Institute of Oceanology, Chinese Academy of Sciences
Article Information
- ZHANG Zhipeng(张智鹏), TANG Xuexi(唐学玺), TANG Haitian(唐海田), SONG Jingjing(宋静静), ZHOU Jian(周健), LIU Hongjun(刘洪军), WANG Qixiang(王其翔)
- Seasonal variations in the phytoplankton community and the relationship between environmental factors of the sea around Xiaoheishan Island in China
- Chinese Journal of Oceanology and Limnology, 35(1): 163-173
- http://dx.doi.org/10.1007/s00343-016-5241-8
Article History
- Received Sep. 19, 2015
- accepted for publication Oct. 28, 2015
- accepted in principle Nov. 25, 2015
2 Marine Biology Institute of Shandong Province, Qingdao 266104, China;
3 Yantai Marine Environment Monitoring Central Station of State Oceanic Administration, Yantai 264006, China
Phytoplankton is composed of freely floating single celled photosynthetic organisms (Ma et al., 2014), and the community is characterized by high diversity and rapid successional shifts in species composition in response to environmental changes (Gallegos, 1992 ; Glibert et al., 1995). Because of the sensitivity of phytoplankton to environmental factors, changes in the phytoplankton community have long been recognized as a strong indicator of the trophic status and environmental quality of marine areas, rivers, lakes and other water bodies (Reynolds, 1996 ; Thiébaut et al., 2006).
The relationship between the phytoplankton community and environmental factors has been the central focus of aquatic research for decades (Sassi, 1991 ; Pinckney et al., 1998 ; Mamyan et al., 2013 ; Jiang et al., 2014 ; Ma et al., 2014). In recent years marine ecosystems, especially island and bay ecosystems, are attracting more attention. As one of the most important marine ecological systems, island ecosystems are not only different from terrestrial ecosystems but also differ from general marine ecosystems of seawater matrix.
Xiaoheishan Island has an area of 1.36 km2 and a coastline of 5.92 km. It lies in the middle of the Miaodao Archipelago, which is the largest archipelago of Shandong Province. It lies on the dividing line of the Yellow Sea and the Bohai Sea in China and thus has the following three attributes: a location in the mid-latitude temperate area of East Asia, a location in maritime humid climates and a location in a typical ocean archipelago. The phytoplankton community in this area has not been reported, the concentration of chlorophyll a in the adjacent sea area Sanggou Bay is 0.36-9.77 μg/L (Hao et al., 2012). The ecological system of the sea area around Xiaoheishan Island belongs to an ocean archipelago of ecological systems with floating-raft and bottom sowing aquaculture. Thus, it is necessary to understand the phytoplankton community and environmental factors of Xiaoheishan Island. In island ecosystem, because of far from land and the lower population density, the surrounding water of these islands may be free of pollution. Some laboratory measurements and studies found that together with the light, water temperature (Yan et al., 2009 ; Dai et al., 2014) and nutrient (Krom et al., 1991 ; Hou et al., 2006 ; Wang et al., 2009 ; Dou et al., 2011), some heavy metals (Deniseger et al., 1986 ; Girling et al., 2000 ; Zhan et al., 2006 ; Li, 2007 ; Liang et al., 2008 ; Zhang et al., 2009 ; Yang et al., 2010) could promote or inhibit the growth of microalgae significantly. So, we are wondering whether the growth and distribution of microalgae in island ecosystem are influenced by the trace heavy metals. And what is the mainly factor affecting the growth and distribution of microalgae in island ecosystem?
This present study aims to investigate the following:(1) the phytoplankton composition and distribution and the concentration of chlorophyll a in the sea area around Xiaoheishan Island during different seasons and (2) the effect of various environmental factors that have the potential to influence the growth of the phytoplankton, which are evaluated by correlating these factors with the density of phytoplankton.
2 MATERIAL AND METHOD 2.1 Study area and sampling designXiaoheishan Island is located at 37°58′14″N and 120°38′46″E in Shandong Province, China. Four time periods were included in this survey: spring (2013.06.04), summer (2013.08.27), autumn (2013.11.05), and winter (2014.03.19). A total of 10 stations were selected from the sea area around Xiaoheishan Island for sampling and analysis (Fig. 1).
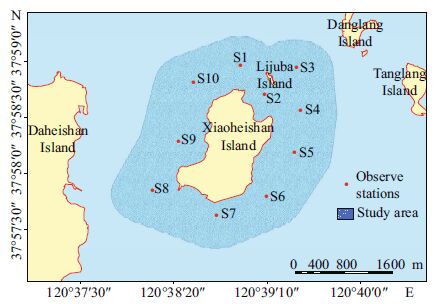
|
| Figure 1 Location of observation stations in the sea area around Xiaoheishan Island |
For taxonomic determination, net hauls (mesh size, 77 μm; inner diameter of net mouth, 37 cm; length, 140 cm) were used at each sampling site to hang the hammer vertically from the bottom to the surface in order to obtain phytoplankton material during sampling (GB 17378.6-2007). Then, a 1-L water sample at each station was fixed with 5% formalin solution for phytoplankton identification and counting. After sedimentation, the phytoplankton was identified and counted on a scaled slide (0.1 mL) with a light microscope (Olympus CX31) at 100× or 400× magnification (Gao et al., 2013). Three replicate samples were collected at each station and each sample was identified and counted 3 times. In this survey, the plankton net with a mesh size of 77 μm cannot catch all of the small-sized phytoplankton species. The vertical distribution of the phytoplankton will be the focus of a future study using water samples.
2.3 Physico-chemical analysisThe concentration of chlorophyll a(Chl a) was determined using spectrophotometry. A total of 17 types of hydrological or water-quality environmental variables, including water temperature (WT), transparency (Tra), salinity (Sal), pH value, dissolved oxygen (DO), chemical oxygen demand (COD), suspend solid (SS), NH4+, NO2-, NO3-, PO43-, Oil, Cu, Pb, Cd, As, and Hg, were measured using the standard methods described in Specifications For Oceanographic Survey (GB/T 12763-2007) and the Specification For Marine Monitoring (GB 17378- 2007) by the general administration of quality supervision, inspection and quarantine of the People's Republic of China (AQSIQ). NH4+ was determined using sodium hypobromite oxidation method; NO3- was determined using zinc cadmium reduction method; PO43- was determined using phosphomolybdate-blue spectrophotometry; Cu, Pb and Cd were determined using anodic stripping voltammetry; As and Hg were determined using atomic fluorescence spectrometry (GB 17378.4-2007; GB/T 12763.4-2007).
2.4 Theory/calculationIn the formulas, n i stands for the individual number of the i -th type (n i) of phytoplankton species, N stands for the number of bionts in the sample, and f i stands for the frequency of the i -th type of individuals appearing in the sample. When the dominance (Y) of a type of phytoplankton was greater than or equal to 0.02(Y ≥0.02), this type of phytoplankton was found to be the dominant species in this survey area (Sun, 2001).
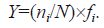
The Shannon-Weaver index (H ') was used to describe the diversity of the phytoplankton of this survey area (Shannon, 1948). The calculation formula of the Shannon-Weaver index is
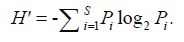
The Pielou index (J) was adopted as the evenness index (Pielou, 1966), whose calculation formula is
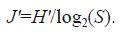
In the formulas, H' represents the diversity index of the species, J ' represents the evenness index of the species, P i represents the ratio of the individual number of the i -th type (n i) of species to the total individual number (N), and S represents the number of species.
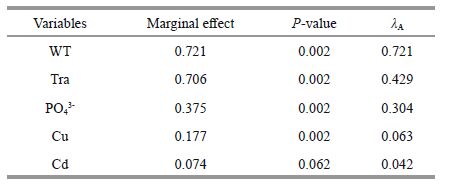
The constrained ordinations were performed using CANOCO for Windows 4.5 software. A constrained ordination method was applied. First, species with a frequency of < 30% of all samples of each season were not included in the analysis in order to eliminate their influence on the ordination results. Constrained ordination (canonical correspondence analysis, CCA) was used to estimate the seasonal variations in the relationship between the community of phytoplankton and environmental factors of the sea area around Xiaoheishan Island (Naqinezhad et al., 2008). Five of the environmental factors were selected by forward selection of CANOCO before analysis to ensure that the environmental factors selected have a higher λ A (conditional effect, it depends on the variables already selected) than the others. A Monte Carlo permutation test with forward selection in CCA was performed to determine the significance of the correlations between the environmental factors and the community of phytoplankton in CCA (Lepš and Šmilauer, 2003). To achieve normality, species data of phytoplankton were log (x +1) transformed prior to analysis (Lacoul and Freedman, 2006). The seasonal variations of the concentration of chlorophyll a were determined using ArcGIS 10.1 software. Seasonal variations in the relationship between nutrients and the concentration of chlorophyll a were performed using SigmaPlot 12.5 software. Analysis of variance (ANOVA) was performed using SPSS 19 software.
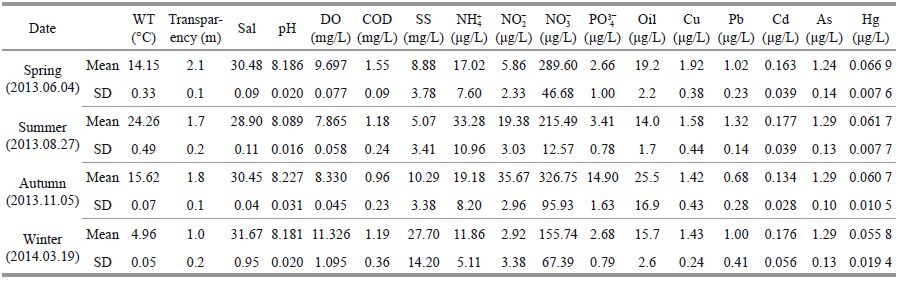
3 RESULT 3.1 Seasonal variations of environmental factors and phytoplankton communitiesA summary of the measured physical and chemical parameters for all seasons is displayed in Table 1. Simple main effects analysis of variance on water temperature (WT), transparency (Tra), salinity (Sal), pH value, dissolved oxygen (DO), chemical oxygen demand (COD), suspend solid (SS), NH4+, NO2-, NO3-, PO43- , and Pb of the sea area around Xiaoheishan Island showed that there was an extremely significant difference between seasons (P < 0.000 1). The water temperature of this area, measured by four cruises at each of the 10st ations, was as low as 4.96±0.05℃ in the winter (2014.03.19), 14.15±0.33℃ in the spring (2013.06.04), 15.62±0.07℃ in the autumn (2013.11.05) and as high as 24.26±0.49℃ in the summer (2013.08.27). There were no significant spatial differences in the water temperature in each cruise.
|
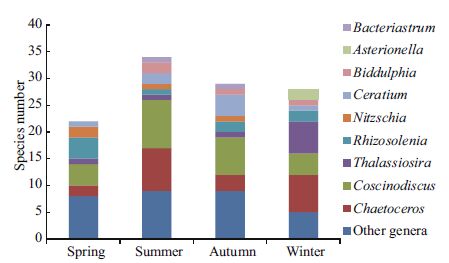
In the samples collected during four cruises at each of the 10st ations around Xiaoheishan Island, a total of 65 species of phytoplankton belonging to three phyla and 27 genera were identified (Fig. 2). Among these species, 56 species belonging to 21 genera in Bacillariophyta represented 86.2% of the total species, eight species belonging to five genera in Pyrrophyta represented 12.3% of the total, and one species belonging to one genus in Chrysophyta represented 1.5% of the total. The species composition and seasonal variations of phytoplankton in the sea area around Xiaoheishan Island are shown in Fig. 2. Chaetoceros contains 13 species representing 20.0% of the total, and Coscinodiscus contains nine species representing 13.8% of the total. The total species number was lowest in spring; there were only 22 species belonging to two phyla and 14 genera, and most of these species belonged to Bacillariophyta (86.4%). Both Coscinodiscus and Rhizosolenia contain four species representing 18.2% each. The greatest number of phytoplankton species were observed during the summer (34 species from three phyla and 17 genera). Among these species, Bacillariophyta represented 88.2% of the total, Pyrrophyta represented 8.8% of the total, and Chrysophyta represented 2.9% of the total. Coscinodiscus contains nine species (26.5%), and Chaetoceros contains eight species (23.5%). There are 29 species belonging to 17 genera that were identified in the autumn. Coscinodiscus contains the most species, with seven total species representing 24.1%. During the winter, 28 species from 12 genera were observed; among them, seven species belong to Chaetoceros(25.0%), and six species belong to Thalassiosira(21.4%).
|
| Figure 2 Species composition and seasonal variations of phytoplankton in the sea area around Xiaoheishan Island Spring: 2013.06.04; Summer: 2013.08.27; Autumn: 2013.11.05; Winter: 2014.03.19. |
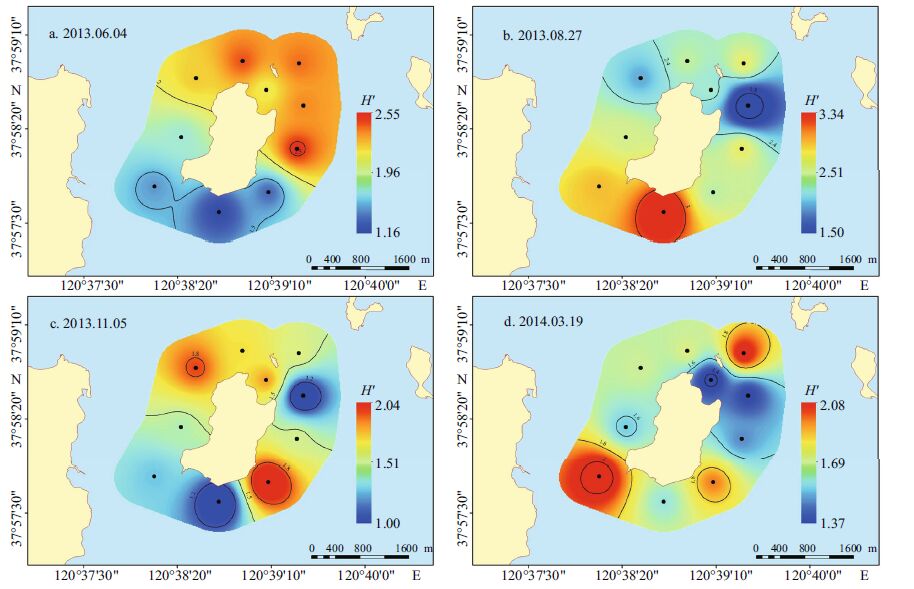
The Shannon-Weaver diversity index (H ') of phytoplankton ranged from 1.00 to 3.34 with an average of 2.37; the Pielou evenness index (J ') ranged from 0.30 to 0.85 with an average of 0.53(Fig. 3). Both of the indexes were highest in the summer. In the spring, the variation in H ' was 1.16-2.55 with a mean of 1.96, and that in J ' was 0.39-0.73 with a mean of 0.58. In the summer, the variation in H ' was 1.50-3.34 with a mean of 2.51, and that in J ' was 0.50-0.85 with a mean of 0.68. In the autumn, the variation in H ' was 1.00-2.04 with a mean of 1.51, and that in J ' was 0.30-0.55 with a mean of 0.42. In the winter, the variation in H ' was 1.37-2.08 with a mean of 1.68, and that in J ' was 0.36-0.54 with a mean of 0.45.
|
| Figure 3 The spatial distribution of the phytoplankton Shannon-Weaver index varied substantially among the seasons a. spring (2013.06.04); b. summer (2013.08.27); c. autumn (2013.11.05); d. winter (2014.03.19). |
The annual average concentration of chlorophyll a for the sea area around Xiaoheishan Island was 3.11 μg/L, 3.52 μg/L in the spring, 1.34 μg/L in the summer, 0.74 μg/L in the autumn and 6.83 μg/L in the winter (Fig. 4). The concentration of chlorophyll a in the winter was higher than that in the other seasons. The spatial distribution of the chlorophyll a concentration varied substantially among the seasons. In the spring, a high chlorophyll a concentration (4.35 μg/L) occurred at the northwest of Xiaoheishan Island (S10) and decreased from the west to the east. In the summer, the chlorophyll a concentration decreased from the south (S7: 1.87 μg/L) to the north, except in the bay between Xiaoheishan Island and Lijuba Island (S2), which had the second highest chlorophyll a concentration (1.59 μg/L). And the chlorophyll a concentration decreased from the southwest (S8: 1.14 μg/L) to the northeast in the autumn.In the winter, the highest chlorophyll a concentration (15.61 μg/L) was at the north of Xiaoheishan Island (S1), and the chlorophyll a concentration of the south (S7) were lower than that of the others.

|
| Figure 4 The spatial distribution of the chlorophyll a concentration varied substantially among the seasons a. spring (2013.06.04); b. summer (2013.08.27); c. autumn (2013.11.05); d. winter (2014.03.19). |
The seasonal variations of dominant species are shown in Table 2. The main dominant species in the sea area around Xiaoheishan Island were Skeletonema costatum, Thalassiosira nordenskioldii and Asterionella kariana(species of the top three highest dominance value Y as 2.72, 0.61 and 0.35, respectively), all belonging to Bacillariophyta and appearing in the winter. The number of dominant species was highest in the summer (14 species), followed by autumn (eight species), spring (six species) and winter (five species). Melosira sulcata was present in all four seasons. In the spring, the genus Rhizosolenia was dominant. In the summer, the genus Chaetoceros was dominant. In the autumn, the genus Coscinodiscus was dominant. Finally, in the winter, S. costatum was dominant.
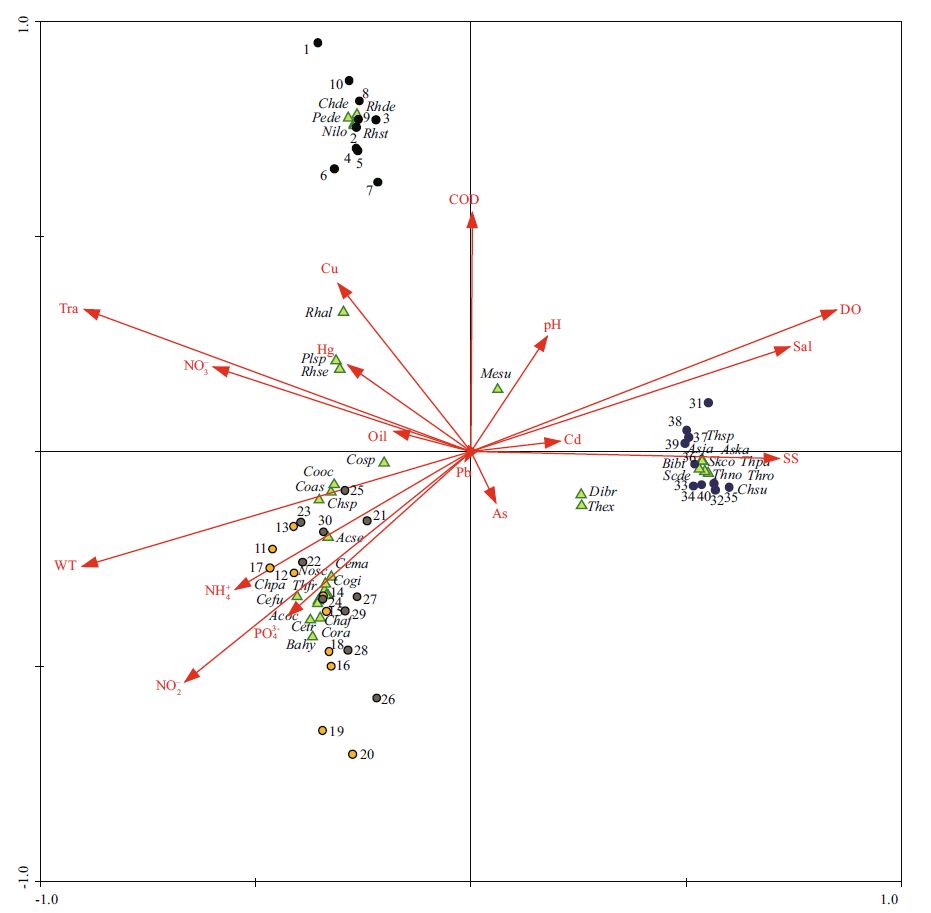
The CCA ordination triplot of the sea area around Xiaoheishan Island in the four seasons, including samples, environmental factors and species, is shown in Fig. 5.
|
| Figure 5 CCA triplots of phytoplankton at the sampling stations, environmental factors and species in the four seasons Sampling sites are represented by circles: black for spring (1-10), yellow for summer (11-20), gray for autumn (21-30) and blue for winter (31-40). Species are represented by green triangles. Nosc: Noctiluca scintillans ; Cefu: Ceratium fusus ; Cetr: Ceratium tripos ; Cema: Ceratium macoceros ; Pede: Peridinium depressum ; Mesu: Melosira sulcata ; Cosp: Coscinodiscus sp.; Coas: Coscinodiscus asteromphalus ; Cogi: Coscinodiscus gigas ; Cooc: Coscinodiscus oculus - iridis ; Cora: Coscinodiscus radiatus ; Asja: Asterionella japonica ; Aska: Asterionella kariana ; Dibr: Ditylum brightwellii ; Scde: Schroederella delicatula ; Plsp: Pleurosigma sp.; Nilo: Nitzschia lorenziana ; Chaf: Chaetoceros affinis ; Chpa: Chaetoceros paradox ; Chsu: Chaetoceros subsecumdus ; Chde: Chaetoceros densus ; Chsp: Chaetoceros sp.; Bibi: Biddulphia biddulphiana ; Thro: Thalassiosira rotula ; Thpa: Thalassiosira pacifica ; Thno: Thalassiosira nordenskioldii ; Thex: Thalassiosira excentrica ; Thsp: Thalassiosira sp.; Skco: Skeletonema costatum ; Rhal: Rhizosolenia alata f. indica ; Rhst: Rhizosolenia stolterfothii ; Rhde: Rhizosolenia delicatula ; Rhse: Rhizosolenia setigera ; Acse: Actinoptychus senarius ; Acoc: Actinocyclus octonarius ; Bahy: Bacteriastrum hyalinum ; Thfr: Thalassiothrix frauenfeldii. |
The eigenvalues of SPE AX1 and SPE AX2 were 0.804 and 0.520, respectively. The first two SPE axes explained 55.4% of the total variance of species data and 70.1% of the species-environment relation. Together, all four of the SPE axes explained 69.3% of the total variance of species data and 87.7% of the species-environment relation. The speciesenvironment correlations for SPE AX1 and SPE AX2 were 0.991 and 0.968, respectively. As shown in Fig. 5, all of the stations from the spring (1-10) located in the upper left of the axis and the stations from the summer and autumn (11-30) have similar environment and phytoplankton compositions, and all of them are located at the lower left of the axis. The rest of the stations are from the winter (31-40) and are located to the right of the axis. There is a significant difference between the stations from each season except summer and autumn. The results of the CCA applied to the environmental factors indicated that water temperature (WT), transparency (Tra), PO43- and Cu significantly influenced the phytoplankton community (P < 0.01; Monte Carlo permutation test). After forward selection of CANOCO, the selection of water temperature (WT), transparency (Tra), PO43- , Cu and Cd includes CCA because of the higher marginal effect or λ A (Table 3). The selected variables explained 82.6% of all variables (1.559 of 1.888). SPE AX1 was negatively correlated with water temperature, transparency, PO43- and Cu (R =-0.90, -0.88, -0.42, -0.30) but positively correlated with Cd (0.20), whereas SPE AX2 was negatively correlated with water temperature and PO43- (-0.26, -0.34) and positively correlated with transparency and Cu (0.33, 0.38). The phytoplankton P. depressum, N. lorenziana , C. densus, R. stolterfothii and R. delicatula are located in the upper left of the axis, the location is near, the niche is similar, and they are typical spring phytoplankton. A. japonica, A. kariana, S. delicatula, C. subsecumdus, B. biddulphiana, T. rotula, T. pacifica, T. nordenskioldii, Thalassiosira sp. and S. costatum are located to the right of the axis, the location is near, the niche is similar, and they are typical winter phytoplankton.
|
In the investigation of the phytoplankton around Xiaoheishan Island, Bacillariophyta contained the most species and had the highest abundance in all four seasons. The results of this investigation are consistent with the results of investigations in both Laizhou Bay in the Bohai Sea (Li et al., 2006) and Sanggou Bay in the Yellow Sea (Mu et al., 2009). In this investigation, S. costatum blooms occur in the winter, but winter is not the peak season for this type of algae. Due to the S. costatum bloom, the concentration of chlorophyll a in the winter is higher than that in other seasons. There are no significant differences among the other three seasons; their data are slightly lower than the historical data but are consistent with the results of the investigation of Laizhou Bay (Li et al., 2006). However, the results of this survey were significantly higher than those of Sanggou Bay (P < 0.01)(Mu et al., 2009).
The diversity index in the summer is higher than that in the other seasons, and the annual average value of the survey results is consistent with the data obtained for Sanggou Bay (Mu et al., 2009). The annual average value of the survey results is higher than that of Laizhou Bay (Li et al., 2006), but the difference is not significant (P > 0.05). M. sulcata is the dominant species in all four seasons.
This study determined that the CCA analysis chart of the year demonstrated that water temperature has the highest marginal effect of all environmental factors, as shown in Fig. 5 the sea water temperature have a higher impact on the distribution of Coscinodiscus (Coscinodiscus sp., C. asteromphalus, C. gigas, C. oculus - iridis, C. radiatus), Ceratium(C. fusus, C. tripos, C. macoceros), and Chaetoceros(C. affinis, C. paradox, C. densus, Chaetoceros sp.), and the water temperature during the summer (24.26±0.49℃) is more suitable for their growth. In the laboratory experiment, research by Yan et al.(Yan et al., 2009) demonstrated that the optimum temperature of Chaetoceros gracilis is 25℃. Research by Dai et al.(Dai et al., 2014) found that the optimum temperature of Chaetoceros curvisetus is 20℃; both of the laboratory experiment results are similar to the results of our survey. Figure 5 shows that S. costatum, Asterionella (A. japonica, A. kariana), and Thalassiosira (T. rotula, T. pacifica, T. nordenskioldii, T. excentrica, Thalassiosira sp.) have less of an impact on the sea water temperature, and the water temperature during the winter (4.96±0.05℃) still did not inhibit their growth. Some research has demonstrated that both S. costatum and Thalassiosira are eurythermic algae (Wang, 1993 ; Zhu et al., 2003). Research by Wang et al.(2006) shows that S. costatum has wide adaptability to temperature, and under conditions of limited nitrogen or phosphorus, there is a difference between the population growth processes of S. costatum at different temperatures. These laboratory experiment results are similar to the results of our survey.
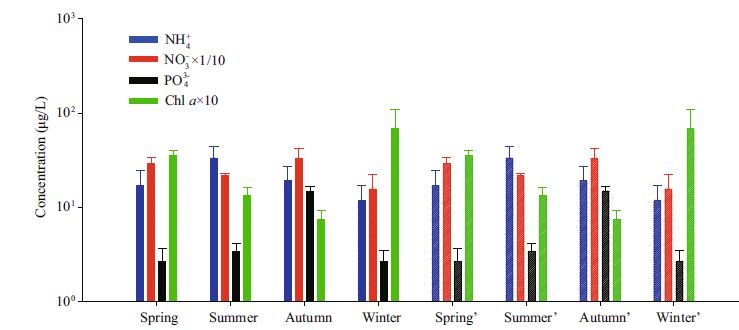
Most studies focus on only one season, and only a few of them focus on seasonal variations. From the seasonal variations of this study, we also observed seasonal variations in the phytoplankton, mainly in terms of changes in nutrients, especially PO43- . The concentration of chlorophyll a is an important symbol of primary productivity and the dynamic changes of phytoplankton. In addition, the distribution of chlorophyll a not only reflects the abundance and the variation of phytoplankton but also serves as the main factor of characterization of the phytoplankton community in the water (Fei et al., 1997 ; Yu et al., 2009). In addition to seasonal changes in the nutrient concentrations and chlorophyll a(Fig. 6), one can easily determine that during the spring, summer and autumn, with the reduced amount of chlorophyll a, the consumption of nutrients is also reduced, leading to an increase in the nutrient concentrations. Among the nutrients, the concentration of PO43- in the three seasons continued to increase, NO3- decreased first and then increased, and NH4+ first increased and then decreased. This result shows that phytoplankton in summer mainly absorb NO3-, phytoplankton from the autumn mainly absorb NH4+, and just before the winter, the concentration of PO43- reached the highest level of the entire year; therefore, a small bloom of S. costatum occurred. With the bloom came reductions in the concentrations of the three types of nutrients in the following spring, the concentration of chlorophyll a began to decrease, while the nutrient concentrations increased. The mesocosm experiment in the Donghai Sea by Hou et al.(2006) proved that under high phosphorus conditions, S. costatum experiences rapid proliferation, and when phosphorus is limited, cell proliferation was inhibited. Both investigations by Krom et al.(1991) in the eastern Mediterranean Sea and Wang et al.(Dou et al., 2011 ; Wang et al., 2009) in the Yellow Sea proved that a lower abundance of phytoplankton in one season is caused by phosphorus limitation but not nitrogen limitation.
|
| Figure 6 Seasonal changes in the concentrations of nutrients and chlorophyll a The values for spring', summer', autumn' and winter' are not the real but, rather, are virtual values for the spring, summer, autumn and winter. |
Most studies on the relationship between biomes and environmental factors of the sea did not take the effect of heavy metals into account. However, our investigation indicated that although the heavy metals (Cd, Cu, and Pb) are already below the first seawater quality standard of China (GB 3097-1997), they still display significant correlations with some types of algae. As shown in Fig. 5, although Cd is trace, it can still significantly inhibit the growth of Rhizosolenia (R. alata f. i ndica, R. stolterfothii, R. delicatula, and R. setigera), Chaetoceros( C. affinis, C. paradox, C. densus, Chaetoceros sp.), Coscinodiscus(Coscinodiscus sp., C. asteromphalus, C. gigas, C. oculus - iridis, C. radiatus) and Ceratium(C. fusus, C. tripos, C. macoceros). This result is consistent with the laboratory experiment results of the effects of Cd on the growth of C. cur visetus by Liang et al.(2008) and Zhan et al.(2006). However, the results of the correlation between Cd and S. costatum, Asterionella(A. japonica, A. kariana), Thalassiosira(T. rotula, T. pacifica, T. nordenskioldii, T. excentrica, Thalassiosira sp.) demonstrate that trace Cd promoted their growth. The laboratory experiment by Zhan et al.(2006) proved that a Cd concentration of 100 μg/L can still promote the growth of S. costatum. The laboratory experiment by Li (2007) proved that the low concentration of Cd can stimulate the growth of Thalassiosira sp. and maintain cell reproduction and growth at a high rate. The trace Cu can promote the growth of Rhizosolenia(R. alata f. indica, R. stolterfothii, R. delicatula, and R. setigera) while inhibiting the growth of S. costatum, Asterionella(A. japonica, A. kariana) and Thalassiosira(T. rotula, T. pacifica, T. nordenskioldii, T. excentrica, Thalassiosira sp.). Research by Deniseger et al.(1986) showed that Rhizosolenia sp. became the dominant species in the Buttle Lake, which has a higher concentration of Cu. While the laboratory experiment by Zhang et al.(2009) shows that a Cu concentration of 100 μg/L can still promote the growth of S. costatum, the field experiment by Wang et al.(2007) in the Jiaozhou Bay of Yellow Sea indicated that even with the addition of 4 μg/L Cu, the growth of S. costatum can be significantly inhibited, with increasing Cu concentration increasing the toxic effect on S. costatum(Zhang et al., 2009). Research by Girling et al.(2000) also noted that the ecotoxicity of Cu in the laboratory is much lower than that in the actual environment. The results demonstrate that trace Pb promotes the growth of Chaetoceros(C. affinis, C. paradox, C. densus, Chaetoceros sp.) but inhibits the growth of Coscinodiscus( Coscinodiscus sp., C. asteromphalus, C. gigas, C. oculus - iridis, C. radiatus). The concentration of Pb in this field survey is far below the optimum growth concentration of Pb for C. curvsieust(454 μg/L), as determined by Zhang (2004) in the laboratory. Yang et al.(2010) found that a Pb concentration of 1 000 μg/L can still play a promoting role in Chaetoceros muelleri growth. It can also be determined from Fig. 5 that there are negative relationships between Cu, Cd and PO43- . This result has been demonstrated by Kuang et al.(1996), as his research indicated that PO43- can play a very important role in reducing the toxicity of Cu and other heavy metals in algae. He also noted that there is an antagonistic effect between Cu and Cd in biology.
5 CONCLUSIONIn this investigation, Bacillariophyta contained the largest number of species all year round. The results of the Shannon-Weaver index and the Pielou index demonstrate that there is a high biodiversity of phytoplankton in the sea area around Xiaoheishan Island; the community structure of phytoplankton is relatively stable. The distribution of the phytoplankton community is mainly determined by water temperature, nutrients (especially PO43- ) and heavy metals (Cd, Cu, and Pb). Although the concentration of heavy metals is well up to the state standards of the first grade of China, these metals still impact the phytoplankton community from this area.
| Dai A Q, Shi X Y, Ding Y Y, Tang H J, Wang L S, Wang X L, 2014. Effects of temperature on the growth and nitrate reductase activity of Chaetoceros curvisetus and Karenia mikimotoi. Progress in Biochemistry and Biophysics, 41 (9) : 896 –903. |
| Deniseger J, Austin A, Roch M, Clark M J R, 1986. A persistent bloom of the diatom Rhizosolenia eriensis (Smith) and other changes associated with decreases in heavy metal contamination in an Oligotrophic lake, Vancouver Island. Environmental and Experimental Botany, 26 (3) : 217 –226. Doi: 10.1016/0098-8472(86)90033-X |
| Dou Y, Tang X X, Yang Z, Wang Y, 2011. Effect of nutrients structure on primary production in offshore of Shandong Province. Marine Environmental Science, 30 (2) : 177 –181. |
| Fei Z L, Tree C C, Li B H, 1997. Estimating primary productivity using chlorophyll data. Journal of Oceanography of Huanghai & Bohai Seas, 15 (1) : 35 –47. |
| Gallegos C L, 1992. Phytoplankton photosynthesis, productivity, and species composition in a eutrophic estuary: comparison of bloom and non-bloom assemblages. Marine Ecology Progress Series, 81 (3) : 257 –267. |
| Gao Y, Jiang Z B, Liu J J, et al, 2013. Seasonal variations of net-phytoplankton community structure in the southern Yellow Sea. Journal of Ocean University of China, 12 (4) : 557 –567. Doi: 10.1007/s11802-013-2258-x |
| Girling A E, Pascoe D, Janssen C R, Peither A, Wenzel A, Schäfer H, Neumeier B, Mitchell G C, Taylor E J, Maund S J, Lay J P, Jüttner I, Crossland N O, Stephenson RR, Persoone G, 2000. Development of methods for evaluating toxicity to freshwater ecosystems. Ecotoxicology and Environmental Safety, 45 (2) : 148 –176. Doi: 10.1006/eesa.1999.1847 |
| Glibert P M, Conley D J, Fisher T R, et al, 1995. Dynamics of the 1990 winter/spring bloom in Chesapeake Bay. Marine Ecology Progress Series, 122 : 27 –43. Doi: 10.3354/meps122027 |
| Hao L H, Sun P X, Hao J M, Du B B, Zhang X J, Xu Y S, Bi J H, 2012. The spatial and temporal distribution of chlorophyll-a and its influencing factors in Sanggou Bay. Ecology and Environmental Sciences, 21 (2) : 338 –345. |
| Hou J L, Zhang C S, Shi X Y, Lu R, Wang X L, 2006. Effect of phosphate on two typical HAB species in East China Sea by mesocosm experiments. Periodical of Ocean University of China, 36 (S) : 163 –169. |
| Jiang Y J, He W, Liu W X, Qin N, Ouyang H L, Wang Q M, Kong X Z, He Q S, Yang C, Yang B, Xu F L, 2014. The seasonal and spatial variations of phytoplankton community and their correlation with environmental factors in a large eutrophic Chinese lake (Lake Chaohu). Ecological Indicators, 40 : 58 –67. Doi: 10.1016/j.ecolind.2014.01.006 |
| Krom M D, Kress N, Brenner S, Gordon L I, 1991. Phosphorus limitation of primary productivity in the eastern Mediterranean Sea. Limnology and Oceanography, 36 (3) : 424 –432. Doi: 10.4319/lo.1991.36.3.0424 |
| Kuang Q J, Xia Y, Hui Y, 1996. Toxic effects of heavy metals on algae. Acta Hydrobiologica Sinica, 20 (3) : 277 –283. |
| Lacoul P, Freedman B, 2006. Relationships between aquatic plants and environmental factors along a steep Himalayan altitudinal gradient. Aquatic Botany, 84 (1) : 3 –16. Doi: 10.1016/j.aquabot.2005.06.011 |
| Lepš J, Šmilauer P. 2003. Multivariate Analysis of Ecological Data Using CANOCO. Cambridge University Press, Cambridge, UK. p.149-162. |
| Li C D. 2007. The biological absorption and response of marine algae and mussels on trace metal (Zn, Cd, Cu).South China Sea Institute of Oceanology Academy of Sciences, Guangzhou, China. (in Chinese) |
| Li G L, Chen B J, Cui Y, Ma S S, Tang X X, 2006. Ecological characteristics of phytoplankton in the Laizhou Bay. Journal of Fishery Sciences of China, 13 (2) : 292 –299. |
| Liang Y, Wang S, Feng L X, Tian C Y, 2008. Effects of heavy metal stress on the growth and chlorophyll fluorescence of Chaetoceros gracilis. Periodical of Ocean University of China, 38 (1) : 59 –67. |
| Ma Y, Li G B, Li J, Zhou H, Jiang B, 2014. Seasonal succession of phytoplankton community and its relationship with environmental factors of North Temperate Zone water of the Zhalong Wetland, in China. Ecotoxicology, 23 (4) : 618 –625. Doi: 10.1007/s10646-014-1231-9 |
| Mamyan A S, Hambaryan L R, Martirosyan A E, 2013. Phytoplankton community growth interconnectivity with some abiotic environmental factors in Pambak and Tandzut Rivers. Electronic Journal of Natural Sciences, 20 (1) : 33 –37. |
| Mu J D, Dong W, Chen B J, Wang W, Fang J G, Tang X X, 2009. Ecological characteristics of phytoplankton in Sanggou Bay. Progress in Fishery Sciences, 30 (3) : 91 –96. |
| Naqinezhad A, Hamzeh'ee B, Attar F, 2008. Vegetationenvironment relationships in the Alderwood Communities of Caspian Lowlands. N. Iran (toward an ecological classification). Flora- Morphology, Distribution, Functional Ecology of Plants, 203 (7) : 567 –577. Doi: 10.1016/j.flora.2007.09.007 |
| National Marine Environmental Monitoring Center. 2007. GB 17378-2007 The specification for marine monitoring.China Standard Press, Beijing. (in Chinese) |
| Pielou E C, 1966. The measurement of diversity in different types of biological collections. Journal of Theoretical Biology, 13 : 131 –144. Doi: 10.1016/0022-5193(66)90013-0 |
| Pinckney J L, Paerl H W, Harrington M B, Howe K E, 1998. Annual cycles of phytoplankton community-structure and bloom dynamics in the Neuse River Estuary, North Carolina. Marine Biology, 131 (2) : 371 –381. Doi: 10.1007/s002270050330 |
| Reynolds C S, 1996. The plant life of the pelagic. Verhandlungen-Internationale Vereinigung für Theoretische und angewandte Limnologie, 26 : 97 –113. |
| Sassi R, 1991. Phytoplankton and environmental factors in the Paraíba do Norte River Estuary, northeastern Brazil:composition, distribution and quantitative remarks. Boletim do Instituto Oceanográfico, 39 (2) : 93 –115. |
| Shannon C E, 1948. A mathematical theory of communication. The Bell System Technical Journal, 27 (3) : 379 –423. Doi: 10.1002/bltj.1948.27.issue-3 |
| Sun R Y, 2001. Principles of Animal Ecology. Beijing Normal University Press, Beijing. |
| Thiébaut G, Tixier G, Guérold F, Muller S, 2006. Comparison of different biological indices for the assessment of river quality: application to the upper river Moselle (France). Hydrobiologia, 570 (1) : 159 –164. Doi: 10.1007/s10750-006-0176-2 |
| Third Institute of Oceanography, State Oceanic Administration. 1997. GB 3097-1997 Marine water quality standard.Environmental Science Press, Beijing. (in Chinese) |
| Third Institute of Oceanography, State Oceanic Administration. 2007. GB/T 12763-2007 Specifications for oceanographic survey. China Standard Press, Beijing. (in Chinese) |
| Wang C Y, Wang X L, Sun B Y, Su R G, 2007. In situ test of copper on Skeletonema costatuma ecotoxicological effect. China Environmental Science, 27 (5) : 703 –706. |
| Wang S P, 1993. Preliminary study on the culture of Skeletonema costatum. Fisheries Science, 12 (10) : 9 –11. |
| Wang Y, Dou Y, Tang X X, Yang Z, 2009. The spatial and temporal distribution of primary production in the Yellow Sea nearshore area of Shandong Province. Periodical of Ocean University of China, 39 (4) : 633 –640. |
| Wang Z L, Li R X, Zhu M Y, Chen B Z, Hao Y J, 2006. Study on population growth processes and interspecific Competition of Prorocentrum donghaiense and Skeletonema costatum in semi-continuous dilution experiments. Advances in Marine Science, 24 (4) : 495 –503. |
| Yan A J, Wu H X, Xue J Z, Wang J H, Liu Y, 2009. The research of optimal factors on the growth of Chaetoceros gracilis population. Journal of Aquaculture, 30 (4) : 38 –41. |
| Yang Y H, Luo B, Zhao Y Z, Wei P Y, Wang D P, Chen X H, 2010. Effects of three heavy metal ions on growth of diatom Chaetoceros muelleri. Journal of Dalian Fisheries University, 24 (S) : 1 –7. |
| Yu Q Y, Tang X X, Du E Z, Yang Z, Wang Y, 2009. The distribution and annual fluctuation of Chl a in Jiaozhou Bay. Periodical of Ocean University of China, 39 (S1) : 120 –126. |
| Zhan Y J, Wang X L, Yang R J, Zhang Y Y, 2006. Effects of Cd(Ⅱ) on the growth of 8 species of marine microalgae. Chinese Journal of Environmental Science, 27 (4) : 720 –726. |
| Zhang Y Y, Wang X L, Zhan Y J, 2009. Effects of Cu (Ⅱ) on the growth of marine algae. Asian Journal of Ecotoxicology, 4 (1) : 114 –122. |
| Zhang Y Y. 2004. Effects of Pb (Ⅱ) on the growth of marine algae. Ocean University of China, Qingdao, China. (in Chinese) |
| Zhu M, Zhang X C, Mao Y X, Yan B L, Teng Y J, Lu Z H, 2003. Effects of temperature, salinity and illumination on the growth of Thalassiosira sp. Marine Sciences, 27 (12) : 58 –61. |
 2017, Vol. 35
2017, Vol. 35



