AI exhibited a general rising trend until March, peaking at 9 185.7±1 887.4, and three significant decreases were observed: from July (1 916.6±283.0) to August (803.9±158.0), September (3 678.4±659.5) to October (2 166.0±397.6) and March (9 185.7±1 887.4) to May (1 208.2±335.2).

|
| Figure 5 Variations in Chlorophyll- a, pheophytin and AI (autotrophic index) in biofilms in the succession experiment Values are given as means (±S.D.)(n =3). Different letters indicate statistically significant difference among months. Capital letters are linked to chlorophylla (ANOVA for Chlorophyll- a, F 11, 24 =43.184, P<0.001). Lowercase letters are linked to pheophytin (ANOVA for pheophytin, F 11, 24 =27.146, P<0.001). Lowercase letters with an asterisk are associated with AI (ANOVA for lg AI, F 11, 24 =74.978, P<0.001). |
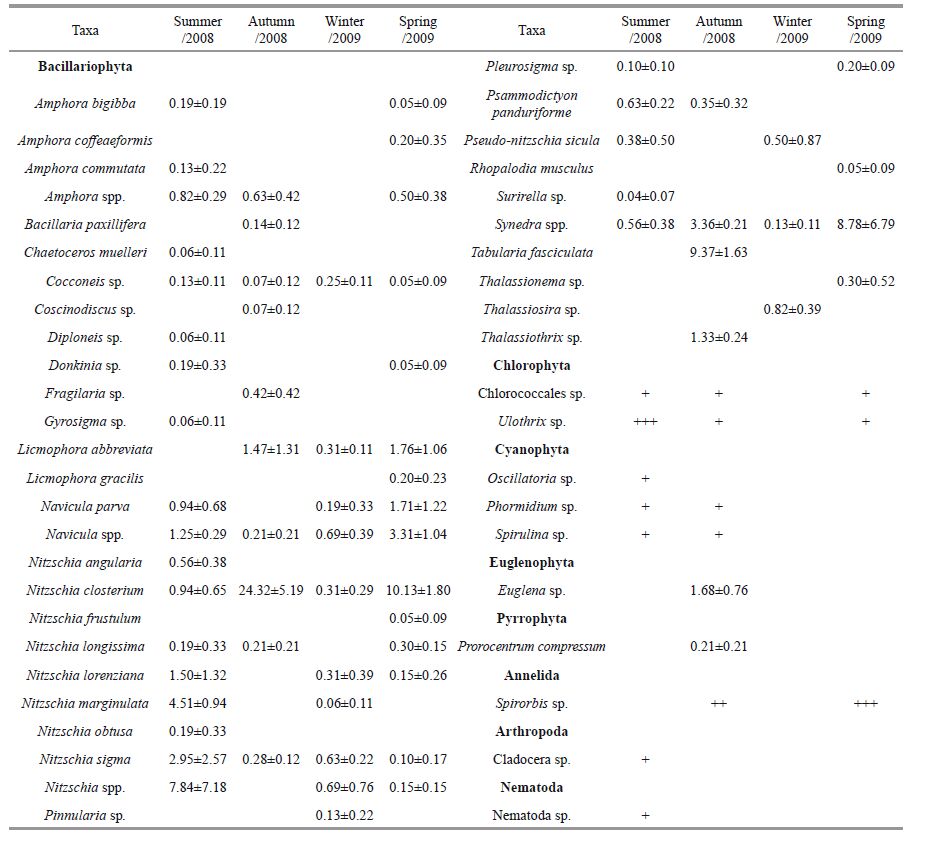
As shown in Table 3, more than 46 species were recorded from biofilms of seasonal test panels, comprising 23 genera and >36 species of Bacillariophyta, 2 species of Chlorophyta, 3 genera and 3 species of Cyanophyta, 1 species of Euglenophyta, 1 species of Pyrrophyta, 1 species of Annelida, 1 species of Arthropoda and 1 species of Nematoda. Photos for the main algal species are shown in Plate I.
|
Several species of genus Nitzschia, including Nitzschia marginulata, Nitzschia sigma, Nitzschia lorenziana and Nitzschia spp., were frequently observed in the summer. The booming of Nitzschia closterium, Tabularia fasciculata, Synedra spp. and Licmophora abbreviata occurred in the autumn, with only species of the genera Thalassiosira, Nitzschia and Navicula found to be relatively abundant in the winter. The dominant species were Nitzschia closterium, Synedra spp., Navicula spp. and Licmophora abbreviata in the spring. Ulothrix sp., a filamentous green alga, was seen in all seasons, except the winter. Species of Cyanophyta appeared mainly in the summer and/or autumn and the annelid Spirorbis sp. was found only on the spring and autumn panels.
The total number of diatom species (S) and richness index (d) both attained their highest values in the summer and lowest values in the winter. The density of diatoms (N) was higher in the autumn than in other seasons, due to the booming of Nitzschia closterium and Tabularia fasciculata. Indices of Shannon-Wiener (H ') and Evenness (J) were both significantly higher in the summer and winter than in the spring and autumn. An opposing trend was observed for the dominance index (D), with the highest value appearing in the autumn (Fig. 6).
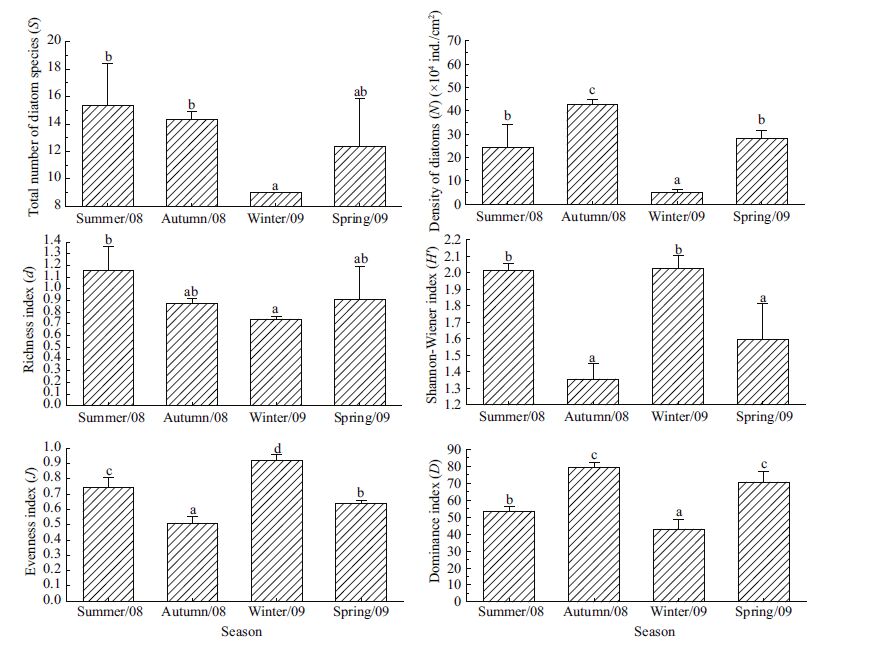
|
| Figure 6 Seasonal variations in the total number of diatom species (S), the total density of diatoms (N), species richness (d), Shannon-Wiener (H '), evenness (J) and dominance (D) indices Values are given as means (±S.D.)(n =3). Bars with different letters indicate significant differences among seasons (ANOVA for S, F 3, 8 =4.258, P =0.045; ANOVA for lg N, F 3, 8 =132.982, P<0.001; ANOVA for d, F 3, 8 =2.817, P =0.107; ANOVA for Hꞌ, F 3, 8 =19.598, P<0.001; ANOVA for J, F 3, 8 = 47.734, P<0.001; ANOVA for D, F 3, 8 =35.589, P<0.001). |
The MDS ordination for seasons, in terms of similarity of diatom community structure, are illustrated in Fig. 7. The global R value from the ANOSIM test was 0.932(P<0.001), which indicated that the diatom community composition of the 4 seasons was significantly different at the P =0.05 significance level.
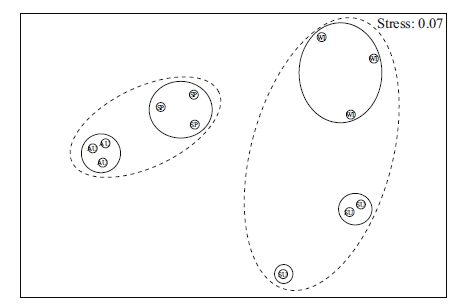
|
| Figure 7 MDS ordination of the 4 seasons, based on square root transformed diatom abundance and Bray- Curtis similarities, with five groups (continuous line) determined from the cluster analysis at similarity levels of 50.3% and two groups (dashed line) at 31%(stress=0.07) For the bubble marker, SP: spring, SU: summer, AU: autumn, WI: winter. |
As shown in Fig. 8, both the DW and AFDW of biofilms on test panels were significantly different among the four seasons, except between the summer and the autumn. Biofilm DW reached its highest value of 2.48±0.72 mg/cm2 in the summer and the lowest value of 0.24±0.06 mg/cm2 in the winter. The DW was also significantly higher in the autumn than in the spring. The maximal value of 0.69±0.06 mg/cm2 for AFDW was recorded in the autumn, significantly higher than in the spring (0.31±0.07 mg/cm2) and the winter (0.13±0.01 mg/cm2). These results indicated that the highest organic matter content of biofilm was in the autumn and the highest inorganic content in the summer. The mean AFDW/DW was higher in the winter (0.479±0.026) and the spring (0.483±0.062) than the summer (0.236±0.019) and the autumn (0.342±0.006).
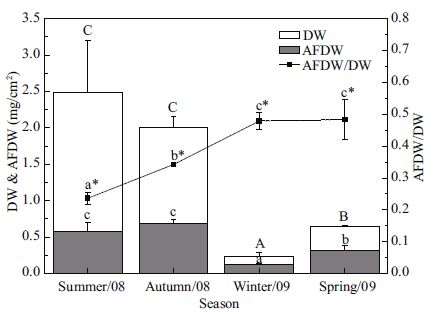
|
| Figure 8 Seasonal variations in DW (dry weight), AFDW (ash free dry weight), and AFDW/DW (ratio of AFDW vs. DW) in biofilms Values are given as means (±S.D.)(n =3). Different letters indicate statistically significant difference among seasons. Capital letters are linked to DW (ANOVA for lg (DW+1), F 3, 8 =52.211, P<0.001). Lowercase letters are linked to AFDW (ANOVA for lg (AFDW+1), F 3, 8 =41.148, P<0.001). Lowercase letters with an asterisk are associated with AFDW/DW (ANOVA for AFDW/DW, F 3, 8 =36.694, P<0.001). |
The Chlorophyll- a concentration was also significantly different among the four seasons. The highest value was recorded in the autumn, and was 14 times the value reported in the winter (Fig. 9). The highest concentration of pheophytin, (0.076± 0.048 μg/cm2) was detected in the summer, significantly higher than in the winter (0.026±0.006 μg/ cm2). On both the spring and autumn panels, the pheophytin concentration was almost undetectable in the biofilms (Fig. 9). The means of AI were winter > summer > autumn > spring (Fig. 9).
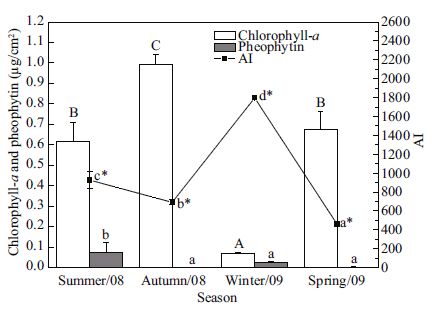
|
| Figure 9 Seasonal variations in Chlorophyll- a, pheophytin and AI (autotrophic index) in biofilms Values are given as means (±S.D.)(n =3). Different letters indicate statistically significant difference among seasons. Capital letters are linked to Chlorophyll- a (ANOVA for Chlorophyll- a, F 3, 8 =96.019, P<0.001). Lowercase letters are linked to pheophytin (ANOVA for pheophytin, F 3, 8 =6.380, P =0.016). Lowercase letters with an asterisk |
The present succession experiment revealed that the dominant diatoms were Cocconeis sp., Amphora spp. and Nitzschia closterium in June, Navicula spp., Cocconeis sp. and Nitzschia spp. from July to September, and Licmophora abbreviata, Nitzschia closterium and Synedra spp. in the following months, respectively. Previous studies have shown that prostrate diatoms, such as Cocconeis and Achnanthes, colonized surfaces during the initial period of biofilm development, followed by the attachment of genera possessing mucilagenous pads or stalks (e.g. Fragilaria and Synedra)(Patrick, 1976 ; Korte and Blinn, 1983). Diatoms are normally regarded as important components of the pioneer species, which develop photic biofilms (Underwood, 1984 ; Rao et al., 1997 ; Hillebrand and Sommer, 2000 ; Liess and Hillebrand, 2004). While studying diatom succession on sand grains, Miller et al.(1987) found increased attachment of low profile diatoms, compared to stalked diatoms. In the present study, the attachment of pioneer species, such as the green algae Chlorococcales sp., was observed in the early months, in addition to the diatoms Cocconeis sp. and Amphora spp. The stalked diatom Licmophora abbreviata, the rosette forming diatom Synedra spp. and filamentous green alga such as Chaetomorpha sp., Cladophora sp. and Ulothrix sp. frequently colonized the plates during the later months. It has been reported that diatoms with a closely adhering nature and the ability to produce mucilage, such as Achnanthes and Cocconeis, would facilitate the attachment of pioneer species during the early stages of colonization (Siver, 1977 ; Hudon and Bourget, 1981 ; Korte and Blinn, 1983). The dominance of Cocconeis sp. might be due to their fast multiplication rate (Oemke and Burton, 1986 ; Stevenson and Peterson, 1989) and high competitiveness in the presence of organic exudates (Tuchman and Blinn, 1979 ; Siver, 1980). Sekar et al.(2004) showed that, in the low energy lentic freshwater environment, biofilm development progressed from initially being dominated by unicellular and small colonial green algae, cyanobacteria and horizontally positioned diatoms, to a community composed of filamentous green algae and cyanobacteria, and vertically positioned rosette and long stalked diatoms. Most filamentous green algae attach themselves using a holdfast (Scott et al., 1996), and their growth rate is slow compared to unicellular green algae and diatoms. In our experiment, the exuberance of filamentous green algae was mainly observed in July-September. Our results were largely similar to the reports of Sekar et al.(2004), excluding the differing development of the initial colonizer Chlorococcales sp. during the later stages of biofilm succession. Instead of declining with time, Chlorococcales sp. increased to form a thick and cohesive mucilage layer on the surface of the panels, and then decayed with the rising temperature towards the late spring. It was suggested that the reduction of early colonizers during the later stages was possibly due to the increased abundance of stalked diatoms and filamentous species, which restricted light penetration (to underlying species) or hindered nutrient transport by interrupting passage of water current to the underlying cells (Oemke and Burton, 1986). In the present study, sustained growth and proliferation of the pioneer species Chlorococcales sp. during later months may be due to the sparse distribution of upper filamentous green algae in the late summer.
Seasonal temperature change is a major factor affecting the diversity of benthic communities (Stevenson et al., 1996). In the present experiment, the pond was exposed to high temperatures during the summer and nearly freezing conditions during the winter (Fig. 1). The temperatures in the spring and autumn facilitated the propagation of some diatoms, such as Nitzschia closterium, Synedra, Tabularia and Licmophora. This accounted for a relatively higher Dominance index (D) and lower Shannon-Wiener (H′) and Evenness (J) indices in the spring and autumn than in the summer and winter (Figs. 2, 5). Higher temperature in the summer favoured the growth of small fast-growing diatoms, such as Navicula parva and Nitzschia sp.(Qian et al., 2009). A fairly low temperature of 1-2 ℃ led to the low density of diatoms (N) in the winter. Despite the significant difference in the number of diatom species (S) between the summer and winter, the high summer and low winter temperatures restrained the mass proliferation of dominant diatoms and resulted in higher Shannon- Wiener (H ') and Evenness (J) indices and a lower Dominance index (D)(Fig. 6). At lower temperatures, a reduction in overall diversity has been previously reported (Stevenson et al., 1996) but was not observed in our study (Fig. 6). The dominant species in the seasonal experiment were similar to those in the succession experiment, particularly diatom species. However, different incubation periods may be responsible for some differences in taxonomical composition between the biofilms. Reid et al.(1995) showed that artificial substrata must be in place for sufficient time to allow representative diatom communities to develop on the substrate surface. Due to their lower growth rates, the later colonizers such as filamentous green algae and cyanobacteria usually settle on the substrate following some pioneer colonizers and may need a longer incubation time. Therefore, filamentous green algae and cyanobacteria, although emerged on the substrate, did not appear as dominant species in the biofilms of seasonal test panels, which differed from the succession experiment. The MDS analysis in the present study revealed significant differences in diatom community composition among months and seasons due to temporal changes, and the grouping of months and seasons also demonstrated differences in the aforementioned dominant species.
Although diatoms dominated in the biofilm communities, biomass was influenced by the growth of some macroalgae. In the succession experiment, the rapid increase in the abundance of filamentous green algae Chaetomorpha sp. and Cladophora sp. in July-September, the incrassation of the membranous green algae Chlorococcales sp. layer, and the outburst of the phaeophyte Scytosiphon lomentaria in March, should be regarded as major contributors to the abrupt increase in biomass in those seasons (Fig. 4). However, the situation was dissimilar in the seasonal experiment. Higher water temperature in the summer favoured the growth of some filamentous green algae, and higher SPM in water (Fig. 1) facilitated the attachment of SPM on the biofilm. Both temperature and SPM increases could lead to increased DW, and the decreasing temperature and decline in green algae could result in an autumn bloom of diatoms (Table 3) and a maxima of AFDW (Fig. 8). It was reported that suspended solids and visibility played crucial roles in periphytic colonization (Albay and Akcaalan, 2003), and the settlement of suspended matter plays an important role in determining periphyton mass (van Dijk, 1993). The settlement of SPM on biofilms is common during resuspension caused by wind driven turbulence. For biofilms on the vertical surface of test panels, the settlement of suspended matter should be dependent on both SPM concentration and the absorptive capacity of the biofilms itself. The reticular structure of the summer biofilms, which contain filamentous green algae, made it easier to trap suspended particles compared to the thinner biofilm in the winter (Fig. 1). Therefore, the inorganic SPM trapped by filamentous green algae, Scytosiphon lomentaria and Spirorbis sp., could account for the significantly lower values of periphyton AFDW/DW in both experiments. The presence of photosynthetic pigments (Chlorophyll- a and pheophytin) could indicate flora colonization of artificial substrates, and the presence of phaeophytin indicated that the flora observed was partly degraded (Richard et al., 2009). The mean AI ranged between 468.8 and 9 185.7 in the year-round experiment (Fig. 5), which indicated that the epilithon contained mainly heterotrophic organisms and dead organic matter, as specified by Huchette et al.(2000) for an AI above 200. The three significant decreases in AI are probably due to the exuberance of filamentous green algae (August), diatoms (October) and the membranous assemblage of Chlorococcales sp.(February). In the seasonal experiment, the variation in Chlorophyll- a concentration indicated vigorous growth of algae in all seasons except the winter, and pheophytin levels denoted the higher mortality of algae in the summer and winter biofilms (Fig. 9). Van Dijk (1993) found that the relatively low Chlorophyll- a content of epiphytic biofilms indicated relatively large amounts of detrital matter in the biofilms. This is also the case for the present epilithic biofilms. The summer biofilm (Chlorophyll- a content 0.025%) contained more detrital matter than the autumn biofilm (Chlorophyll- a content 0.05%). The lower AI in biofilms in the autumn and spring indicated a higher autotrophic level than in summer and winter. This could be interpreted by the vigorous growth of diatoms in the former, and more trapped inorganic SPM in the latter.
To facilitate the nocturnal behaviour of A. japonicus, suitable artificial substrate are usually provided in large-scale sea cucumber culture (Dong et al., 2010). Apostichopus japonicus is an omnivore that feeds on almost everything in the substrate, including microorganisms, diatoms, poriferans, bryozoans, mollusks, crustaceans, echinoderms, macroalgae, vascular plants and/or their fragments/ detritus, as well as sand and mud in substrate (Kinoshita and Tanaka, 1939 ; Zhang et al., 1995). An artificial diet for A. japonicus was also recommended to contain inorganic materials like sea mud and grinded mollusk shells (Tian et al., 2008). Dong et al.(2010) reported that pre-incubated shelters could attract significantly more sea cucumbers than nonincubated shelters during the feeding time (night), due to the potential for feeding on the biofilm. This may be attributed to the effects of the diatoms in biofilms. As the dominant organisms in the community, diatoms consist of abundant fatty acids, some sugars and proteins (Dunstan et al., 1994 ; Simental-Trinidad et al., 2001), as well as feeding attractants for aquatic animals, such as EPS (extracellular polymeric substances), polyunsaturated fatty acids, and amino acids (Hoagland et al., 1993 ; Daume et al., 1999, 2000). More recently, a field trophic study with stable isotope analysis revealed that particulate organic matter, macroalgae, benthic microalgae, nematode and copepod are important food sources for sea cucumbers (Sun et al., 2013). Therefore, epilithic biofilms, which are mainly composed of diatoms, macroalgae, some invertebrates, organic and inorganic detritus, can be optimal food sources for cultured sea cucumbers.
The present results may provide a reference for the management of sea cucumber culture. In the spring and autumn, the pond condition is suitable for the growth of diatoms, like stalked Licmophora, rosette forming Synedra and Nitzschia closterium. The diatom bloom in the autumn biofilm coincided well with the main growth period of sea cucumbers. A laboratory experiment demonstrated that the addition of benthic matter (mainly containing diatom) to the macroalgae powder, which is used as a food source, could improve the absorption of macroalgae and the growth of A. japonicus(Gao et al., 2011). Therefore, during these seasons, regulation of the aquatic environment and sediment is recommended to promote the growth of benthic diatoms and the contribution of biofilms in the diet of sea cucumbers. In seasons when juvenile A. japonicus are put in culture waters, the preferred pre-incubation time of substrate should be 4-6 weeks to allow diatoms the time to thrive in biofilms. Further, Dong et al.(2010) recommended a period of at least 1 month for attracting sea cucumbers. Although A. japonicus cease feeding at high (20℃) and low (3℃) temperatures (Tian et al., 2008), the periphyton in aquaculture ponds may also affect the culture of sea cucumbers in the summer and winter. In the summer, the blooms of filamentous green algae may act as snares for sea cucumbers. Further, the decay of macroalgae may cause deteriorations in water quality (Tian et al., 2008), demonstrated by the abrupt rising of total ammonia in late August (Fig. 1), and thus excessive filamentous green algae should be eliminated. Traditionally, superfluous green algae is manually removed. In recent years however, a sunshade net was generally used to control water temperature and light intensity, and thus to restrain the overgrowth of filamentous green algae. It was confirmed that most macroalgae may end up as ingestible detritus and consumed by suspension and deposit feeders, including sea cucumbers (Nadon and Himmelman, 2006). Hence, the moderate amount of macroalgae developing during the summer could have a lag effect during the autumn, since decayed debris during this season can be an important food source for A. japonicus. In the winter, the growth of benthic diatoms as well as Scytosiphon lomentaria may help to maintain the dissolved oxygen and to control the ammonia (Fig. 1), which may be particularly important when culture ponds are covered with ice. Future studies on the epilithic biofilms in sea cucumber culture waters should include quantitative determination of the contribution of epilithic biofilms in the food of A. japonicus, the growth and succession of epilithic biofilms under the predation of sea cucumbers, the potential roles of epilithic biofilms in regulating the water quality of sea cucumber ponds, and the regulation of epilithic biofilms in sea cucumber culture ponds.
5 ACKNOWLEDGEMENTWe are grateful to Homey Company for providing great help in field study. We would like to thank Prof. YANG Xiulan for technical assistance in identifying the algae. Constructive comments from anonymous reviewers are also appreciated.
| Albay M, Akcaalan R, 2003. Comparative study of periphyton colonisation on common reed (Phragmites australis) and artificial substrate in a shallow lake, Manyas, Turkey. Hydrobiologia, 506 . |
| American Public Health Association. 1992. Standard Methods for the Examination of Water and Wastewater. 18th ed.APHA, Washington, DC. |
| Ballester E L C, Wasielesky Jr W, Cavalli R O, Abreu P C, 2007. Nursery of the pink shrimp Farfantepenaeus paulensis in cages with artificial substrates: biofilm composition and shrimp performance. Aquaculture, 269 (1-4) : 355 –362. Doi: 10.1016/j.aquaculture.2007.04.003 |
| Battaglene S C, Seymour J E, Ramofafia C, 1999. Survival and growth of cultured juvenile sea cucumbers, Holothuria scabra. Aquaculture, 178 (3-4) : 293 –322. Doi: 10.1016/S0044-8486(99)00130-1 |
| Berger W H, Parker F L, 1970. Diversity of planktonic foraminifera in deep-sea sediments. Science, 168 (3937) : 1 . |
| Bray J R, Curtis J T, 1957. An ordination of the upland forest communities of Southern Wisconsin. Ecological Monographs, 27 (4) : 325 –349. Doi: 10.2307/1942268 |
| Callow M E. 1986. A world-wide survey of slime formation on anti-fouling paints. In: Evans L V, Hoagland K D eds.Algal Biofouling. Elsevier Science Publishers, Amsterdam, The Netherlands. p.1-20. |
| Chang Y Q, Sui X L, Li J, 2006. The current situation, problem and prospect on the Apostichopus japonicus aquaculture. Fisheries Science, 25 (4) : 198 –201. |
| Cheng Z D, Gao Y H. 2012. Flora Algarum Marinarum Sinicarum. Tomus V. Bacillariophyta, No. Ⅱ Pennatae I.Science Press, Beijing. (in Chinese) |
| Cheng Z D, Gao Y H. 2013. Flora Algarum Marinarum Sinicarum. Tomus V. Bacillariophyta, No. Ⅲ Pennatae Ⅱ.Science Press, Beijing. (in Chinese) |
| Chessman B C, 1985. Artificial-substratum periphyton and water quality in the Lower La Trobe River, Victoria. Australian Journal of Marine and Freshwater Research, 36 (6) : 855 –871. Doi: 10.1071/MF9850855 |
| Clarke K R, Warwick R M. 2001. Changes in Marine Communities: An Approach to Statistical Analysis and Interpretation. 2nd ed. PRIMER-E Ltd., Plymouth. |
| Cooksey K E, Wigglesworth-Cooksey B, 1995. Adhesion of bacteria and diatoms to surfaces in the sea: a review. Aquatic Microbial Ecology, 9 : 87 –96. Doi: 10.3354/ame009087 |
| Daume S, Brand-Gardner S, Woelkerling W J, 1999. Preferential settlement of abalone larvae: diatom films vs. non-geniculate coralline red algae. Aquaculture, 174 (3-4) : 243 –254. |
| Daume S, Krsinich A, Farrell S, Gervis M, 2000. Settlement, early growth and survival of Haliotis rubra in response to different algal species. Journal of Applied Phycology, 12 (3-5) : 479 –488. |
| Dong G C, Dong S L, Wang F, Tian X L, 2010. Effects of materials, incubation time and colors of artificial shelters on behavior of juvenile sea cucumber Apostichopus japonicus. Aquacultural Engineering, 43 (1) : 1 –5. Doi: 10.1016/j.aquaeng.2010.01.002 |
| Dunstan G A, Volkman J K, Barrett S M, Leroi J M, Jeffrey S W, 1994. Essential polyunsaturated fatty acids from 14 species of diatom (Bacillariophyceae). Phytochemistry, 35 (1) : 155 –161. |
| Fairchild G W, Sherman J W, 1992. Linkage between epilithic algal growth and water column nutrients in softwater lakes. Canadian Journal of Fisheries and Aquatic Sciences, 49 (8) : 1641 –1649. Doi: 10.1139/f92-183 |
| Ford T E, Walch M, Mitchell R, Kaufman M J, Vestal J R, Ditner S A, Lock M A, 1989. Microbial film formation on metals in an enriched arctic river. Biofouling, 1 (4) : 301 –311. Doi: 10.1080/08927018909378118 |
| Gao Q F, Wang Y S, Dong S L, Sun Z L, Wang F, 2011. Absorption of different food sources by sea cucumber Apostichopus japonicus (Selenka) (Echinodermata:Holothuroidea): evidence from carbon stable isotope. Aquaculture, 319 (1-2) : 272 –276. Doi: 10.1016/j.aquaculture.2011.06.051 |
| Hamel J F, Hidalgo R Y, Mercier A, 2003. Larval development and juvenile growth of the galapagos sea cucumber Isostichopus fuscus. SPC Beche-de-Mer Information Bulletin, 18 : 3 –8. |
| Hillebrand H, Sommer U, 2000. Diversity of benthic microalgae in response to colonization time and eutrophication. Aquatic Botany, 67 (3) : 221 –236. Doi: 10.1016/S0304-3770(00)00088-7 |
| Hoagland K D, Roemer S C, Rosowski J R, 1982. Colonization and community structure of two periphyton assemblages, with emphasis on the diatoms (Bacillariophyceae). American Journal of Botany, 69 (2) : 188 –213. Doi: 10.2307/2443006 |
| Hoagland K D, Rosowski J R, Gretz M R, Roemer S C, 1993. Diatom extracellular polymeric substances: function, fine structure, chemistry, and physiology. Journal of Phycology, 29 (5) : 537 –566. Doi: 10.1111/jpy.1993.29.issue-5 |
| Huchette S M H, Beveridge M C M, Baird D J, Ireland M, 2000. The impacts of grazing by tilapias (Oreochromis niloticus L. ) on periphyton communities growing on artificial substrate in cages. Aquaculture, 186 (1-2) : 45 –60. |
| Hudon C, Bourget E, 1981. Initial colonization of artificial substrate: community development and structure studied by scanning electron microscopy. Canadian Journal of Fisheries and Aquatic Sciences, 38 (11) : 1 . |
| Ishida N, Mitamura O, Nakayama M, 2006. Seasonal variation in biomass and photosynthetic activity of epilithic algae on a rock at the upper littoral area in the north basin of Lake Biwa, Japan. Limnology, 7 (3) : 175 –183. Doi: 10.1007/s10201-006-0181-1 |
| Jin D X, Cheng Z D, Lin J M, Liu S C. 1982. The Marine Benthic Diatoms in China, Volume 1. Ocean Press, Beijing. (in Chinese) |
| Jin D X, Cheng Z D, Liu S C, Ma J X. 1992. The Marine Benthic Diatoms in China, Volume 2. Ocean Press, Beijing. (in Chinese) |
| Kelly M G, Cazaubon A, Coring E, Dell'Uomo A, Ector L, Goldsmith B, Guasch H, Hürlimann J, Jarlman A, Kawecka B, Kwandrans J, Laugaste R, Lindstrøm E A, Leitao M, Marvan P, Padisák J, Pipp E, Prygiel J, Rott E, Sabater S, van Dam H, Vizinet J, 1998. Recommendations for the routine sampling of diatoms for water quality assessments in Europe. Journal of Applied Phycology, 10 (2) : 215 –224. Doi: 10.1023/A:1008033201227 |
| Khatoon H, Yusoff F, Banerjee S, Shariff M, Bujang J S, 2007. Formation of periphyton biofilm and subsequent biofouling on different substrates in nutrient enriched brackishwater shrimp ponds. Aquaculture, 273 (4) : 470 –477. Doi: 10.1016/j.aquaculture.2007.10.040 |
| Kinoshita T, Tanaka S, 1939. Hokkaido san namako no syokuji ni tsuite (A report of the feeding pattern of the sea cucumber Stichopus japonicus in Hokkaido). Suisankenkyushi, 34 : 32 –35. |
| Korte V L, Blinn D W, 1983. Diatom colonization on artificial substrata in pool and riffle zones studied by light and scanning electron microscopy. Journal of Phycology, 19 (3) : 332 –341. Doi: 10.1111/jpy.1983.19.issue-3 |
| Kutka F J, Richards C, 1996. Relating diatom assemblage structure to stream habitat quality. Journal of the North American Benthological Society, 15 (4) : 469 –480. Doi: 10.2307/1467799 |
| Lane C M, Taffs K H, Corfield J L, 2003. A comparison of diatom community structure on natural and artificial substrata. Hydrobiologia, 493 (1-3) : 65 –79. |
| Liess A, Hillebrand H, 2004. Invited review: direct and indirect effects in herbivore-periphyton interactions. Archiv für Hydrobiologie, 159 (4) : 433 –453. Doi: 10.1127/0003-9136/2004/0159-0433 |
| Margalef R, 1958. Information theory in ecology. General Systems Yearbook, 3 : 36 –71. |
| Miller A R, Lowe R L, Rotenberry J T, 1987. Succession of diatom communities on sand grains. Journal of Ecology, 75 (3) : 693 –709. Doi: 10.2307/2260200 |
| Nadon M O, Himmelman J H, 2006. Stable isotopes in subtidal food webs: have enriched carbon ratios in benthic consumers been misinterpreted?. Limnology and Oceanography, 51 (6) : 2828 –2836. Doi: 10.4319/lo.2006.51.6.2828 |
| Oemke M P, Burton T M, 1986. Diatom colonization dynamics in a lotic system. Hydrobiologia, 139 (2) : 153 –166. Doi: 10.1007/BF00028099 |
| Patrick R, 1976. The formation and maintenance of benthic diatom communities. Proc. Am. Phil. Soc., 120 : 475 –484. |
| Pielou E C, 1966. The measurement of diversity in different types of biological collections. Journal of Theoretical Biology, 13 : 131 –144. Doi: 10.1016/0022-5193(66)90013-0 |
| Qian Z M, Xing R L, Wu C X, Wang C H, Tang N, 2009. Effects of temperature on growth and physiological biochemical compositions of eight benthic diatoms. Journal of Yantai University (Natural Science and Engineering Edition), 22 (1) : 30 –34. |
| Rao T S, Rani P G, Venugopalan V P, Nair K V K, 1997. Biofilm formation in a freshwater environment under photic and aphotic conditions. Biofouling, 11 (4) : 265 –282. Doi: 10.1080/08927019709378336 |
| Reid M A, Tibby J C, Penny D, Gell P A, 1995. The use of diatoms to assess past and present water quality. Australian Journal of Ecology, 20 (1) : 57 –64. Doi: 10.1111/aec.1995.20.issue-1 |
| Ren Y C, Dong S L, Qin C X, Wang F, Tian X L, Gao Q F, 2012. Ecological effects of co-culturing sea cucumber Apostichopus japonicus (Selenka) with scallop Chlamys farreri in earthen ponds. Chinese Journal of Oceanology and Limnology, 30 (1) : 71 –79. Doi: 10.1007/s00343-012-1038-6 |
| Ren Y C, Wang F, Dong S L, Liu F, 2010. Seasonal characteristics of primary production of sea cucumber(Apostichopus japonicus) culture ponds in Jinghai Bay, Rongcheng. Periodical of Ocean University of China, 40 (3) : 24 –28. |
| Richard M, Trottier C, Verdegem M C J, Hussenot J M E, 2009. Submersion time, depth, substrate type and sampling method as variation sources of marine periphyton. Aquaculture, 295 (3-4) : 209 –217. Doi: 10.1016/j.aquaculture.2009.07.005 |
| Robinson C T, Rushforth S R, 1987. Effects of physical disturbance and canopy cover on attached diatom community structure in an Idaho stream. Hydrobiologia, 154 (1) : 49 –59. Doi: 10.1007/BF00026830 |
| Scott C, Fletcher R L, Bremer G B, 1996. Observations on the mechanisms of attachment of some marine fouling bluegreen algae. Biofouling, 10 (1-3) : 161 –173. Doi: 10.1080/08927019609386277 |
| Sekar R, Venugopalan V P, Nandakumar K, Nair K V K, Rao V N R, 2004. Early stages of biofilm succession in a lentic freshwater environment. Hydrobiologia, 512 (1-3) : 97 –108. Doi: 10.1023/B:HYDR.0000020314.69538.2c |
| Shannon C E, Weaver W. 1963. The Mathematical Theory of Communication. University of Illinois Press, Urbana, IL. |
| Shapiro S S, Wilk M B, 1965. An analysis of variance test for normality (complete samples). Biometrika, 52 (3-4) : 591 –611. Doi: 10.1093/biomet/52.3-4.591 |
| Simental-Trinidad J A, Sánchez-Saavedra M D P, CorreaReyes J G, 2001. Biochemical composition of benthic marine diatoms using as culture medium a common agricultural fertilizer. Journal of Shellfish Research, 20 (2) : 611 –617. |
| Siver P A, 1977. Comparison of attached diatom communities on natural and artificial substrates. Journal of Phycology, 13 (4) : 402 –406. |
| Siver P A, 1980. Microattachment patterns of diatoms on leaves of Potamogeton robbinsii Oakes. Transactions of the American Microscopical Society, 99 (2) : 217 –220. Doi: 10.2307/3225710 |
| Slater M J, Jeffs A G, 2010. Do benthic sediment characteristics explain the distribution of juveniles of the deposit-feeding sea cucumber Australostichopus mollis?. Journal of Sea Research, 64 (3) : 241 –249. Doi: 10.1016/j.seares.2010.03.005 |
| Stevenson R J, Bothwell M L, Lowe R L. 1996. Algal Ecology:Freshwater Benthic Ecosystems. Aquatic Ecology Series.Academic Press, California. |
| Stevenson R J, Peterson C G, 1989. Variation in benthic diatom(Bacillariophyceae) immigration with habitat characteristics and cell morphology. Journal of Phycology, 25 (1) : 120 –129. Doi: 10.1111/jpy.1989.25.issue-1 |
| Strickland J D H, Parsons T R. 1977. A Practical Handbook of Seawater Analysis. Canadian Government Publishing Centre, Ottawa. |
| Sui X L, Liu X G, Wang J, 2010. The status and key issues of sea cucumber culture in Liaoning Province. Fisheries Science, 29 (11) : 688 –690. |
| Sun Z L, Gao Q F, Dong S L, Shin P K S, Wang F, 2013. Seasonal changes in food uptake by the sea cucumber Apostichopus japonicus in a farm pond: evidence from C and N stable isotopes. Journal of Ocean University of China, 12 (1) : 160 –168. Doi: 10.1007/s11802-012-1952-z |
| Tian C Y, Li Q, Liang Y. 2008. Healthy Culture of the Sea Cucumber Apostichopus japonicus. Ocean University Press, Qingdao. (in Chinese) |
| Tseng C K. 1983. Common Seaweeds of China. Science Press, Beijing, China. |
| Tuchman M, Blinn D W, 1979. Comparison of attached algal communities on natural and artificial substrata along a thermal gradient. British Phycological Journal, 14 (3) : 243 –254. Doi: 10.1080/00071617900650281 |
| Underwood A J, 1984. Microalgal food and the growth of the intertidal gastropods Nerita atramentosa Reeve and Bembicium nanum (Lamarck) at four eights on a shore. Journal of Experimental Marine Biology and Ecology, 79 (3) : 277 –291. Doi: 10.1016/0022-0981(84)90201-6 |
| van Dijk G M, 1993. Dynamics and attenuation characteristics of periphyton upon artificial substratum under various light conditions and some additional observations on periphyton upon Potamogeton pectinatus L. Hydrobiologia, 252 (2) : 143 –161. Doi: 10.1007/BF00008152 |
| Wetzel R G, 1964. A comparative study of the primary productivity of higher aquatic plants, periphyton, and phytoplankton in large, shallow lake. Internationale Revue der gesamten Hydrobiologie und Hydrographie, 49 (1) : 1 –61. Doi: 10.1002/(ISSN)1522-2632 |
| Wetzel R G. 1983. Periphyton of Freshwater Ecosystems.Springer, Netherlands. http://dx.doi.org/10.1007/978-94-009-7293-3. |
| Williams D M, Round F E, 1986. Revision of the genus Synedra Ehrenb. Diatom Research, 1 (2) : 313 –339. Doi: 10.1080/0269249X.1986.9704976 |
| Wu Y F, Antoine S E, Bowker D W, 1999. Colonisation of epilithic diatom population of the River Taff, South Wales, U. K. Limnologica, 29 (2) : 174 –185. Doi: 10.1016/S0075-9511(99)80065-6 |
| Yang H S, Yuan X T, Zhou Y, Mao Y Z, Zhang T, Liu Y, 2005. Effects of body size and water temperature on food consumption and growth in the sea cucumber Apostichopus japonicus (Selenka) with special reference to aestivation. Aquaculture Research, 36 (11) : 1085 –1092. Doi: 10.1111/are.2005.36.issue-11 |
| Zhang B L, Sun D Y, Wu Y Q, 1995. Preliminary analysis on the feeding habit of Apostichopus japonicus in the rocky coast waters off Lingshan Island. Marine Sciences (3) : 11 –13. |
 2017, Vol. 35
2017, Vol. 35


