Institute of Oceanology, Chinese Academy of Sciences
Article Information
- FENG Song(冯颂), LIN Jianing(林佳宁), SUN Song(孙松), ZHANG Fang(张芳)
- Artificial substrates preference for proliferation and immigration in Aurelia aurita (s. l.) polyps
- Chinese Journal of Oceanology and Limnology, 35(1): 153-162
- http://dx.doi.org/10.1007/s00343-016-5230-y
Article History
- Received Sep. 20, 2015
- accepted for publication Nov. 10, 2015
- accepted in principle Dec. 29, 2015
2 Laboratory for Marine Ecology and Environmental Science, Qingdao National Laboratory for Marine Science and Technology, Qingdao 266071, China;
3 Jiaozhou Bay Marine Ecosystem Research Station, Institute of Oceanology, Chinese Academy of Sciences, Qingdao 266071, China;
4 State Key Laboratory of Environmental Criteria and Risk Assessment, Chinese Research Academy of Environmental Sciences, Beijing 100012, China;
5 Laboratory of Riverine Ecological Conservation and Technology, Chinese Research Academy of Environmental Sciences, Beijing 100012, China
Aurelia aurita, or moon jellyfish, is a scyphozoan species found worldwide (Kramp, 1961 ; Lucas, 2001). Aurelia has attracted increased attention because of the deleterious socioeconomic and ecological impacts caused by wide-ranging jellyfish blooms (Purcell et al., 2007 ; Richardson et al., 2009 ; Graham et al., 2014). A notorious regional case occurred in the East Asian Sea, where massive A. aurita(s. l.) blooms developed in harbor and bays, including Tokyo Bay, Shiwa Lake, and Jiaozhou Bay (Toyokawa et al., 2000 ; Han and Uye, 2010 ; Wang et al., 2012 ; Hong et al., 2013). High-density water aggregations of A. aurita(s. l.) clogged the cooling water intakes of thermal and nuclear power plants, bringing about billions of dollars in economic losses (Purcell et al., 2007 ; Dong et al., 2010 ; Graham et al., 2014 ; Korean Nuclear Power Plant Operational Performance Information System, http://opis.kins.re.kr/). In addition, research has shown that frequent Aurelia outbreaks may vary the marine food chain by competing for zooplankton with fish or ingesting fish eggs and larvae (Purcell, 1997 ; Hansson et al., 2005). In several areas, higher biomass of Aurelia spp. topdown regulated the zooplankton population (Makabe et al., 2012). Frequent outbreaks of jellyfish might cause the changes of marine ecosystem from fish dominate to jellyfish dominate (Uye, 2011 ; Mcnamara et al., 2013). The decomposition of Aurelia spp. blooms could lead to the significant releases of organic and inorganic nutrients, thereby modulate microbial food web (Tinta et al., 2012). Multiple explanations have been put forward for the possible causes of the jellyfish blooms, including climate change, overfishing, eutrophication, and development of aquaculture (Purcell et al., 2007 ; Uye, 2011 ; Purcell, 2012).
The life cycle of A. aurita(s. l.) comprises of the pelagic medusa and the benthic polyp (Lucas, 2001). Polyp population dynamics play a determinant role in the medusa outbreaks (Lucas et al., 2012 ; Purcell, 2012). Polyps proliferate on hard substrates by asexual reproduction, and the “multi-mode” reproductive strategies of A. aurita are the most complicated among the scyphozoan species (Schiariti et al., 2014). Six kinds of modes have been recorded to date: buds, stolons, podocysts, fission, planuloids, and motile bud-like tissue particles (Berrill, 1949 ; Kakimura, 1975 ; Aria, 1997 ; Lucas, 2001 ; Vagelli, 2007 ; Schiariti et al., 2014). Buds, stolons, and podocysts are the most common. Buds and stolons not only increased the nearby polyp population with rapid speed and high production (Adler and Jarms, 2009 ; Purcell et al., 2012 ; Schiariti et al., 2014), but also allowed new-generated polyps to settle onto additional substrates, namely immigration of polyps (Willcox et al., 2008 ; Hoover and Purcell, 2009). Migrant also occurred through motile bud-like tissue particles and detachment of polyps (Schiariti et al., 2014, 2015). Podocysts are produced in response to food shortage as a means of asexual reproduction in unfavorable environments (Arai, 2009 ; Thein et al., 2012). Moreover, multiple ephyrae were liberated by a polyp through strobilation, and then developed into meduase, further leading to jellyfish blooms.
Among numerous hypotheses on jellyfish blooms, increasing artificial marine substrates has been suggested as a pivotal anthropogenic driver, as a result of providing additional substrates for the settlement and proliferation of jellyfish polyps (Richardson et al., 2009 ; Purcell, 2012 ; Duarte et al., 2013). Marine construction is increasing along with socioeconomic development worldwide and massive amounts of garbage are being deposited in the sea (Uye, 2011 ; Purcell, 2012). Some of these artificial materials may afford suitable substrates to be the habitats of polyps for their settlement, growth, propagation and immigration. Several specific cases have indicated that medusa abundance was positively related to newly-added artificial substrates in some regions (Lo et al., 2008 ; Malej et al., 2012 ; Janßen et al., 2013). Scuba diving investigation has discovered that massive assemblages of Aurelia spp. polyps settle onto some artificial material constructions such as concrete, wharfs, cross-sea bridges, and agriculture rafts (Miyake, 2002 ; Willcox et al., 2008 ; Purcell et al., 2009 ; Di Camillo et al., 2010 ; Ishii and Katsukoshi, 2010 ; Toyokawa et al., 2011 ; Makabe et al., 2014). Laboratory studies on substrate preference only focused on the settlement of some jellyfish planulae at present, suggesting planulae prefer some artificial substrates for attachment, such as concrete, wood, and foam plastics (Holst and Jarm, 2007 ; Hoover and Purcell, 2009). However, until now, the substrate preferences for polyp proliferation and immigration were unclear. Investigations by scuba diving in the natural environment and in situ experiments have not completely illuminated the above issues due to interference from other factors, including interspecific competition with fouling organisms (Watanabe and Ishii, 2001 ; Ishii et al., 2008 ; Ishii and Katsukoshi, 2010 ; Toyokawa et al., 2011), predators (Hoover et al., 2012 ; Takao et al., 2014), and accidental deaths. Hence, we examined the artificial substrate preferences for polyp proliferation and immigration via a series of laboratory experiments. The null hypothesis that the abundance of propagated and immigrated polyps did not significantly differ among substrates was tested to further understand the role of artificial substrates in jellyfish blooms.
2 MATERIAL AND METHOD 2.1 Stock of polyp populationTen mature A. aurita(s. l.) medusae (six female and four male with mean umbrella diameter of 20 cm) were captured with hand nets through a rubber boat in Jiaozhou Bay in July 2013, when massive medusae occurred, and then transferred into 200-L glass aquariums in the laboratory of Chinese Academy of Sciences, Qingdao, China. The seawater temperature in the aquariums was 24℃, in consistence with ambient temperature. After transitory cultivation of a week, millions of planulae were sexually produced; then, planulae attached onto polyethylene plates and gave rise to polyps. Polyps were fed with Artemia sp. nauplii for 1–2 h every 4 days. After feeding, water was replaced with freshly-filtered seawater. The seawater in aquariums was pumped from Luxun Park in Jiaozhou Bay, which was filtered by a set of nets, and the temperature varied with the ambient water. Polyps developed into the 16-tentacle stage after 1 month as a stock of polyp population.
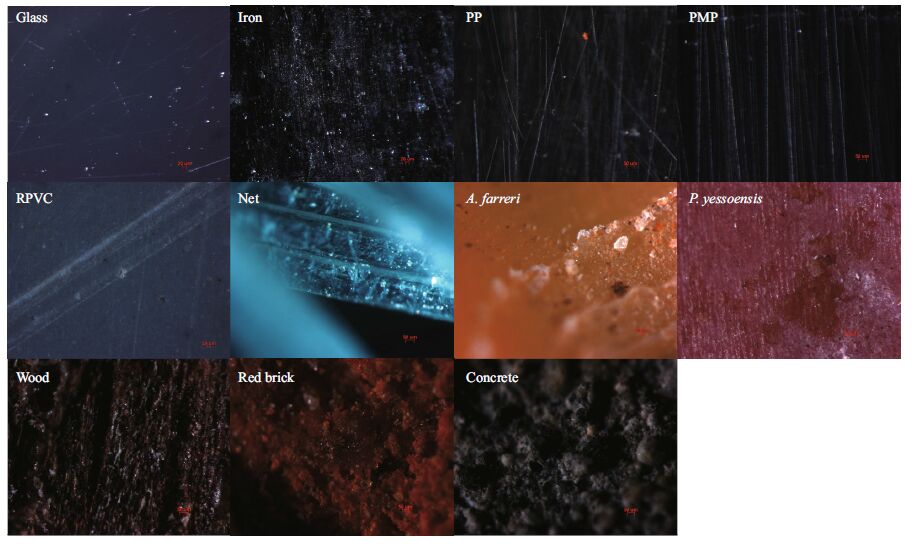
Pictures were taken under the stereomicroscope (Zeiss Stereo Discovery V20). The magnification of glass, iron, polyethylene plate (PP), polymethyl methacrylate plate (PMP), rigid polyvinyl chloride plate (RPVC), shell of A. farreri, shell of P. yessoensis, and wood is 10×6.6. The magnification of concrete and red brick is 10×5.
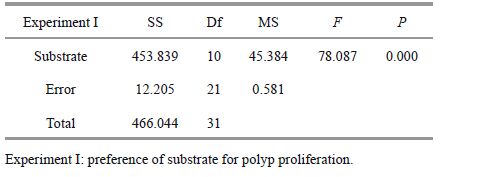
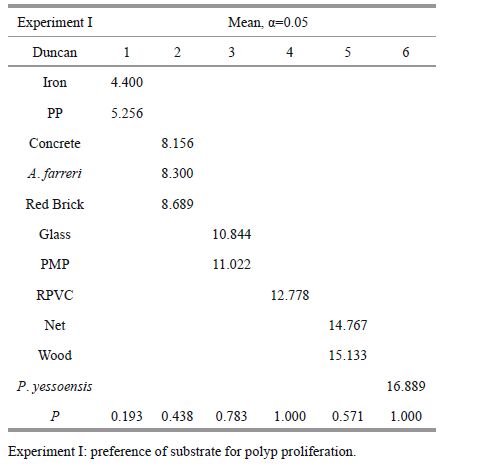
2.2 Experiment I: preference of substrate for polyp proliferationTwo natural and nine artificial substrates were chosen for the experiment (Table 1), whose essential characteristics were described in Table 2. Figure 1 showed the magnified textures of each type of substrates. These substrates were cut into 6 cm×5 cm pieces, except for some shells with sectors, but their surface areas were similar (30 cm2). Each substrate contained three replicates, totally 6 pieces of natural substrates and 27 of the artificial ones. The experiment used 16-tentacle polyps in good condition, which were detached from the polyethylene plates with dissecting needles. The detached polyps (n. 495) were transferred to the 33 experimental plates putting 15 polyps on each substrate. Re-settlement occurred after five days.
|
| Figure 1 The magnified texture pictures of each type of substrates |
Polyps on each piece were cultured in a 2-L glass beaker, filled with 1 L of 0.45 μm-hybrid-fiber membrane filtered seawater. Adequate Artemia sp. nauplii were added into glass beakers every 3 days to guarantee that polyps were fully fed. Two hours later, water was replaced with freshly-filtered seawater. All of the beakers were placed in incubators at 20±0.5℃. The salinity was 30±0.5. The number of polyps was recorded under a dissecting microscope (Olympus SZ61) every week. The experiment started on September 2, 2013 and lasted 35 days.
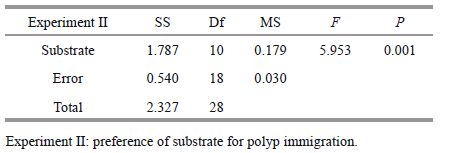
2.3 Preference of substrate for polyp immigrationeA 200-L glass aquarium, whose bottom was attached by fully-developed A. aurita(s. l.) polyps, was chosen to observe the natural polyp population immigration. A. aurita(s. l.) polyps were cultivated as the methods described above. The initial density of polyps was 9.3±2.5 ind./cm2, which was determined via a magnifier. The seawater temperature and salinity remained constant at 20±0.5℃ and 30±0.5, respectively. The experimental materials consisted of two live shellfish, two natural substrates, and seven artificial substrates (Table 1). These substrates were 6×5 cm pieces except some shells, while their surface areas were closely 30 cm2. For each kind of substrates, three replicates were done. They were randomly put into the bottom of the aquarium. After 1 week, the number of polyps on each piece was observed under a dissecting microscope (Olympus SZ61).
2.4 StatisticsData were analyzed with Excel 2013, Origin 8.5, and SPSS 16.0 software packages. Data were tested for normality and homogeneity, and the difference in the number of polyps among substrates was examined by one-way ANOVA. If the overall ANOVA results were significant, Duncan's test was performed to test among experimental combinations. Nonparametric analogues (Kruskal-Wallis one-way ANOVA tests on ranks) were chosen for some data that did not meet normality and homogeneity after transformation. Pair-wise comparisons were analyzed by the Mann- Whitney U method (non-parametric).
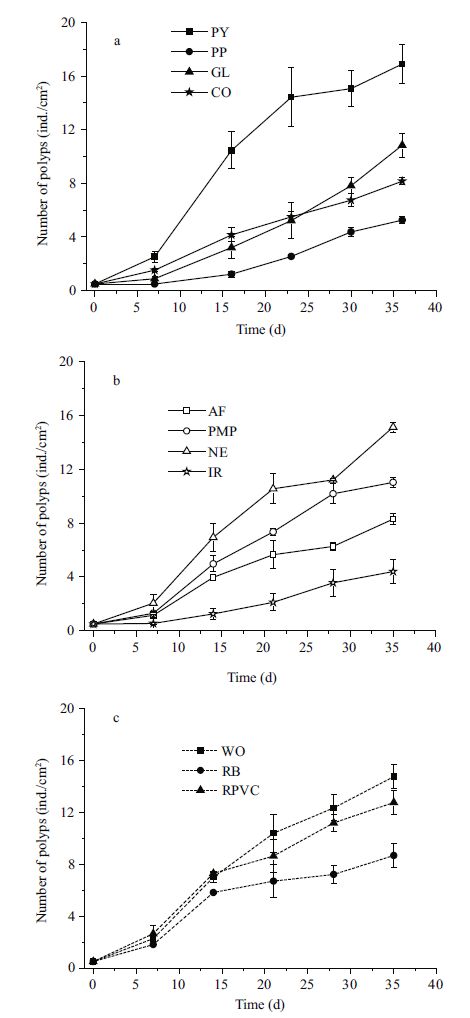
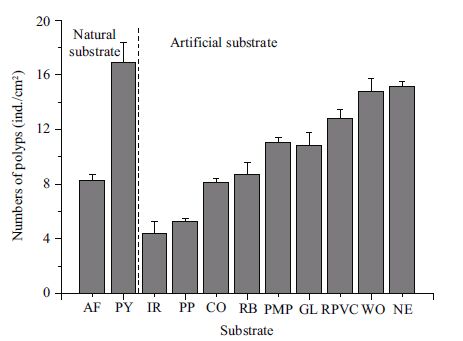
3 RESULT 3.1 Polyp proliferation on various substratesThe number of polyps on all the substrates increased exponentially over the first 20 days, then increased relatively slowly (Fig. 2). The final number of polyps was significantly different among substrates (Table 3). The maximum occurred on P. yesoensis shells (16.9±1.5 ind./cm2), followed by the artificial net (15.1±0.4 ind./cm2), and wood (14.8±0.9 ind./cm2) (Fig. 3), with no significant differences between the two (Table 4). The abundance of polyps was lowest on the iron plates (4.4±0.9 ind./cm2) (Fig. 3). Compared to natural P. yesoensis shells, A. farreri shells retained significantly fewer polyps.
|
| Figure 2 Changes in the number of A. aurita (s. l.) polyps on various substrates over the course of 35 days PY: shells of Patinopecten yessoensis ; PP: polyethylene plate; GL: glass; CO: concrete; AF: shells of Azumapecten farreri ; PMP: polymethyl methacrylate plate; NE: net; IR: iron; WO: wood; RB: red brick; RPVC: rigid polyvinyl chloride plate. |
|
|
|
| Figure 3 Final number of A. aurita (s. l.) polyps proliferating on different substrates AF: shell of Azumapecten farreri; PY: shell of Patinopecten yessoensis ; IR: iron; PP: polyethylene plate; CO: concrete; RB: red brick; PMP: polymethyl methacrylate plate; GL: glass; RPVC: rigid polyvinyl chloride plate; WO: wood; NE: net. |
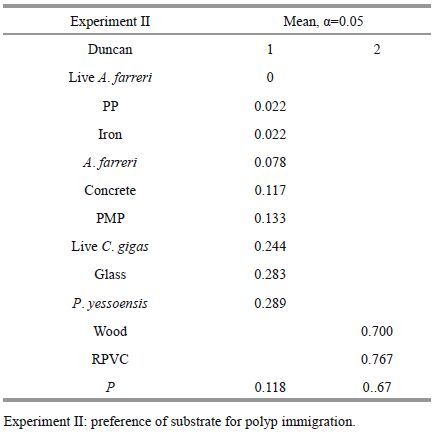
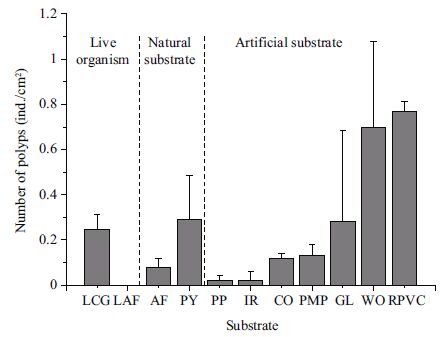
ANOVA results showed that the density of polyps varied significantly among substrates used for immigration (Table 5). PVC and wood were the favorite substrates for polyp immigration, and there were no significant differences between the two in terms of density of polyps (Table 6), which was 0.77±0.05/cm2 and 0.70±0.04/cm2, respectively (Fig. 4). The lowest density appeared on the polyethylene and the iron plates (0.02±0.02/cm2 and 0.02±0.04/cm2, respectively), with no significant difference between the two (Table 6). The density of polyps on P. yesoensis shells was notably higher than A. farreri shells. A. aurita(s. l.) polyps did not immigrate onto the live A. farreri, but some were discovered on the live oyster (0.24±0.07/cm2).
|
|
|
| Figure 4 Final number of A. aurita (s. l.) immigrating onto various substrates in a week LCG: live C. gigas; LAF: live Azumapecten farreri ; AF: shell of Azumapecten farreri ; PY: shell of Patinopecten yessoensis ; PP: polyethylene plate; IR: iron; CO: concrete; PMP: polymethyl methacrylate plate; GL: glass; WO: wood; RPVC: rigid polyvinyl chloride plate. |
As one of the causal drivers for jellyfish outbreaks (Purcell et al., 2007 ; Purcell, 2012 ; Duarte et al., 2013), studies on marine artificial substrates previously concentrated more on the substrate preference of planulae settlement. Hoover and Purcell (2009) reported that the Aurelia labiate planulae preferred plastics (EPS foams) to rubber and treated wood. Another study showed that more A. aurita, Cyanea capillata, Cyanea lamarckii and Chrysaora hysoscella planulae settled onto polyethylene plates than shells (Holst and Jarms, 2007). Also, Rhopilema nomadica planulae attached on PVC, ceramic, and glass, but not on natural rocks covered in epibiota (Lotan et al., 1992). Planulae of Catostylus mosaicus prefer glass over seagrass for settlement (Pitt, 2000).
Our experiment showed the substrate preferences for polyp proliferation and immigration. Significant differences were observed in the density of polyps among both artificial and natural substrates. Higher increase rates of A. aurita(s. l.) polyps were observed on P. yesoensis shells than on A. farreri shells. The optimal artificial substrates for polyp propagation were net, wood, and RPVC. Concerning immigration, Aurelia labiate polyps from Cornet Bay preferred immigrating to plasm, compared to treated wood and perduren (Hoover and Purcell, 2009). Our study discovered that A. aurita(s. l.) polyps could immigrate to live oyster shells, instead of live A. farreri shells. In comparison to natural substrates, more A. aurita(s. l.) polyps immigrated to the artificial substrates, PVC and wood in this case. The lowest immigration rates were observed for iron and polyethylene plates. Hence, it was proved to be significantly substrate specific when polyps propagated and immigrated on artificial materials.
4.2 Possible cause of variations in substrates preferenceThe surface texture of substrates might determine the settlement choice of planulae (Brewer, 1978 ; Hoover and Purcell, 2009). Higher settlement rates of planulae have been reported with increases in the surface density of roughness for the substrates (Eckman, 1990). Hence, the substrate preference of A. aurita(s. l.) polyp proliferation and immigration may also be associated with surface roughness. In our experiment, a higher density of roughness was observed on the net, wood, and PVC plates than on the glass, organic glass, and iron plates. Similarly, more varied texture was found on P. yesoensis shells than on C. farreri . The reproduction and immigrant rate of polyps were lower on concrete and red brick in spite of complex surface texture. This may be closely related with chemicals treatments of substrates, considered by Hoover and Purcell (2009), reporting that wood, treated by Ammoniacal Copper Zinc Arsenate, exhibited significantly lower rates of recruitment and immigration than the synthetic plastics. The aluminate in concrete, or aluminum oxide and iron trioxide in red brick, may inhibit the propagation of polyps. The least amount of increase in polyps was observed on iron plates. Apart from the lowest roughness, iron is easily corroded in the seawater, and this may damage the polyps' attachment surface. On the other hand, Lucas and Horton (2014) demonstrated that high concentrations of metals had more significant impacts on polyp condition such as copper, silver. Thus, large amount of the rust and iron ion in the seawater might also be unfavorable to the proliferation and immigrant of A. aurita(s. l.) polyps.
The attractiveness of substrates may vary over time due to the coverage of biofilms or pioneer species, which modify roughness and weaken the effects of chemical compounds. Holst and Jarms (2007) reported that the various bacterial films found on different substrates can also affect planulae settlement choices. Biofilms may permanently present on the substrates and increase the strength of attachment (Hadfield, 2011), owing to the enhancive hydrophobicity of the film-covered surface (Brewer, 1984 ; Müller and Leitz, 2002). Cassiopea spp.(Scyphozoa: Rhizostomeae) polyps naturally prefer to colonize on degrading mangrove leaves, whose surfaces were found to contain the diverse biofilms (Fleck and Fitt, 1999 ; Hofmann and Crow, 2002). Thus, biofilms on the substrates likewise may also contribute the proliferation and immigration of A. aurita(s. l.) polyps. Other researchers have proposed that planulae can detect some physical and chemical properties from substrate-borne bacteria or other biogenic cues which present on a substrate, and these may trigger the final metamorphic stimulus that leads to settlement (Müller and Leitz, 2002). The biofilms, as well as chemical properties and roughness of artificial substrates influence asexual reproduction and immigration of polyps. Even the inclination of the substrate is important to avoid or limit sedimentation or light exposition (Miyake et al., 2002 ; Holst and Jarms, 2007 ; Hoover and Purcell, 2009).
The multi-mode reproduction strategy of Aurelia spp. polyps acts a determinant role in the medusae dynamics. Plentiful environmental factors had been defined to affect polyp proliferation. Among them, previous reports had confirmed that temperature and food supply were the foremost impact factors (Purcell, 2007 ; Han and Uye, 2010 ; Lucas et al., 2012 ; Purcell et al., 2012 ; Schiariti et al., 2014). Our study verified that substrate preference also regulate the colonization propagation. Under favorable thermal condition and adequate food availability, low-density polyps amplified through buds and stolons with a trend of the sigmoid curve (Coyne, 1973 ; Schiariti et al., 2015 ; this study), when substrate preference may dominate the asexual reproduction rate at the equal inclination. As the density of polyps increased, the densitydependent factor activated producing more motile bud-like tissue particles, as well as induced the detachment of polyps and stolon occurrence to migrate additional substrates (Hoover and Purcell, 2009 ; Schiariti et al., 2015 ; this study), although it decreased the reproduction rate of the local colonization due to the intraspecific competition for space and food resource, on the basis of our experiment and early reports (Coyne, 1973 ; Schiariti et al., 2015). During the immigrant process, the newly-added specific artificial substrates make crucial promotion accompanied by the disappearance of densitydependent factor, to achieve the population amplification on a larger scale.
As a cosmopolitan species, Aurelia spp. frequently outbreaks in harbors and bays of the coastal seas in recent decades, which was compactly associated with the increased artificial marine substrates (Richardson et al., 2009 ; Purcell, 2012 ; Duarte et al., 2013). This had been testified by a series of scuba diving investigations on polyps in natural environment (Miyake et al., 2002 ; Willcox et al., 2008 ; Purcell et al., 2009 ; Di Camillo et al., 2010 ; Ishii and Katsukoshi, 2010 ; Toyokawa et al., 2011) and particular cases in situ (Lo et al., 2008 ; Malej et al., 2012 ; Makabe et al., 2014). Our study demonstrated that vacant artificial or natural substrates discretely influence the proliferation and the immigration of polyps in the laboratory. By this taken, even in the natural environment, various materials and texture may regulate the composition and the abundance of the fouling communities and the assemblages of Aurelia spp. polyps and, indirectly, have effects on the amounts of liberated medusae.
5 ACKNOWLEDGEMENTWe are very grateful to Dr. WANG Yantao for help with sampling of A. aurita(s. l.) adults for fertilization in the field. The experiment was completed with the help of Dr. LIN Jianing. Drs. SUN Song and ZHANG Fang equally contributed to this manuscript, and should be considered as co-corresponding authors.
| Adler L, Jarms G, 2009. New insights into reproductive traits of scyphozoans: special methods of propagation in Sanderia malayensis GOETTE, 1886 (Pelagiidae, Semaeostomeae) enable establishing a new classification of asexual reproduction in the class Scyphozoa. Mar. Biol., 156 (7) : 1411 –1420. Doi: 10.1007/s00227-009-1181-6 |
| Arai M N, 1997. A Functional Biology of Scyphozoa. Chapman & Hall, London. 316p. |
| Arai M N, 2009. The potential importance of podocysts to the formation of scyphozoan blooms: a review. Hydrobiologia, 616 (1) : 241 –246. Doi: 10.1007/s10750-008-9588-5 |
| Berrill N J, 1949. Developmental analysis of Scyphomedusae. Biol. Rev., 24 (4) : 393 –410. |
| Brewer R H, 1978. Larval settlement behavior in the jellyfish Aurelia aurita (Linnaeus) (Scyphozoa: semaeostomeae). Estuaries, 1 (2) : 121 –122. |
| Brewer R H, 1984. The influence of orientation, roughness and wettability of solid surfaces on the behavior and attachment of planulae of Cyanea (Cnidaria: scyphozoa). Biol. Bull., 166 (1) : 11 –21. Doi: 10.2307/1541426 |
| Coyne J A, 1973. An investigation of the dynamics of population growth and control in scyphistomae of the scyphozoan Aurelia aurita. Chesapeake Science, 14 (1) : 55 –58. Doi: 10.2307/1350704 |
| Degarmo E P, Black J, Kohser R. A. 2003. Materials and Processes in Manufacturing. 9th ed. Wiley, Hoboken, NJ, 223p. |
| Di Camillo C G, Betti F, Bo M, Martinelli M, Puce S, Bavestrello G, 2010. Contribution to the understanding of seasonal cycle of Aurelia aurita (Cnidaria: scyphozoa)scyphopolyps in the northern Adriatic Sea. Journal of the Marine Biological Association of the United Kingdom, 90 (6) : 1105 –1110. Doi: 10.1017/S0025315409000848 |
| Dong Z, Liu D, Keesing J K, 2010. Jellyfish blooms in China:Dominant species, causes and consequences. Mar. Pollut.Bull, 60 (7) : 954 –963. Doi: 10.1016/j.marpolbul.2010.04.022 |
| Duarte C M, Pitt K A, Lucas C H, Purcell J E, Uye S, Robinson K, Brotz L, Decker M B, Sutherland K R, Malej A, Madin L, Mianzan H, Gili J M, Fuentes V, Atienza D, Pagés F, Breitburg D, Malek J, Graham W M, Condon R H, 2013. Is global ocean sprawl a cause of jellyfish blooms? Front. Ecol. Environ., 11 (2) : 91 –97. Doi: 10.1890/110246 |
| Eckman J E, 1990. A model for passive settlement by planktonic larvae onto bottoms of differing roughness. Limnol. Oceanogr., 35 (4) : 887 –901. Doi: 10.4319/lo.1990.35.4.0887 |
| Fleck J, Fitt W K, 1999. Degrading mangrove leaves of Rhizophora mangle Linne provide a natural cue for settlement and metamorphosis of the upside down jellyfish Cassiopea xamachana Bigelow. Journal of Experimental Marine Biology and Ecology, 234 (1) : 83 –94. Doi: 10.1016/S0022-0981(98)00140-3 |
| Graham W M, Gelcich S, Robinson K L, Duarte C M, Brotz L, Purcell J E, Madin L P, Mianzan H, Sutherland K R, Uye S I, Pitt K A, Lucas C H, Bøgeberg M, Brodeur R D, Condon R H, 2014. Linking human well-being and jellyfish: ecosystem services, impacts and socital responses. Front. Ecol. Environ., 12 (9) : 515 –523. Doi: 10.1890/130298 |
| Hadfield M G, 2011. Biofilms and marine invertebrate larvae:what bacteria produce that larvae use to choose settlement sites. Annu. Rev. Mar. Sci., 3 (1) : 453 –470. Doi: 10.1146/annurev-marine-120709-142753 |
| Han C H, Uye S I, 2010. Combined effects of food supply and temperature on asexual reproduction and somatic growth of polyps of the common jellyfish Aurelia aurita s. l.Plankton and Benthos Research, 5 (3) : 98 –105. Doi: 10.3800/pbr.5.98 |
| Hansson L J, Moeslund O, Kiørboe T, Riisgård H U. 2005.Clearance rates of jellyfish and their potential predation impact on zooplankton and fish larvae in a neritic ecosystem (Limfjorden, Denmark). Mar. Ecol. Prog. Ser. 304: 117-131. |
| Hofmann D K, Crow G L, 2002. Induction of larval metamorphosis in the tropical scyphozoan Mastigias papua: striking similarity with upside down-jellyfish Cassiopea spp. (with notes on related species). Vie et Milieu, 52 (4) : 141 –147. |
| Holst S, Jarms G, 2007. Substrate choice and settlement preferences of planula larvae of five Scyphozoa (Cnidaria) from German Bight, North Sea. Mar. Biol., 151 (3) : 863 –871. Doi: 10.1007/s00227-006-0530-y |
| Hong H P, Han C H, Yoo J K, 2013. Population dynamics of jellyfish Aurelia aurita (s. l.) in Sihwa Lake. Ocean and Polar Research, 35 (3) : 205 –217. Doi: 10.4217/OPR.2013.35.3.205 |
| Hoover R A, Armour R, Dow I, Purcell J E, 2012. Nudibranch predation and dietary preference for the polyps of Aurelia labiata (Cnidaria: scyphozoa). Hydrobiologia, 690 (1) : 199 –213. Doi: 10.1007/s10750-012-1044-x |
| Hoover R A, Purcell J E, 2009. Substrate preferences of scyphozoan Aurelia labiata polyps among common dockbuilding materials. Hydrobiologia, 616 (1) : 259 –267. Doi: 10.1007/s10750-008-9595-6 |
| Ishii H, Katsukoshi K, 2010. Seasonal and vertical distribution of Aurelia aurita polyps on a pylon in the innermost part of Tokyo Bay. J. Oceanogr., 66 (3) : 329 –336. Doi: 10.1007/s10872-010-0029-5 |
| Ishii H, Ohba T, Kobayashi T, 2008. Effects of low dissolved oxygen on planula settlement, polyp growth and asexual reproduction of Aurelia aurita. Plankton and Benthos Research, 3 (S) : 107 –113. |
| Janßen H, Augustin C B, Hinrichsen H H, Kube S, 2013. Impact of secondary hard substrate on the distribution and abundance of Aurelia aurita in the western Baltic Sea. Mar. Pollut. Bull., 75 (1-2) : 224 –234. Doi: 10.1016/j.marpolbul.2013.07.027 |
| Kakimura, Y, 1975. An experimental study of the life cycle and organ differentiation of Aurelia aurita Lamarck. The Bulletin of the Marine Biological Station of Asamushi, 15 (3) : 101 –113. |
| Korean Nuclear Power Plant Operational Performance Information System. 2009. Annual report. Available at:http://opis.kins.re.kr/(accessed on 8 August 2009). |
| Kramp P L, 1961. Synopsis of the medusae of the world. Journal of the Marine Biological Association of the United Kingdom, 40 : 1 –469. |
| Lo W T, Purcell J E, Hung J J, Su H M, Hsu P K, 2008. Enhancement of jellyfish (Aurelia aurita) populations by extensive aquaculture rafts in a coastal lagoon in Taiwan. ICES J. Mar. Sci., 65 (3) : 453 –461. Doi: 10.1093/icesjms/fsm185 |
| Lotan A, Ben-Hillel R, Loya Y, 1992. Life cycle of Rhopilema nomadica: a new immigrant scyphomedusan in the Mediterranean. Mar. Biol., 112 (2) : 237 –242. Doi: 10.1007/BF00702467 |
| Lucas C H, Graham W M, Widmer C, 2012. Jellyfish life histories: role of polyps in forming and maintaining scyphomedusa populations. Adv. Mar. Biol., 63 : 133 –196. Doi: 10.1016/B978-0-12-394282-1.00003-X |
| Lucas C H, Horton A A, 2014. Short-term effects of the heavy metals, Silver and copper, on polyps of the common jellyfish, Aurelia aurita. Journal of Experimental Marine Biology and Ecology, 461 : 154 –161. Doi: 10.1016/j.jembe.2014.08.003 |
| Lucas C H, 2001. Reproduction and life history strategies of the common jellyfish, Aurelia aurita, in relation to its ambient environment. Hydrobiologia, 451 (1-3) : 229 –246. |
| Makabe R, Furukawa R, Takao M, Uye S I, 2014. Marine artificial structures as amplifiers of Aurelia aurita s. l. blooms: a case study of a newly installed floating pier. J.Oceanogr., 70 (5) : 447 –455. |
| Makabe R, Kurihara T, Uye S I, 2012. Spatio-temporal distribution and seasonal population dynamics of the jellyfish Aurelia aurita s. l. studied with Dual-frequency IDentification SONar (DIDSON). J. Plankton Res., 34 (11) : 936 –950. |
| Malej A, Kogovšek T, Ramšak A, Catenacci L, 2012. Blooms and population dynamics of moon jellyfish in the northern Adriatic. Cahiers de Biologie Marine, 53 (3) : 337 –342. |
| McNamara M E, Lonsdale D J, Cerrato R M, 2013. Top-down control of mesozooplankton by adult Mnemiopsis leidyi influences microplankton abundance and composition enhancing prey conditions for larval ctenophores. Estuarine, Coastal and Shelf Science, 133 : 2 –10. Doi: 10.1016/j.ecss.2013.04.019 |
| Miyake H, Terazaki M, Kakinuma Y, 2002. On the polyps of common jellyfish Aurelia aurita in Kagoshima Bay. J.Oceanogr., 58 (3) : 451 –459. Doi: 10.1023/A:1021628314041 |
| Müller W A, Leitz T, 2002. Metamorphosis in the Cnidaria. Canadian Journal of Zoology, 80 (10) : 1755 –1771. Doi: 10.1139/z02-130 |
| Pitt K A, 2000. Life history and settlement preferences of the edible jellyfish Catostylus mosaicus (Scyphozoa:rhizostomeae). Mar. Biol., 136 (2) : 269 –279. Doi: 10.1007/s002270050685 |
| Purcell J E, Atienza D, Fuentes V, Olariaga A, Tilves U, Colahan C, Gili J M, 2012. Temperature effects on asexual reproduction rates of scyphozoan species from the northwest Mediterranean Sea. Hydrobiologia, 690 (1) : 169 –180. Doi: 10.1007/s10750-012-1047-7 |
| Purcell J E, Hoover R A, Schwarck N T, 2009. Interannual variation of strobilation by the scyphozoan Aurelia labiata in relation to polyp density, temperature, salinity, and light conditions in situ. Mar. Ecol. Prog. Ser., 375 : 139 –149. Doi: 10.3354/meps07785 |
| Purcell J E, Uye S I, Lo W T, 2007. Anthropogenic causes of jellyfish blooms and their direct consequences for humans:a review. Mar. Ecol. Prog. Ser., 350 : 153 –174. Doi: 10.3354/meps07093 |
| Purcell J E, 1997. Pelagic cnidarians and ctenophores as predators: selective predation, feeding rates, and effects on prey populations. Annales de L'Institut Océanographique, 73 (2) : 125 –137. |
| Purcell J E, 2007. Environmental effects on asexual reproduction rates of the scyphozoan Aurelia labiata. Mar. Ecol. Prog. Ser., 348 : 183 –196. Doi: 10.3354/meps07056 |
| Purcell J E, 2012. Jellyfish and ctenophore blooms coincide with human proliferations and environmental perturbations. Annu. Rev. Mar. Sci., 4 (1) : 209 –235. Doi: 10.1146/annurev-marine-120709-142751 |
| Richardson A J, Bakun A, Hays G C, Gibbons M J, 2009. The jellyfish joyride: causes, consequences and management responses to a more gelatinous future. Trends Ecol. Evol., 24 (6) : 312 –322. Doi: 10.1016/j.tree.2009.01.010 |
| Schiariti A, Melica V, Kogovšek T, Malej A, 2015. Densitydependent effects control the reproductive strategy and population growth of Aurelia aurita s. l. scyphistomae.Mar. Biol., 162 (8) : 1665 –1672. Doi: 10.1007/s00227-015-2704-y |
| Schiariti A, Morandini A C, Jarms G, Paes R V G, Franke S, Mianzan H, 2014. Asexual reproduction strategies and blooming potential in Scyphozoa. Mar. Ecol. Prog. Ser., 510 : 241 –253. Doi: 10.3354/meps10798 |
| Takao M, Okawachi H, Uye S I, 2014. Natural predators of polyps of Aurelia aurita s. l. (Cnidaria: scyphozoa:semaeostomeae) and their predation rates. Plankton Benthos Research, 9 (2) : 105 –113. |
| Thein H, Ikeda H, Uye S I, 2012. The potential role of podocysts in perpetuation of the common jellyfish Aurelia aurita s. l. (Cnidaria: scyphozoa) in anthropogenically perturbed coastal waters. Hydrobiologia, 690 (1) : 157 –167. |
| Tinta T, Kogovšek T, Malej A, Turk V, 2012. Jellyfish modulate bacterial dynamic and community structure. PLoS One, 7 (6) : e39274 . Doi: 10.1371/journal.pone.0039274 |
| Toyokawa M, Aoki K, Yamada S, Yasuda A, Murata Y, Kikuchi T, 2011. Distribution of ephyrae and polyps of jellyfish Aurelia aurita (Linnaeus 1758) sensu lato in Mikawa Bay, Japan. J. Oceanogr., 67 (2) : 209 –218. Doi: 10.1007/s10872-011-0021-8 |
| Toyokawa M, Furota T, Terazaki M, 2000. Life history and seasonal abundance of Aurelia aurita medusae in Tokyo Bay, Japan. Plankton Biol. Ecol., 47 (1) : 48 –58. |
| Uye S I, 2011. Human forcing of the copepod-fish-jellyfish triangular trophic relationship. Hydrobiologia, 666 (1) : 71 –83. Doi: 10.1007/s10750-010-0208-9 |
| Vagelli A A, 2007. New observations on the asexual reproduction of Aurelia aurita (Cnidaria, Scyphozoa) with comments on its life cycle and adaptive significance. Invertebrate Zoologiya, 4 (2) : 111 –127. |
| Wang S W, Zhang G T, Sun S, Wang Y T, Zhao Z X, 2012. Population dynamics of three scyphozoan jellyfish species during summer of 2011 in Jiaozhou Bay. Oceanologia et Limnologia Sinica, 43 (3) : 471 –479. |
| Watanabe T, Ishii H, 2001. In situ estimation of ephyrae liberated from polyps of Aurelia aurita using settling plates in Tokyo Bay, Japan. Hydrobiologia, 451 (1-3) : 247 –258. |
| Willcox S, Moltschaniwskyj N A, Crawford C M, 2008. Population dynamics of natural colonies of Aurelia sp. scyphistomae in Tasmania, Australia. Mar. Biol., 154 (4) : 661 –670. |
 2017, Vol. 35
2017, Vol. 35