Institute of Oceanology, Chinese Academy of Sciences
Article Information
- SUN Chengjun(孙承君), JIANG Fenghua(蒋凤华), GAO Wei(高伟), LI Xiaoyun(李小云), YU Yanzhen(于延珍), YIN Xiaofei(尹晓斐), WANG Yong(王勇), DING Haibing(丁海兵)
- Scanning electron microscopy coupled with energydispersive X-ray spectrometry for quick detection of sulfuroxidizing bacteria in environmental water samples
- Chinese Journal of Oceanology and Limnology, 35(1): 185-191
- http://dx.doi.org/10.1007/s00343-016-5175-1
Article History
- Received Jun. 22, 2015
- accepted for publication Aug. 18, 2015
- accepted in principle Oct. 19, 2015
2 College of Chemistry and Chemical Engineering, Ocean University of China, Qingdao 266100, China;
3 Qingdao Hao Ao Environmental Engineering Ltd., Qingdao 266100, China;
4 Key Laboratory of Marine Chemistry Theory and Technology, Ministry of Education, Ocean University of China, Qingdao 266100, China;
5 Qingdao Collaborative Innovation Center of Marine Science and Technology, Ocean University of China, Qingdao 266100, China
Sulfur oxidizers can oxidize reduced sulfur compounds, such as hydrogen sulfide, and transiently store elemental sulfur in their bodies. Purple sulfur bacteria are typical sulfur oxidizers. They are genetically-related species that can carry out anoxygenic photosynthesis in anoxic aquatic environments in the presence of hydrogen sulfide and light (Pfennig and Trüper, 1992). They can be found in water and mud of shallow lakes, ditches, and stagnant waters. They play critical roles in the sulfur cycle. In shallow-water habitats, accumulation of phototrophic sulfur bacteria often occurs during summer or fall, when hydrogen sulfide is formed from decaying organic matter. To date, 16S rRNA gene sequencing and targeted gene sequencing are the most efficient and widely used methods for sulfur bacteria detection (Amann et al., 1995 ; Mori et al., 2010 ; Zhao et al., 2011 ; Rabus et al., 2013 ; Zhong et al., 2015). Both methods heavily depend on the quality of the total genomic DNA extracted. In addition, qPCR (quantitative polymerase chain reaction) can be used for quantitative bacteria detection. However, this method requires specific primers, which are not readily available for unknown species. The complexity of natural samples increases the difficulties in identifying all of their microbes, with some important microbes being left undetected. Greater challenges arise when a quick result is required for time-sensitive environmental samples.
One distinguishing feature of sulfur oxidizers is that they can store elemental sulfur in membranebound sulfur globules within their cells (Maki, 2013). Both X-ray absorption near-edge structure (XANES) spectroscopy (Prange et al., 2002) and X-ray absorption spectroscopy (Franz et al., 2007) have been used to analyze the form of sulfur in these globules from pure cell cultures. Keim et al.(2005) used EDS coupled with high-resolution TEM (transmission electron microscopy) to study the ultrastructure and elemental composition of intracellular inclusions from uncultured marine magnetotactic bacteria. They concluded that the presence and composition of intracellular structures could provide information on the physiology and the microenvironments of these bacteria. Jogler et al.(2010) applied high-resolution SEM and EDS to study the intracellular organization of the magnetotactic bacterium Candidatus Magnetobacterium bavaricum. Very recently, Eder et al.(2014) used confocal Raman micro-spectrometry to map magnetite crystals and sulfur globules in magnetotactic bacteria. Oren et al.(2015) used Raman spectroscopy to identify sulfur and carotenoids in purple sulfur bacteria Allochromatium vinosum and Allochromatium warmingii. As a convenient technique, EDS has been used to detect anion and cation concentrations in single marine microplankton cells (Segura-Noguera et al., 2012). It has also been applied to detect the presence of sulfur in purecultured sulfur oxidizers (Marshall and Morris, 2013). SEM coupled with EDS has the potential to be used for sulfur-oxidizing bacteria detection based on single-cell elemental analysis.
In this study, SEM coupled with EDS mapping was applied to quickly detect sulfur-oxidizing bacteria in natural water samples. The bacteria were detected within a short time after sample collection, with minimal sample treatment. Later, 16S rRNA sequencing was used to confirm the identity of these bacteria. Combining SEM, EDS, and gene sequencing methods were proved to be a powerful strategy to overcome difficulties related to 16S rRNA sequencing of environmental samples and to obtain quick results.
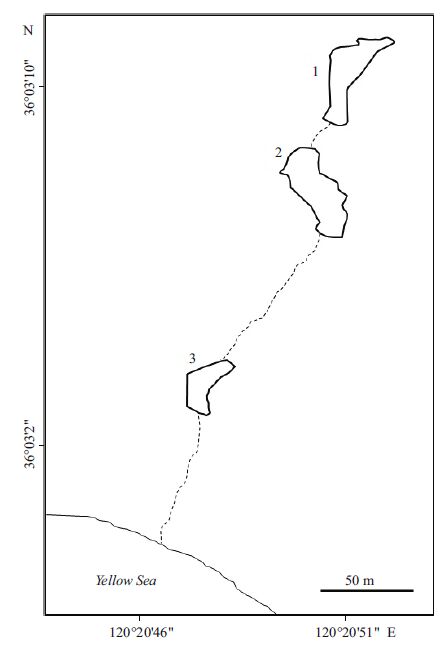
2 MATERIAL AND METHOD 2.1 Sample preparationIn February 2014, the surface water of the Badaguan Lake (Fig. 1) in Qingdao, China suddenly turned a light-violet color (Fig. 2a). To investigate the origin of this violet color, surface water samples were obtained from the lake. Dissolved O2 was measured site. Water temperature and pH were measured with a PH-010 pH meter on site. One liter-water samples were collected from the lake and sealed in two 500-mL plastic bottles, which were taken to the laboratory within 1 h. Two aliquots of 100 mL of a water sample were centrifuged at 700× g on a benchtop centrifuge (D1008, Dragonlab, Beijing, China) for 2 min to pellet the large pinkish cells. One pellet was microscope, while the other was frozen at -80℃ for further analysis. For 16S rRNA gene sequencing, another 300 mL of the water sample was let stand for 2 h to allow the colored cells to self-settle to the bottom of the bottle. The cells at the bottom of the bottle were harvested by decanting the top layer, followed by slow centrifugation at 700× g for 2 min. The pelleted cells were resuspended in 200 μL of Milli-Q water (Millipore Corp., Germany).
|
| Figure 1 Diagram showing the three-connected lakes at the sampling site, Badaguan Lake, Qingdao, China Water samples were taken from Lake #2(36°3′8″N and 120°20′51″E). |
|
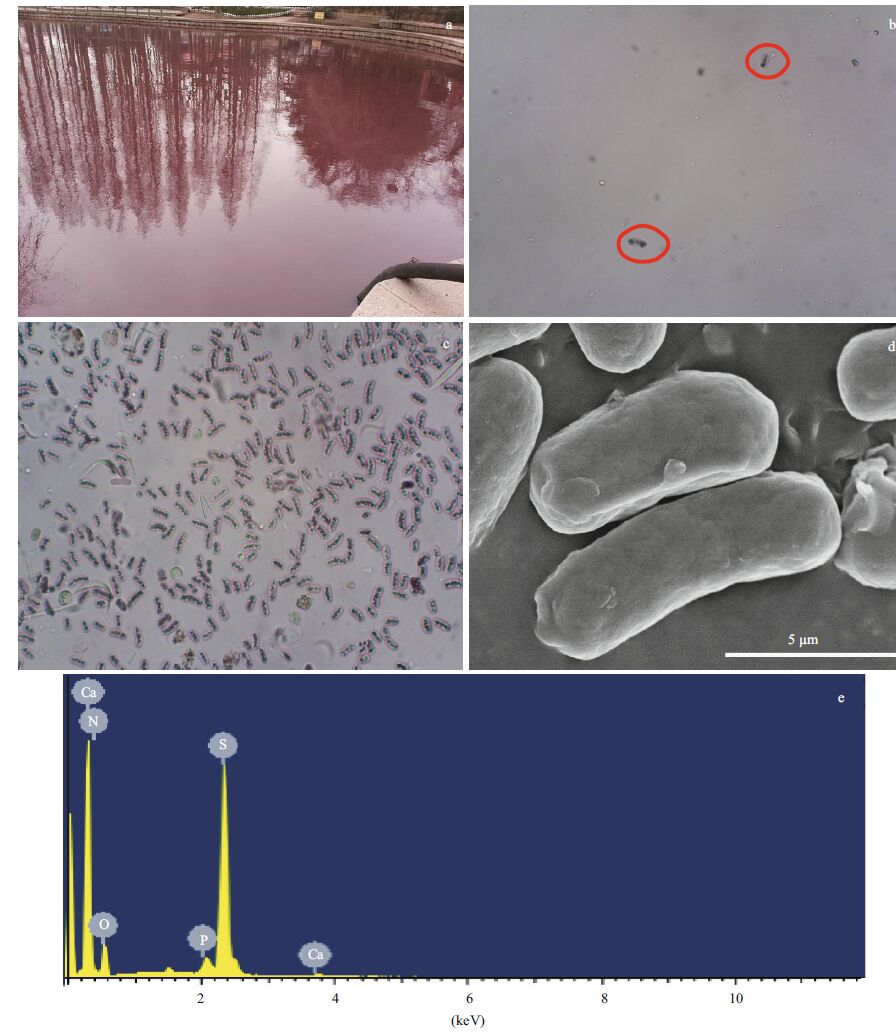
| Figure 2 Images and elemental component mapping of the sulfur bacteria found in the lake a. picture of the lake at the time of sampling. The lake has an area of around 0.2 km2, which was violet colored for a couple of weeks during February 2014; b. light microscopy image of microbes in the water sample, showing different cell types that were present. Red circles indicate the visible, large rod-shaped cells, which have a purple reflective outline and dense internal particles; c. concentrated purple bacterial cells under the light microscope; d. scanning electron microscopy image of the freeze-dried purple cells; e. energy-dispersive X-ray spectrometry analysis of a single cell showing its high sulfur content. |
One drop each of the water sample and the resuspended cell pellet were deposited on a glass slide. After putting on a cover slide, the sample was viewed under an N-180M biological microscope (All Pro, China), equipped with a HDCE-10C camera (Lead-Optical Ltd., China).
2.3 Scanning electron microscopy and energydispersive X-ray spectrometryFor electron microscopy, 50 μ L of the Milli-Q resuspended cells were frozen at -80℃ for 4 h and then freeze-dried using a Christ ALPHA 1-2 LD plus freeze-dryer (Martin Christ, GmbH, Germany). After freeze-drying, the cells were carefully transferred to conductive carbon tabs (Ted Pella; Redding, CA, USA) on SEM posts. Samples were sputter-coated with gold using a Hitachi E-1045 coater (Hitachi High-Tech Science Corp., Japan) and examined with an Hitachi S-4800 emission scanning electron microscope equipped with a HORIBA 7593-H energy-dispersive X-ray spectrometer (Horiba Ltd., UK). The SEM acceleration voltage was 15 kV. EDS working distance was set to 15 mm, while data acquisition time was set to 300 s, with a speed of 2 000 cps.
2.4 16S r RNA gene sequencingGenomic DNA was extracted from 50 μ L of resuspended cells using an AxyPrep™ Bacterial Genomic DNA Miniprep kit (Axygen Scientific, CA, USA). PCR amplification of the 16S rRNA gene was conducted under the following thermal cycling conditions: initial denaturation at 95℃ for 3 min; 24 cycles of 95℃ denaturation for 1 min, 55℃ annealing for 30 s, 72℃ extension for 1.5 min; and final extension at 72℃ for 10 min. Universal bacterial primers 27F (5′-AGAGTTTGAT-CCTGGCTCAG-3′) and 1492R (5′-TACGGCTACCTTGTTACGACTT-3′) were used in the PCR. PCR products were gel-purified using AxyPrep PCR Cleanup kit (Axygen Scientific). The cleaned PCR product was then cloned with a TOPO TA cloning kit (Life Technologies, CA, USA), following the manufacturer's standard protocol. Colonies were picked to grow for plasmid DNA extraction. Extracted plasmid DNA was analyzed on a model 3730XL automated DNA analyzer (Applied Biosystems, CA, USA) at Majorbio (Shanghai, China). Gene sequences obtained were deposited at the GenBank and searched against the GenBank DNA database using BLASTN search, available at http:// www.ezbiocloud.net.
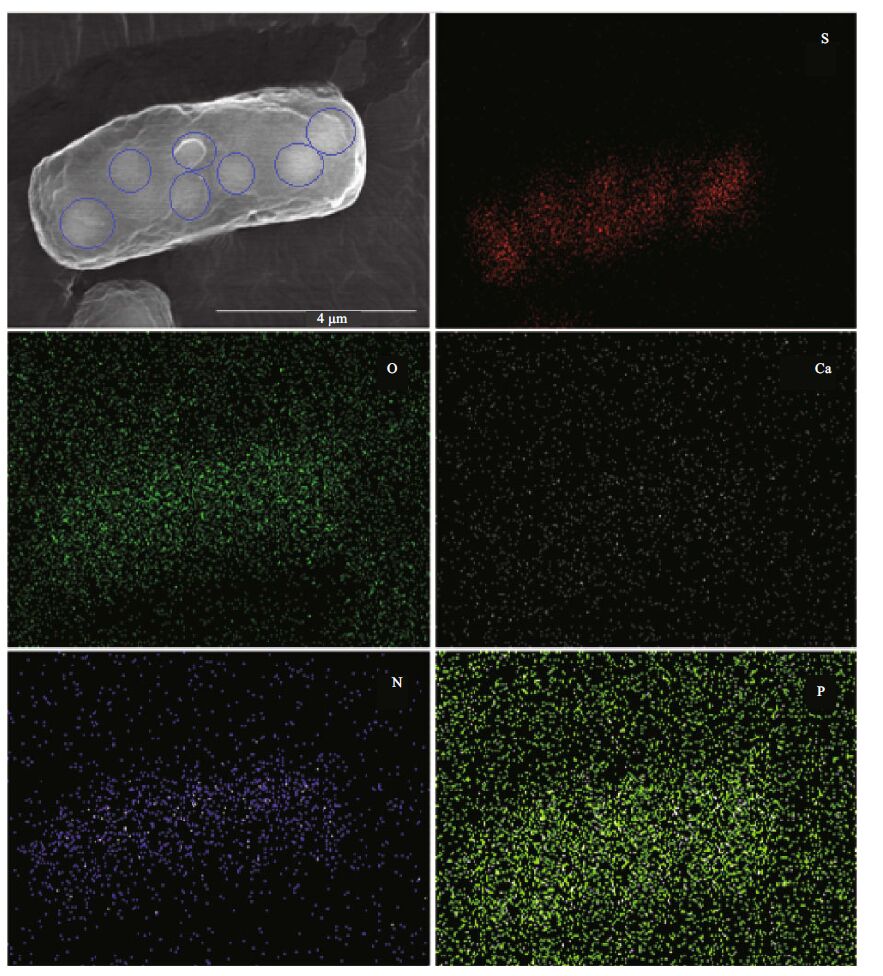
3 RESULT AND DISCUSSION 3.1 Presence of sulfur-oxidizing bacteriaThe sampling site consists of three interconnected lakes, with water flowing from lake #1 to #2 to #3 and finally into the Yellow Sea (Fig. 1). Lake #2 was the main cause of public concern, with its violet color and unpleasant hydrogen sulfide smell. In situ water analyses showed that the water temperature was 6.1℃ and its dissolved oxygen content was 5.15 mg/L (less than 50% saturation). A few rod-shaped microbial cells with a pink reflective body outline were observed under the light microscope (Fig. 2a and b). These cells had a size range from 2 to 12 μ m, with varied numbers of internal globules. These particlelike globules suggested the presence of sulfur bacteria. To quickly identify the microbes to facilitate effective measures to treat the water, the pinkish cells were concentrated on a low-speed benchtop spinner (D1008, Dragonlab, Beijing, China). This low speed centrifugation pelleted mainly rod-shaped pink cells, with a few algae cells (Fig. 2c). SEM coupled with EDS analysis showed that the rod-shaped cells contained a large amount of sulfur (Fig. 2d, e). The atomic ratio of sulfur and phosphorus in the sulfurrich cell was calculated to be 33:1. In the E. coli cell control, the sulfur to phosphorous ratio was 0.78:1(data not shown). In normal cells, the phosphorous content is usually slightly higher than sulfur. Therefore, the sulfur content in these rod-shaped cells was extremely high in comparison to our control. We used EDS mapping to localize major elements in the cell (carbon was not analyzed because of the underlying carbon tape used for analyses). Results showed that oxygen, calcium, nitrogen, and phosphorous were evenly distributed within the cell, while, sulfur was concentrated in the observed globules within the cell (Fig. 3). Because a distinguishing feature of sulfur oxidizers is that they can store elemental sulfur in membrane-bound sulfur globules inside the cell, we surmised that these cells were sulfur bacteria.
|
| Figure 3 Scanning electron microscopy and energy-dispersive X-ray spectrometry mapping of major elements in the sulfurrich bacteria cells Blue circles in the scanning electron microscopy image indicate the locations of presumed sulfur granules. |
Other methods can also be applied to detect cell components. For instance, Oren et al.(2015) recently used Raman spectroscopy to simultaneously monitor the presence of biomarkers (carotenoids, elemental sulfur) within single cells of the cultured photosynthetic purple sulfur bacteria, Allochromatium vinosum and A. warmingii. They found that Raman spectroscopy was a convenient and sensitive technique to assess compounds of interest within single bacterial cells. Sulfur inclusions in the uncultured magnetotactic bacterium Candidatus Magnetobacterium bavaricum were also mapped with high-resolution SEM and EDS (Jogler et al., 2010). Likewise, confocal Raman micro-spectrometry was used to map magnetite crystals and sulfur globules in magnetotactic bacteria (Eder et al., 2014). In this study, the existence of sulfur oxidizers could be identified from complex natural environmental samples within 24 h using SEM and EDS methods.
3.2 DNA sequencingTo confirm the presence of purple sulfur bacteria, we extracted genomic DNA from concentrated cells and used traditional 16S rRNA gene sequencing to identify their strain. Sequences of around 1 500 bps (searched against the database available at http:// www.ezbiocloud.net) showed that these bacteria belong to the genus of Chromatium(Table 1). Most of the sequences had over 99% similarity to Chromatium okenii(Table 1). C. okenii(GenBank accession # NR 025315.1) is a kind of purple sulfur bacteria, often found in stagnant waters (Hageage and Gherna, 1971 ; Pfennig and Trüper, 1992 ; Marshall and Morris, 2013). Purple sulfur bacteria can oxidize hydrogen sulfide and produce sulfur granules. They can use hydrogen sulfide as an energy source (Hageage and Gherna, 1971 ; Pfennig and Trüper, 1992), playing an important role in the sulfur cycle.
Based on SEM imaging, EDS mapping and 16S rRNA sequencing, the existence of the purple sulfur oxidizer from the genus Chromatium was confirmed at the Badaguan Lake. Thus, the violet color of the lake was derived from the presence of purple sulfur bacteria. The lake has been stagnant for some time because of lack of rain; meanwhile, lake sediments have not been desilted for over 10 years. Anoxic degradation of organic matter in lake sediments could provide hydrogen sulfide to support purple sulfur bacteria blooms. The deepest part of the lake is only 2.8 m; hence, sun light can penetrate to the bottom of the water body. Sunlight and accumulation of reduced hydrogen sulfide at the bottom of the lake can trigger blooms of purple sulfur bacteria, capable of anoxygenic photosynthesis.
Sequencing results showed the bacteria had over 99% similarity to C. okenii. According to previous studies, C. okenii is usually found near the chemocline, in waters deeper than 6 m (Fischer et al., 1996 ; Gervais, 1997). Because the deepest part of the Badaguan Lake is only 2.8 m, when the pinkish color first occurred, an algal bloom was suspected to be the cause. Our results indicate that Chromatium species can thrive in shallow lake waters under the right conditions. A low oxygen concentration in the water as well as hydrogen sulfide in the sediment helped the bacteria to grow at the sediment-water interface. Subsequently, water mixing likely brought the bacteria to the surface of the lake. Surface cooling is one process that promotes diffusive water column mixing (Pjevac et al., 2015). The surface water temperatures in the study area fluctuated from below freezing to 5–6℃ in January and February of 2014. Since maximum water density occurs at about 4℃, such temperature variations would cause the heavier surface water to sink to the bottom of the lake, while the lighter bottom water was moved to the surface, bringing sulfur oxidizers to the lake surface. Such mixing processes may have occurred many times, promoting the distribution of the bacteria throughout the lake, and turning it violet.
4 SUMMARYSEM coupled with EDS mapping quickly detected and localized sulfur in sulfur bacteria from environmental water samples. In this study, combining SEM, EDS, and 16S rRNA technologies, we confirmed the existence of a sulfur-oxidizing bacterial strain Chromatium in a shallow lake with less than 2.8-m water depth. Based on the sulfur features of sulfur oxidizers, this technique was proved to be a powerful, fast (within 24 h) and accurate tool to identify sulfur-oxidizing bacteria in complex natural water samples with minimal sample treatment. Thus, SEM and EDS coupled with 16S rRNA gene sequencing has great potential for analyzing sulfur bacteria in other complex environmental samples.
5 ACKNOWLEDGEMENTC. Sun would also like to acknowledge support from Human Resource and Social Security of China and Taishan Scholar.
| Amann R I, Ludwig W, Schleifer K H, 1995. Phylogenetic identification and in situ detection of individual microbial cells without cultivation. Microbiol. Rev., 59 (1) : 143 –169. |
| Eder S H K, Gigler A M, Hanzlik M, Winklhofer M, 2014. Sub-micrometer-scale mapping of magnetite crystals and sulfur globules in magnetotactic bacteria using confocal raman micro-spectrometry. PLoS One, 9 (9) : e107356 . Doi: 10.1371/journal.pone.0107356 |
| Fischer C, Wiggli M, Schanz F, Hanselmann K W, Bachofen R, 1996. Light environment and synthesis of bacteriochlorophyll by populations of Chromatium okenii under natural environmental conditions. FEMS Microbiol.Ecol., 21 (1) : 1 –9. Doi: 10.1111/fem.1996.21.issue-1 |
| Franz B, Lichtenberg H, Hormes J, Modrow H, Dahl C, Prange A, 2007. Utilization of solid ‘elemental’ sulfur by the phototrophic purple sulfur bacterium Allochromatium vinosum: a sulfur K-edge X-ray absorption spectroscopy study. Microbiology, 153 (4) : 1268 –1274. Doi: 10.1099/mic.0.2006/003954-0 |
| Gervais F, 1997. Diel vertical migration of Cryptomonas and Chromatium in the deep chlorophyll maximum of a eutrophic lake. J. Plankton Res., 19 (5) : 533 –550. Doi: 10.1093/plankt/19.5.533 |
| Hageage Jr G J, Gherna R L, 1971. Surface structure of Chromatium okenii and Chromatium weissei. J. Bacteriol., 106 (2) : 687 –690. |
| Jogler C, Niebler M, Lin W, Kube M, Wanner G, Kolinko S, Stief P, Beck A J, de Beer D, Petersen N, Pan Y, Amann R, Reinhardt R, Schüler D, 2010. Cultivation-independent characterization of ‘Candidatus Magnetobacterium bavaricum’ via ultrastructural, geochemical, ecological and metagenomic methods. Environ. Microbiol., 12 (9) : 2466 –2478. Doi: 10.1111/j.1462-2920.2010.02220.x |
| Keim C N, Solórzano G, Farina M, Lins U, 2005. Intracellular inclusions of uncultured magnetotactic bacteria. Int.Microbiol., 8 (2) : 111 –117. |
| Maki J S, 2013. Bacterial intracellular sulfur globules: structure and function. J. Mol. Microbiol. Biotechnol., 23 (4-5) : 270 –280. Doi: 10.1159/000351335 |
| Marshall K T, Morris R M, 2013. Isolation of an aerobic sulfur oxidizer from the SUP05/Arctic96BD-19 clade. ISME J., 7 (2) : 452 –455. Doi: 10.1038/ismej.2012.78 |
| Mori Y, Purdy K J, Oakley B B, Kondo R, 2010. Comprehensive detection of phototrophic sulfur bacteria using PCR primers that target reverse dissimilatory sulfite reductase gene. Microbes Environ., 25 (3) : 190 –196. Doi: 10.1264/jsme2.ME10109 |
| Oren A, Mana L, Jehlička J, 2015. Probing single cells of purple sulfur bacteria with Raman spectroscopy:carotenoids and elemental sulfur. FEMS Microbiol. Lett., 362 (6) . |
| Pfennig N, Trüper H G. 1992. The family Chromatiaceae. In:Balows A, Trüper H G, Dworkin M, Harder W, Schleifer K H eds. The Prokaryotes, A Handbook on the Biology of Bacteria: Ecophysiology, Isolation, Identification, Applications. 2nd edn. Springer, New York. p.3200-3221. |
| Pjevac P, Korlević M, Berg J S, Bura-Nakić E, Ciglenečki I, Amann R, Orlić S, 2015. Community shift from phototrophic to chemotrophic sulfide oxidation following anoxic holomixis in a stratified seawater lake. Appl.Environ. Microbiol., 81 (1) : 298 –308. Doi: 10.1128/AEM.02435-14 |
| Prange A, Chauvistré R, Modrow H, Hormes J, Trüper H G, Dahl C, 2002. Quantitative speciation of sulfur in bacterial sulfur globules: X-ray absorption spectroscopy reveals at least three different species of sulfur. Microbiology, 148 (1) : 267 –276. Doi: 10.1099/00221287-148-1-267 |
| Rabus R, Hansen T A, Widdel F. 2013. Dissimilatory sulfateand sulfur-reducing prokaryotes. In: Rosenbrug E, DeLong E F, Lory S, Stackebrandt E, Thompson F eds.Prokaryotes Prokaryotic Physiology and Biochemistry.Springer-Verlag, Berlin Heidelberg. p.309-404. |
| Segura-Noguera M, Blasco D, Fortuño J M, 2012. An Improved energy-dispersive X-ray microanalysis method for analyzing simultaneously carbon, nitrogen, oxygen, phosphorus, sulfur, and other cation and anion concentrations in single natural marine microplankton cells. Limnol. Oceanogr. Methods, 10 (9) : 666 –680. Doi: 10.4319/lom.2012.10.666 |
| Zhao J Y, Fu Y N, Zhao C G, Yang S P, Qu Y B, Jiao N Z, 2011. Identification and characterization of a purple sulfur bacterium from mangrove with rhodopin as predominant carotenoid. Acta Microbiological Sinica, 51 (10) : 1318 –1325. |
| Zhong F, Wu J, Dai Y R, Yang L H, Zhang Z H, Cheng S P, Zhang Q, 2015. Bacterial community analysis by PCRDGGE and 454-pyrosequencing of horizontal subsurface flow constructed wetlands with front aeration. Appl.Microbiol. Biotechnol., 99 (3) : 1499 –1512. Doi: 10.1007/s00253-014-6063-2 |
 2017, Vol. 35
2017, Vol. 35



