Institute of Oceanology, Chinese Academy of Sciences
Article Information
- QIAN Kuimei(钱奎梅), LIU Xia(刘霞), CHEN Yuwei(陈宇炜)
- Effect of hydrological variability on diatom distribution in Poyang Lake, China
- Chinese Journal of Oceanology and Limnology, 35(1): 174-184
- http://dx.doi.org/10.1007/s00343-016-5264-1
Article History
- Received Sep. 19, 2015
- accepted for publication Nov. 10, 2015
- accepted in principle Jan. 8, 2016
2 College of Environmental Engineering, Xuzhou Institute of Technology, Xuzhou 221111, China
Diatoms are ubiquitous in freshwater environments, and along with other algae, they are considered of fundamental importance in the maintenance of optimum freshwater productivity. Diatom communities are a popular tool for monitoring past and present environmental conditions and commonly used in studies of water quality (Prygiel and Coste, 1993 ; Sorvari et al., 2002 ; Dong et al., 2008). The increased frequency of planktonic diatoms in lakes with some turbulence is conspicuous when cyanobacteria decline as a result of reversed eutrophication (Willén, 1991). Diatoms are commonly dominant in deep lakes but frequently less abundant than cyanobacteria in shallow lakes (Chen et al., 2003). Diatoms are sensitive to a number of environmental and biological variables, such as stratification and lake mixing (Lewis Jr, 1978) or high silica and phosphorus concentrations (Tilman et al., 1982, 1986). Diatoms respond to these variables by shifting their community composition and growth forms. Because of their siliceous skeleton, diatoms become scarce when silicon is depleted in lakes (Schelske et al., 1984 ; Martin-Jézéquel et al., 2000). Thus, diatoms are useful biological indicators for investigating ecological responses to natural and anthropogenic hydrological and nutrient regimes in lakes and can be used to track eutrophication (Dong et al., 2008) and detect post-19th century Arctic warming (Sorvari et al., 2002).
Floodplains are characterized by a high level of habitat heterogeneity, including wetlands and shallow lakes as significant biotopes (Mihaljević et al., 2009, 2000). In floodplain lakes and wetlands, floodwater alters the physical habitat and chemical characteristics of these habitat by serving as the major source of water, nutrient and sediments (Forsberg et al., 1988 ; Junk et al., 1989 ; Van den Brink et al., 1992). The regular floodpulse makes floodplain wetlands existing in four phases: low water level phase, when the wetland and shallow lakes are physically separated with no surface water exchange, and high water level phase, where floodwaters dissolve the ecotone between the wetland and shallow lakes and they can be viewed as one hydrologic unit, and two transition phases between the above two. In turn, algal communities in shallow, floodplain wetlands are shaped by the interplay of internal forces and the periodic external forcing of floodwaters (de Emiliani, 1993 ; Weilhoefer et al., 2008). The variable environment made wetlands heterogeneous, posing challenges to wetland monitoring and assessment. Usually, diatom was used as indicators of various environmental parameters, such as phosphorus and water level (Pan and Stevenson, 1996 ; Nogueira, 2000), as their narrow tolerances for many environmental variables, and their rapid responses to environmental change. The study of de Emiliani (1997) indicated that the absolute and relative biomass of diatoms increased with the water retention time, and they were inversely correlated with the water level.
As one of the few lakes that are freely connected to the river, Poyang Lake plays an important role in the maintenance of the unique biota of the Changjiang (Yangtze) River floodplain ecosystem (Wang et al., 2007). A thorough knowledge of freshwater diatom ecology is fundamental to adequate ecological interpretation and assessment of aquatic environments in Poyang Lake. The objective of this study was to investigate the spatial and temporal variations of diatom biomass and species composition through the four different hydrological phases of Poyang Lake. We hypothesized that variations in diatom biomass and community composition were primarily caused by changes in the physical and chemical environmental parameters, with these changes driven by seasonal water-level fluctuations.
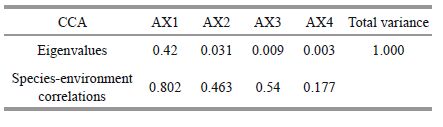
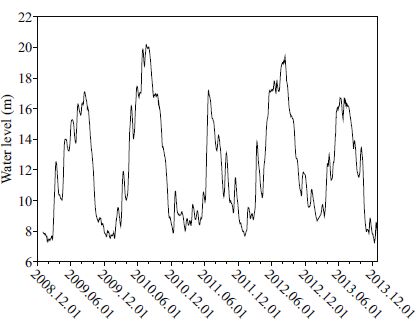
2 MATERIAL AND METHODPoyang Lake (29°7′31.1″N, 116°16′39.7″E) is located at the junction of the middle and lower reaches of the Changjiang River. This lake has a monsooninfluenced humid subtropical climate with four distinct seasons that are characterized by a mean annual precipitation of 1 680 mm and annual average temperature of 17.5℃. Poyang Lake has four distinct recurring seasonal water-level phases (Fig. 1). The lake surface elevation decreases to less than 10 m above sea level (Wu Song) at Xingzi, the lake area typically decreases to less than 1 000 km 2(Fig. 2), and it functions primarily as a river delta from December through March in winter. The water level increases and continues increasing to approximately 14 m above sea level from April to June in spring. The high water-level phase occurs from July to September in summer, with water levels higher than 14 m above sea level, and the lake acts as a large lake, with the surface area capable of exceeding 4 000 km 2. Typically, the water level dramatically decreases to approximately 10 m from October through November in autumn and remains in a low water-level phase in winter. Poyang Lake is influenced by human activities, such as land reclamation, levee construction, fishery operations, sand excavation and transportation.
|
| Figure 1 The water level variations of Poyang Lake from 2009 to 2013 |
|
| Figure 2 Study area: Poyang Lake at the different water levels Light grey: water level 20 m; dark grey: water level 13 m. |
To examine seasonal and hydrological trends, water samples were collected in the four representative phases of regular water-level fluctuations from 2009 to 2013. The water samples were collected at 15st ations by a ‘Ruttner'-sampler at three depths (surface, middle and bottom layers of the lake) in each phase. Stations 1-5 were in the south of Poyang Lake, the area of which changed dramatically between low water level phase and high water level phase. Stations 6-8 were in the outlet of the river or the isolated floodplain lake connecting with Lake Poyang. Stations 9-15 were in the north of Lake Poyang, i.e. the main channel, which changed little all over the year. Integrated samples were blended with water samples collected from three depths for the entire water column of the lake for physico-chemical analyses and phytoplankton examinations. Samples were taken to laboratory for analyzing the nutrient, and preserved with Lugol (4%) iodine solution and stored in the dark at a low temperature (4℃) for phytoplankton enumeration (1 L). Phytoplankton in the subsamples (30 mL) were counted with an inverted microscope after settling for 24-48 h following the method of Utermöhl (1958). Algal taxa were identified according to Hu Wei (2006) and Krammer and Lange- Bertalot (1986, 1988, 1991a, 1991b). A minimum of 500 diatom valves were counted at 1 000× magnification. Biomass was enumerated following microscope measurements of size and the geometrical shapes (Hillebrand et al., 1999). Water transparency was determined using a Secchi disk, and the water temperature, salinity, dissolved oxygen (DO), pH, oxidation-reduction potential (ORP), turbidity and salinity were measured in situ with the multiparameter profiler YSI 6600 V2(Yellow Springs, Ohio, USA). The suspended solids (SS), chlorophyll a (Chl- a), and nutrient concentrations (TN, TP, NO2-N, NO3-N, NH4 -N, and PO4 -P) were analyzed according to the American Public Health Association (APHA, 1998). Both physicochemical parameters and phytoplankton samples were analyzed in triplicate, and mean values were used for quantification.
Figures were constructed using the software program SigmaPlot (version 10.0), and all of the calculations were performed using the statistical package SPSS for Windows (version 17.0). The seasonal average values for the phytoplankton biomass of 15st ations were used in Fig. 3, and the diatom biomass data in the pie charts (Fig. 4) were the average diatom biomass over 5 years per season at each site. An analysis of variance (ANOVA) was used to determine whether the temporal variation in algal biomass was related to the collection periods. A redundancy analysis (RDA) biplot of the main water features and phytoplankton biomass was constructed to determine whether the phytoplankton distribution and composition were correlated with the physicochemical parameters The RDA was performed according to Lepš and Šmilauer (2003). The DCA and RDA were performed with CANOCO version 4.5 for Windows (Biometrics-Plant Research International, the Netherlands).
|
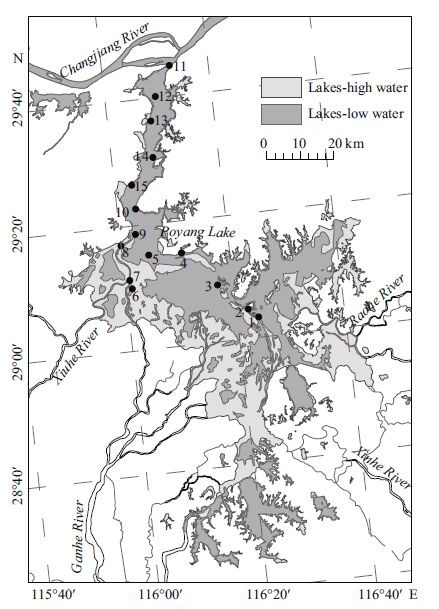
| Figure 3 Variations in the phytoplankton biomass and monthly average values of water level (a), diatom biomass (b) and relative biomass of diatom genera (c) in different sampling time in Poyang Lake The box-plot indicates the median, the 25% and 75% quartile and the range of the values; solid circle indicates outlier. Note: median was calculated for 15st ations. |
|
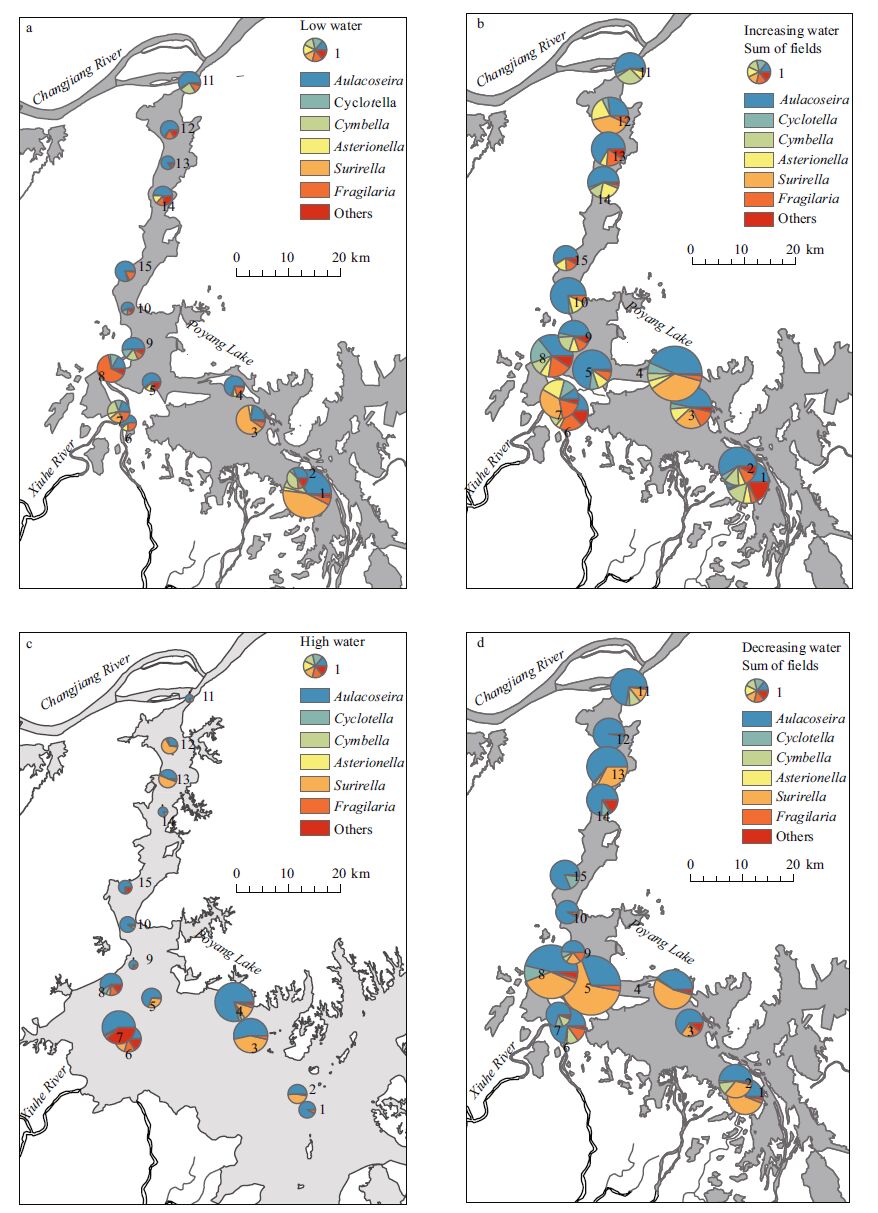
| Figure 4 Diatom biomass and species contribution during the four water level phases The size of the pie charts is equivalent to total diatom biomass. a. low water level phase; b. increasing water level phase; c. high water level phase; d. decreasing water level phase. |
In total, 27 diatom genera and 137 species were identified over the 5-year sampling period, and the list of dominant diatom species (contributing more than 5% of biomass) at different water phases and localities are shown in Table 1. The phytoplankton and diatom communities drastically changed throughout the study period (Fig. 3a), during which the algal assemblage of the open water was abundant and presented an average biomass of 0.04 mg/L in 2009, 0.25 mg/L in 2010, 0.34 mg/L in 2011, 6.46 mg/L in 2012, and 3.95 mg/L in 2013 and the average diatom biomass from 2009-2013 was 0.017, 0.19, 0.23, 3.91, and 2.30 mg/L. Diatoms were generally dominant in Poyang Lake from 2009 to 2013 and accounted for more than 50% of the total phytoplankton biomass except in July 2009(26%) and January 2012(35%)(Fig. 3a, b). Bacillariophyta were primarily dominated by centric diatoms (Aulacoseira granulata and occasionally Cyclotella meneghiniana) and two pennate diatoms (Surirella robusta and Staurosirella pinnata). Fragilaria spp. and Aulacoseira spp. were predominant in 2009-2011, accounting for 5%-77% and 17%-86% of the total phytoplankton biomass, respectively. Aulacoseira spp. and Surirella were predominant in 2012-2013, accounting for 25%-57% and 5%-15% of the total phytoplankton biomass, respectively (Fig. 3c).
|
As shown in Fig. 4, the hydrological phases and locations influenced the diatom biomass and community composition. In general, higher diatom biomass was observed in phases when the water levels increased or decreased, and lower biomass was observed in phases when the water level was low or high, and the biomass of diatom in the outlet and south region was higher than those in the channel. A. granulata and S. robusta were the dominant diatom species in Poyang Lake, and the diatom biomass and ratios of A. granulata were higher during decreasing water-level phases and relatively low during increasing water-level phases.
The temporal development of diatom biomass in the 15 sampling stations is reported in Fig. 4. The diatom biomass at stations 6-8 accounted on average for approximately one third of the total diatom biomass. PY8 is the outlet of Banghu Lake, which is connected to the main channel of Poyang Lake. The excessive and frequent fishery operations in Banghu Lake have caused eutrophication in this region. Similarly, stations PY4 and PY6 are outlets of local regions or river inlets connected to the main rivers and channels of Poyang Lake.
In the low water-level phase, the diatom biomass was relatively low in almost the stations, ranging between 0.31 and 4.47 mg/L. The highest biomass of A. granulata and S. robusta at PY1 in the south were 1.45 and 2.01 mg/L, respectively. In the increasing water-level phase, the diatom biomass increased in almost the stations, ranging from 1.13 to 5.51 mg/L, with the highest biomass of A. granulata and S. robusta at PY4 at 2.40 and 2.01 mg/L, respectively. In the high water-level phase, the diatom biomass ranged between 0.12 and 2.82 mg/L with the highest biomass of A. granulata at PY4(2.24 mg/L) and at PY3(1.20 mg/L). In the decreasing water-level phase, the diatom biomass ranged between 1.00 and 5.35 mg/L, which presented the highest biomass of A. granulata at PY8(2.80 mg/L) and S. robusta at PY5(2.89 mg/L).
Overall, diatom assemblages in the river and wetland differed, coinciding with the water level fluctuations. The channel and south region were dominated by planktic taxa throughout much of the study. A. granulata was the most abundant taxon in the channel and present at each sampling date. During the low water level phase, diatom biomass was low and the relative biomass of A. granulata in the channel and south regions was higher than 50% in most of the sampling stations. S. robusta was dominant in outlet and the relative biomass of S. robusta in outlet was higher than 25%. In increasing water level phase, Aulacoseira granulata was the most abundant taxon in the channel and south region and the relative biomass was higher than 50%. In high water level phase, Aulacoseira granulata was the most abundant taxon in almost all the sampling stations, and the relative biomass ranged between 40%-95%. In decreasing water level phase, Aulacoseira granulata was the most abundant taxon in the channel and the relative biomass was higher than 50%, S. robusta was the most abundant taxon in the outlet and south region and the relative biomass ranged between 14%-65%. The ratios of A. granulata among diatoms in the channel and lentic regions were all high in the high water-level phase, whereas the ratios of S. pinnata among diatoms at most of the sampling stations were higher during the low water-level phases and increasing water-level phases.
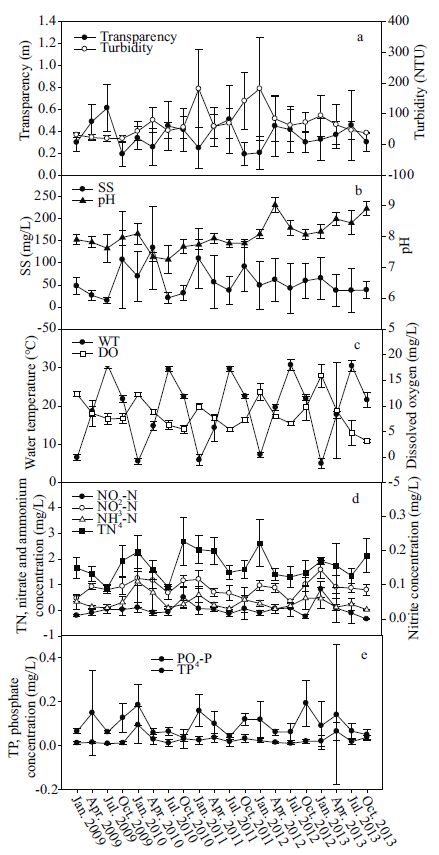
3.2 Relationship of diatom distribution and physicochemical parameters in Poyang LakeThe variations of physicochemical parameters in Poyang Lake were shown in Fig. 5. Fluctuations of the Poyang Lake flooding regime varied significantly on a yearly scale. Regular and frequent flooding characterized the hydrological regime, whereas extremely high and regular flooding caused mixing between lotic and lentic waters during the high waterlevel phases. Different types of environmental changes occurred and induced alterations of the diatom biomass and species distribution. The nutrient concentrations in Poyang Lake were sufficient to support algal growth (Wu et al., 2013). PH value of the Poyang Lake was above 7.5, with the average value of 8.0. Water temperature show a regular seasonal pattern, ranged from 6 to 33℃. Dissolved oxygen fluctuated with the season and displayed a clear pattern; the lowest values were recorded in January (Fig. 5c). Transparency was usually higher in high water level phase, and turbidity show an opposite pattern with transparency. Total nitrogen concentrations show a clear seasonal pattern in the study period, with small values in July (high water level phase). Nitrate and ammonium concentrations exhibited undulating variations in the study period. Total phosphorus concentrations had an irregular fluctuation and phosphate concentrations had a slight fluctuation in the study period.
|
| Figure 5 Water physical and chemical parameters of Poyang Lake through 2009-2013 Average values, with standard error, shown for all the parameters. a. Secchi disk and turbidity; b. SS and pH; c. water temperature and dissolved oxygen; d. total nitrogen, nitrate, nitrate and ammonium; e. total phosphorus and phosphate. |
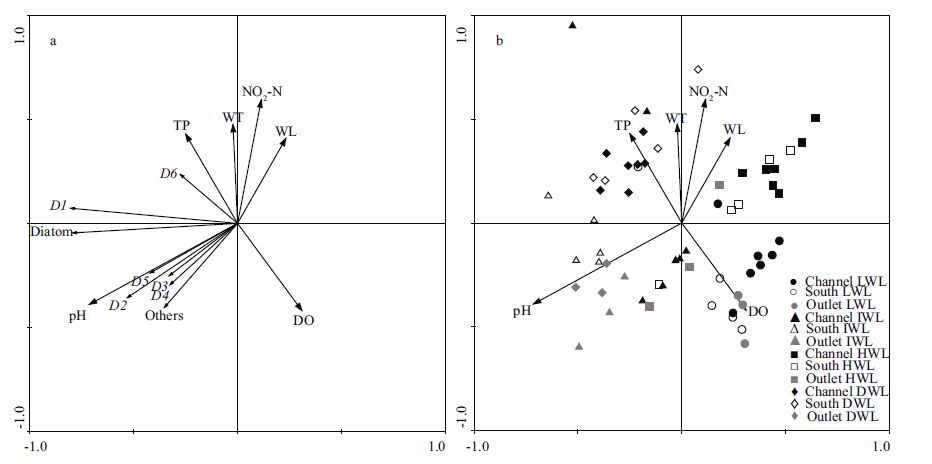
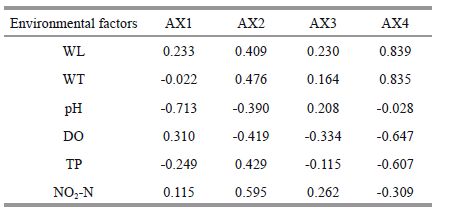
The relationship between phytoplankton biomass and environmental conditions, which was shown by the RDA analysis (Fig. 6), indicates that nutrient fluctuations were related to the water level and temperature. The canonical correspondence analysis (CCA) performed with nine abiotic variables (the ones representing the highest correlation with the first two axes of the CCA, thus avoiding redundant variables and maintaining ecological importance for diatom) and all the diatom species accounted to eigenvalues of 0.420 and 0.031 for the first two axes, respectively (Table 2). The Pearson correlation of environment-species was high for both axes (R =0.802 and R =0.463), and indicated a strong correlation between abiotic variables and species distribution. The Monte Carlo test (99 permutations; P<0.05) demonstrated that the ordination of axes 1 and 2 was statistically significant (P =0.01), and did not occur at random. Canonical coefficient pointed out that pH (-0.713) and DO (0.310) were the most important variables to axis one ordination and NO2 -N (0.595) and water temperature (0.476) to axis two. According to the CCA, pH was the most important environmental factors affecting the diatom species composition Aulacoseira spp.(D1), Cyclotella spp.(D2), Fragilaria spp.(D4), Asterionella sp.(D5) and the other species in Poyang Lake, DO was the most important environmental factors affecting the diatom species Surirella spp.(D6) in Poyang Lake. Surirella spp.(D6) was associated with TP and DO.
|
| Figure 6 RDA biplots based on the diatom biomass (a) and the significant environmental variables (b)(P<0.05) in Poyang Lake in 2009-2013 The environmental variables: water temperature (WT), dissolved oxygen (DO), Salinity (SAL), nitrates (NO 3), nitrites (NO 2), total nitrogen (TN), total phosphorus (TP), oxidation reduction potential (ORP), suspended solids (SS), nitrite (NO 2 -N). The diatom biomass (diatom, Aulacoseira spp.(D1), Cyclotella spp.(D2), Cymbella spp.(D3), Fragilaria spp.(D4), Asterionella sp.(D5), Surirella spp.(D6), others. |
|
The response of the diatom assemblage among four hydrological phases was variable (Fig. 6b). Diatom assemblages in four different hydrological phases were separated by the first and second ordination axis, with LWL sampling points clustering on the bottom-right side of the ordination diagram, IWL sampling points clustering on the bottom-left side, HWL sampling points clustering on the top-right side and DWL sampling points clustering on the topleft side. At the same time, the response of the diatom assemblage were variable among different regions. Diatom assemblages in channel and outlet were separated by the first and second ordination axis, with channel sampling points clustering on the right side of the ordination diagram, and outlet sampling points clustering on the bottom side.
4 DISCUSSIONDuring floods, there is an exchange of water, sediment, chemicals and biota between the main channel and the floodplain lakes, and these exchanges are instrumental in shaping the water quality and diatom assemblage of floodplain wetlands (de Emiliani, 1993 ; Weilhoefer et al., 2008). Diatoms typically represent an important link between river and floodplain algal communities under conditions of flooding (Mihaljević et al., 2013). In our study, floodwaters altered wetland habitat and water chemistry and the resulting changes to the diatom assemblage appear to be related to the water level fluctuations. Floodwater brought nutrient into the main channel from the upstream river and floodplain lakes with excessive agricultural chemical inputs and aquaculture and waste discharge. A higher abundance of diatoms in the lake during the mixing of river and floodplain waters may have been partly caused by their input from potamoplankton communities. The influence of the main channel on the increased diatom biomass in the adjacent floodplain can be strong and has been thoroughly documented in the Lower Rhine and Meuse floodplains (Van den Brink et al., 1994).
The seasonal and annual fluctuation of water level fluctuations lead to the habitat alteration of diatoms in Poyang Lake, such as nutrients, lake area and flow variability (Gasith and Resh, 1999). Diatom seasonality was strongly related to the flood pulse in Poyang Lake. Higher diatom biomass occurred in both increasing water level phases and decreasing water level phases while the lower average biomass occurred in low and high water level phases. The input of nutrients from a flood event produced an increase in diatom biomass in increasing water level phase. Although high temperature is usually accompanied by high transparency and high light intensity in summer, average biomass was lower in the high water level phase mainly because of dilution and washout effects. There were a concentrating of diatom and nutrient in the decreasing water level phases, leading to the accumulation of diatom biomass. Higher biomass in the increasing water phase and decreasing water phase also is a result of resuspension processes in which sediments containing diatoms are made available in the pelagic environment. Minimums also occur in low water level phase, mainly because the low temperature in winter. Thus, the variation in hydrology shifted Poyang Lake from a turbid water state characterized by high phytoplankton biomass to a clear water state characterized by low phytoplankton biomass, with the cycle continuing. Apparently, the major forces driving the cyclic shift were closely related to regular high and long-lasting flood events (Mihaljević and Stević, 2011). Among the environmental parameters, pH, DO, NO2 -N, water temperature, water level and TP were the most important physical and chemical variables with seasonal and annual changes in Poyang Lake. Overall, changes in environmental parameters related to both seasonal variations and water-level fluctuations caused variations in diatom biomass and community composition.
The seasonal pattern of diatom dynamics in Poyang Lake revealed that A. granulata and S. robusta were the dominant components of the diatom community throughout the year. The abundance of Aulacoseira within phytoplankton communities has been related to low growth thresholds for light (Reynolds, 2006). A. granulata can easily sink because of its heavy and thick cell walls; however, it is well adapted for growth in rapidly flowing and turbid channels (O'Farrell, 1994 ; Sherman et al., 1998 ; Unrein, 2002) and can tolerate highly fluctuating mixing intensities (Reynolds, 1994). In our study, the channel was a special river with rapid flowing in all four hydrological phases, and A. granulat a was dominant in the channel of Poyang Lake. A positive correlation has been observed between A. granulata and water temperature in lentic waters (Bahnwart et al., 1999 ; Nogueira, 2000 ; Takano et al., 2001 ; Wang et al., 2009). Water samples were collected from 3 depths to collect diatoms throughout the entire water column; thus, benthic diatoms, such as Surirella robusta, were found with the plankton, suggesting that they are in temporary resuspension likely caused by relocation from their original habitat because of flooding, lake mixing, sand excavation, transportation, etc.
The hydrological variations led to environmental heterogeneity of the different regions in Poyang Lake. The north and south regions form a narrow meandering channel and the lentic regions form separate lakes in low water level phase, and they form a big lake in high water level phase. Diatoms were the most abundant representatives of potamoplankton, which was most likely because they are capable of surviving in the main flow of the surrounding rivers (Devercelli, 2006). The seasonal and annual fluctuation of water level made the different region of Poyang Lake heterogeneous habitat for diatom. There are different variables relating to diatom in different hydrological phases. In increasing water level phase, the influence factor was pH. Many studies indicated that diatoms are very sensitive to pH change in the environment (Cameron et al., 1999). In high water level phase, the influence factor were water level and NO2 -N, because floodwater contains rich nutrients but diluted organisms and suspended solids. In decreasing water level phase, the influence factor was TP, as diatom proliferated with condensed nutrient in this phase. In low water level phase, the influence factor was DO. In the outlet, the factors influencing diatom distribution were pH and DO. It maybe because the heterogeneity of the river and lentic regions connecting with Poyang Lake. Backwater sites are lentic water environments that present slow flow velocity, and because these sites are disconnected from the main channel for half of the year (October to March), the nutrient levels in lentic regions (PY8 and PY6 in Poyang Lake) were high because of excessive agricultural chemical inputs and industrial and waste discharge. Because of the slow-flow water, phytoplankton proliferated in lentic regions. The results of Wu et al.(2014) indicated that phytoplankton Chl- a was influenced by light conditions in the main channel, whereas phytoplankton growth was affected by human activities and presented high heterogeneity in the local lentic regions of Poyang Lake. Thus, extreme hydrological events likely have different influences on diatom communities in the main channel and lentic regions.
5 CONCLUSIONAs a floodplain lake, Poyang Lake is commonly subjected to seasonal hydrological fluctuations, which can strongly regulate the pattern of diatom composition and seasonal succession. The dominant phytoplankton are typically potamoplanktonic species (mainly A. granulata and its varieties), the diatom biomass and community has seasonal variations in Poyang Lake. Significant differences between the main channel and the outlet for diatom biomass demonstrate large spatial heterogeneity in Poyang Lake. The growth of planktonic diatoms in the main channel and south region was likely related to water level fluctuations and nutrient concentrations, while it was related with DO and pH in the outlet. These results indicate that changes in environmental parameters related to both seasonal variations and water-level fluctuations caused variations in diatom biomass and community composition in Poyang Lake. Furthermore, extreme hydrological events can have different influences on the diatom community composition in the main channel and lentic regions. This research indicated that diatom can be used as indicators of water level fluctuations in Poyang Lake.
| APHA (American Public Health Association). 1998. Standard Methods for the Examination of Water and Waste Water. 20th ed. American Public Health Association, Washington, D.C. |
| Bahnwart M, Hübener T, Schubert H, 1999. Downstream changes in phytoplankton composition and biomass in a lowland river-lake system (Warnow River, Germany). Hydrobiologia, 391 (1) : 99 –111. |
| Cameron N G, Birks H J B, Jones V J, Berges F, Catalan J, Flower R J, Garcia J, Kawecka B, Koinig K A, Marchetto A, Sánchez-Castillo P, Schmidt R, Šiško M, Solovieva N, Štefková E, Toro M, 1999. Surface-sediment and epilithic diatom pH calibration sets for remote European mountain lakes (AL: PE Project) and their comparison with the Surface Waters Acidification Programme (SWAP) calibration set. Journal of Paleolimnology, 22 (3) : 291 –317. Doi: 10.1023/A:1008025928509 |
| Chen Y W, Fan C X, Teubner K, Dokulil M, 2003. Changes of nutrients and phytoplankton chlorophyll-a in a large shallow lake, Taihu, China: an 8-year investigation. Hydrobiologia, 506 (1-3) : 273 –279. |
| de Emiliani M O G, 1993. Seasonal succession of phytoplankton in a lake of the Paraná River floodplain, Argentina. Hydrobiologia, 264 (2) : 101 –114. Doi: 10.1007/BF00014097 |
| de Emiliani M O G, 1997. Effects of water level fluctuations on phytoplankton in a river-floodplain lake system (Paraná River, Argentina). Hydrobiologia, 357 (1-3) : 1 –15. |
| Devercelli M, 2006. Phytoplankton of the Middle Paraná River during an anomalous hydrological period: a morphological and functional approach. Hydrobiologia, 563 (1) : 465 –478. Doi: 10.1007/s10750-006-0036-0 |
| Dong X H, Bennion H, Battarbee R, Yang X D, Yang H D, Liu E F, 2008. Tracking eutrophication in Taihu Lake using the diatom record: potential and problems. Journal of Paleolimnology, 40 (1) : 413 –429. Doi: 10.1007/s10933-007-9170-6 |
| Forsberg B R, Devol A H, Richey J E, et al, 1988. Factors controlling nutrient concentrations in Amazon floodplain lakes. Limnology and Oceanography, 33 (1) : 41 –56. Doi: 10.4319/lo.1988.33.1.0041 |
| Gasith A, Resh V H, 1999. Streams in Mediterranean climate regions: abiotic influences and biotic responses to predictable seasonal events. Annual Review of Ecology and Systematics, 30 (1) : 51 –81. Doi: 10.1146/annurev.ecolsys.30.1.51 |
| Hillebrand H, Dürselen C D, Kirschtel D, Pollingher U, Zohary T, 1999. Biovolume calculation for pelagic and benthic microalgae. Journal of Phycology, 35 (2) : 403 –424. Doi: 10.1046/j.1529-8817.1999.3520403.x |
| Hu H J, Wei Y X, 2006. The Freshwater Algae of China:Systematics, Taxonomy and Ecology. Science Press, Beijing. |
| Junk W J, Bayley P B, Sparks R E, 1989. The flood pulse concept in river-floodplain systems. Canadian Special Publication of Fisheries and Aquatic Sciences, 106 : 110 –127. |
| Krammer K, Lange-Bertalot H. 1986. Süsswasserflora van Mitteleuropa. Bacillariophyceae. 1. Teil: Naviculaceae Volume 2. Gustav Fischer Verlag, Stuttgart, US. p.442-874. |
| Krammer K, Lange-Bertalot H. 1988. Süsswasserflora van Mitteleuropa. Bacillariophyceae. 2. Teil: Bacillariaceae, Epthimiaceae, Surirellaceae Volume 2. Gustav Fischer Verlag, Stuttgart, US. p.3-608. |
| Krammer K, Lange-Bertalot H. 1991a. Süsswasserflora van Mitteleuropa. Bacillariophyceae. 3. Teil: Centrales, Fragilariaceae, Eunotiaceae Volume 2. Gustav Fischer Verlag, Stuttgart, US. p.3-596. |
| Krammer K, Lange-Bertalot H. 1991b. Süsswasserflora van Mitteleuropa. Bacillariophyceae. 4. Teil: Achnanthaceae, Achnanthes and Gomphonema, Volume 2. Gustav Fischer Verlag, Stuttgart, US. p.3-466. |
| Lepš J, Šmilauer P. 2003. Multivariate Analysis of Ecological Data Using CANOCO. Cambridge University Press, Cambridge, UK. p.149-166. |
| Lewis Jr W M, 1978. Dynamics and succession of the phytoplankton in a tropical lake: lake Lanao, Philippines. Journal of Ecology, 66 (3) : 849 –880. Doi: 10.2307/2259300 |
| Martin-Jézéquel V, Hildebrand M, Brzezinski M A, 2000. Silicon metabolism in diatoms: implications for growth. Journal of Phycology, 36 (5) : 821 –840. Doi: 10.1046/j.1529-8817.2000.00019.x |
| Mihaljević M, Špoljarić D, Stević F, Cvijanović V, Hackenberger Kutuzović B, 2010. The influence of extreme floods from the River Danube in 2006 on phytoplankton communities in a floodplain lake: shift to a clear state. Limnologica, 40 (3) : 260 –268. Doi: 10.1016/j.limno.2009.09.001 |
| Mihaljević M, Špoljarić D, Stević F, Pfeiffer T Ž, 2013. Assessment of flood-induced changes of phytoplankton along a river-floodplain system using the morphofunctional approach. Environ. Monit. Assess., 185 (10) : 8601 –8619. Doi: 10.1007/s10661-013-3198-z |
| Mihaljević M, Stević F, Horvatić J, Hackenberger Kutuzović B, 2009. Dual impact of the flood pulses on the phytoplankton assemblages in a Danubian floodplain lake(Kopački Rit Nature Park, Croatia). Hydrobiologia, 618 (1) : 77 –88. Doi: 10.1007/s10750-008-9550-6 |
| Mihaljević M, Stević F, 2011. Cyanobacterial blooms in a temperate river-floodplain ecosystem: the importance of hydrological extremes. Aquatic Ecology, 45 (3) : 335 –349. Doi: 10.1007/s10452-011-9357-9 |
| Nogueira M G, 2000. Phytoplankton composition, dominance and abundance as indicators of environmental compartmentalization in Jurumirim Reservoir(Paranapanema River), São Paulo, Brazil. Hydrobiologia, 431 (2-3) : 115 –128. |
| O'Farrell I, 1994. Comparative analysis of the phytoplankton of fifteen lowland fluvial systems of the River Plate Basin(Argentina). Hydrobiologia, 289 (1-3) : 109 –117. Doi: 10.1007/BF00007413 |
| Pan Y D, Stevenson R J, 1996. Gradient analysis of diatom assemblages in western Kentucky wetlands. Journal of Phycology, 32 (2) : 222 –232. Doi: 10.1111/j.0022-3646.1996.00222.x |
| Prygiel J, Coste M, 1993. The assessment of water quality in the Artois-Picardie basin (France) by the use of diatom indices. Hydrobiologia, 269-270 (1) : 343 –349. Doi: 10.1007/BF00028033 |
| Reynolds C S, 1994. The long, the short and the stalled: on the attributes of phytoplankton selected by physical mixing in lakes and rivers. Hydrobiologia, 289 (1-3) : 9 –21. Doi: 10.1007/BF00007405 |
| Reynolds C S. 2006. The Ecology of Phytoplankton.Cambridge University Press, Cambridge, UK. 535p. |
| Schelske C L, Eadie B J, Krause G L, 1984. Measured and predicted fluxes of biogenic silica in Lake Michigan. Limnology and Oceanography, 29 (1) : 99 –110. Doi: 10.4319/lo.1984.29.1.0099 |
| Sherman B S, Webster I T, Jones G J, Oliver R L, 1998. Transitions between Auhcoseira and Anabaena dominance in a turbid river weir pool. Limnology and Oceanography, 43 (8) : 1902 –1915. Doi: 10.4319/lo.1998.43.8.1902 |
| Sorvari S, Korhola A, Thompson R, 2002. Lake diatom response to recent Arctic warming in Finnish Lapland. Global Change Biology, 8 (2) : 171 –181. Doi: 10.1046/j.1365-2486.2002.00463.x |
| Takano K, Ishikawa Y, Mikami H, Ban S, Yoshida T, Aono T, Imada K, Yasutomi R, Takeuchi K, Hino S, 2001. Analysis of the change in dominant phytoplankton species in unstratified Lake Oshima-Ohnuma estimated by a bottle incubation experiment. Limnology, 2 (1) : 29 –35. Doi: 10.1007/s102010170013 |
| Tilman D, Kiesling R, Sterner R W, Kilham S S, Johnson F A, 1986. Green, bluegreen and diatom algae: taxonomic differences in competitive ability for phosphorus, silicon and nitrogen. Archiv für Hydrobiologie, 106 (4) : 473 –485. |
| Tilman D, Kilham S S, Kilham P, 1982. Phytoplankton community ecology: the role of limiting nutrients. Annual Review of Ecology and Systematics, 13 (1) : 349 –372. Doi: 10.1146/annurev.es.13.110182.002025 |
| Unrein F, 2002. Changes in phytoplankton community along a transversal section of the Lower Paraná floodplain, Argentina. Hydrobiologia, 468 (1-3) : 123 –134. |
| Utermöhl H, 1958. Zur vervollkommnung der quantitativen phytoplankton-methodik. Mitt. Int. Ver. Theor. Angew.Limnol., 9 : 1 –38. |
| Van den Brink F W B, De Leeuw J P H M, Van Der Velde G, Verheggen G M, 1992. Impact of hydrology on the chemistry and phytoplankton development in floodplain lakes along the Lower Rhine and Meuse. Biogeochemistry, 19 (2) : 103 –128. Doi: 10.1007/BF00000798 |
| Van den Brink F W B, Van Katwijk M M, Van der Velde G, 1994. Impact of hydrology on phyto- and zooplankton community composition in floodplain lakes along the Lower Rhine and Meuse. J. Plankton Res., 16 (4) : 351 –373. Doi: 10.1093/plankt/16.4.351 |
| Wang C, Li X H, Lai Z N, Tan X C, Pang S X, Yang W L, 2009. Seasonal variations of Aulacoseira granulata population abundance in the Pearl River Estuary. Estuarine, Coastal and Shelf Science, 85 (4) : 585 –592. Doi: 10.1016/j.ecss.2009.09.031 |
| Wang H Z, Xu Q Q, Cui Y D, Liang Y L, 2007. Macrozoobenthic community of Poyang Lake, the largest freshwater lake of China, in the Yangtze floodplain. Limnology, 8 (1) : 65 –71. Doi: 10.1007/s10201-006-0190-0 |
| Weilhoefer C L, Pan Y D, Eppard S, 2008. The effects of river floodwaters on floodplain wetland water quality and diatom assemblages. Wetlands, 28 (2) : 473 –486. Doi: 10.1672/07-114.1 |
| Willén E, 1991. Planktonic diatoms-an ecological review. Algological Studies, 62 : 69 –106. |
| Wu Z S, Cai Y J, Liu X, Xu C P, Chen Y W, Zhang L, 2013. Temporal and spatial variability of phytoplankton in Lake Poyang: the largest freshwater lake in China. J. Great Lakes Res., 39 (3) : 476 –483. Doi: 10.1016/j.jglr.2013.06.008 |
| Wu Z S, Lai X J, Zhang L, Cai Y J, Chen Y W, 2014. Phytoplankton chlorophyll a in Lake Poyang and its tributaries during dry, mid-dry and wet seasons: a 4-year study. Knowledge and Management of Aquatic Ecosystems, 412 (1) : 1 –13. |
 2017, Vol. 35
2017, Vol. 35