Institute of Oceanology, Chinese Academy of Sciences
Article Information
- SUN Zhongmin(孙忠民), WANG Yongqiang(王永强), YAN Pengcheng(严鹏程), GUO Hui(郭辉), YAO Jianting(姚建亭), TANAKA Jiro(田中次郎), KAWAI Hiroshi(川井浩史)
- New record of Lobophora rosacea (Dictyotales; Phaeophyceae) from the South China Sea
- Chinese Journal of Oceanology and Limnology, 35(1): 192-197
- http://dx.doi.org/10.1007/s00343-016-5238-3
Article History
- Received Sep. 16, 2015
- accepted for publication Oct. 28, 2015
- accepted in principle Nov. 10, 2015
2 School of Pharmaceutical Sciences, Wenzhou Medical University, Wenzhou 325035, China;
3 Key Laboratory of Experimental Marine Biological Laboratory, Institute of Oceanology, Chinese Academy of Sciences, Qingdao 266071, China;
4 Grade School of Marine Science and Technology, Tokyo University of Marine Science and Technology, 4-5-7 Konan, Minatoku, Tokyo 108-8744, Japan;
5 Kobe University Research Center for Inland Seas, Rokkodai, Nadaku, Kobe 657-8501, Japan
Lobophora spp. occur in tropical and subtropical seas around the world. The genus is characterized by a marginal row of meristematic cells and a singlelayered large central medulla, and lacks pharaphyses around the sporangial sori (Womersley, 1967, 1987). It is difficult to identify species based on morphological criteria alone. Recent genetic analysis of Lobophora has suggested that the species level divergence was considerably underestimated, and a dozen new species were described (Sun et al., 2012 ; Vieira et al., 2014 ; Schultz et al., 2015). At present, approximately 20 Lobophora species are listed in AlgaeBase (Guiry and Guiry, 2015).
In the present study, we report an additional Lobophora species collected from the South China Sea, based on analysis of the rbcL and cox3 gene sequences and morphological observations.
2 MATERIAL AND METHOD 2.1 Sampling and morphological analysisNew specimens were collected from two sites of Hainan Island (Fig. 1) by snorkelling and SCUBA diving, and immediately stored in a cooler and desiccated in silica gel. Some specimens were dried and mounted on herbarium sheets. All specimens were deposited in the Marine Biological Museum of the Chinese Academy of Sciences (MBMCAS). For the morphological observations, the thalli were sectioned manually using a razor blade, and mounted on glass slides in Karo Syrup/seawater. Photographs were taken with a digital camera (Keyence VB-7010, Tokyo, Japan) attached to a compound microscope (Olympus BX-51, Tokyo, Japan).
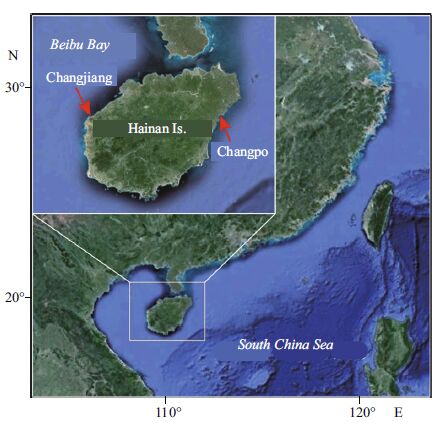
|
| Figure 1 Map showing the collection sites of the new specimens identified in the present study Changjiang: 19°22'N, 108°41'E; Changpo: 19°22'N, 110°40'E. |
Genomic DNA was extracted from the silica geldried specimens and herbaria, whose morphology was analyzed later, using a DNeasy ® Plant Mini Kit (Qiagen, Hiden, Germany), according to the manufacturer's instructions. Polymerase chain reaction (PCR) was carried out with a TaKaRa PCR Thermal Cycler Dice (Takara Shuzo, Shiga, Japan), using a Takara Ex Taq™ reaction kit according to the manufacturer's instructions. Primers and PCR conditions were as those described by Sun et al.(2012). The PCR products were purified with a Gel Extraction Mini Kit (Watson BioTechnologies Inc., Shanghai, China). The purified products were then sequenced on an ABI 3730xl automatic sequencer (Shanghai Sangon Co., Ltd) from both strands with the same primers as those used for the PCR reactions.
The newly generated Lobophora sequences and those from the public databases were aligned with ClustalX (Thompson et al., 1997) and then manually adjusted. The best-fit evolutionary model in each codon position of each gene was determined by comparing different evolutionary models via the corrected Akaike information criterion (AIC; Akaike, 1974) for maximum-likelihood (ML) analysis and the Bayesian analysis (Schwarz, 1978 ; Tanabe, 2007). ML analysis was conducted by the likelihood ratchet method (Vos, 2003), 100 sets of 25% site up-weighted data were created using the pgresampleseq command in Phylogears 1.5.2009.12.29(Tanabe, 2010), and the ML trees with up-weighted data were estimated in Treefinder (Jobb et al., 2004) with the best-fit model applied. The robustness of the resulting phylogenies was tested by bootstrap analysis (Felsenstein, 1985) using 1 000 replications in the ML analysis. Bayesian analysis was conducted in MrBayes v3.1.2(Ronquist and Huelsenbeck, 2003) with the selected evolutionary models. The Bayesian analysis was initiated with a random starting tree and ran four Markov chain Monte Carlo (MCMC) iteration chains simultaneously for 10000000 generations, keeping one tree every 100 generations. The first 10000 trees sampled were discarded as ‘burn-in', based on the stationarity of ln L as assessed in Tracer version 1.4.1(Rambaut et al., 2014); a consensus topology and posterior probability values were calculated for the remaining trees.
3 RESULT 3.1 Molecular phylogenetic analysisAlthough numerous specimens (individual number >30) were examined, only one new rbcL (1 353 bp, KT583622) and one new cox3 sequence (609 bp, KT583623) were obtained. In total, 34 rbcL sequences and 31 cox3 sequences were downloaded from GenBank, including 16 previously described Lobophora species. Zonaria diesingiana J. Agardh and Zonaria sp. clustered as an outgroup in rbcL and cox3 trees. The topological structure of two trees were similar.
All of the Lobophora sequences formed a large clade, and our sequences were embedded in the Lobophora rosacea clade with a high bootstrap value. In the rbcL tree (Fig. 2), the specimen (EU579955) from Guadeloupe, West Indies branched basally, and the specimen (EU579953) that identified as L. papenfussii(W. R. Taylor) Farghaly from the Solomon Islands (Bittner et al., 2008) were closely related to L. rosacea. In the cox3 tree (Fig. 3), the L. rosacea clade branched basally in the larger Lobophora clade.
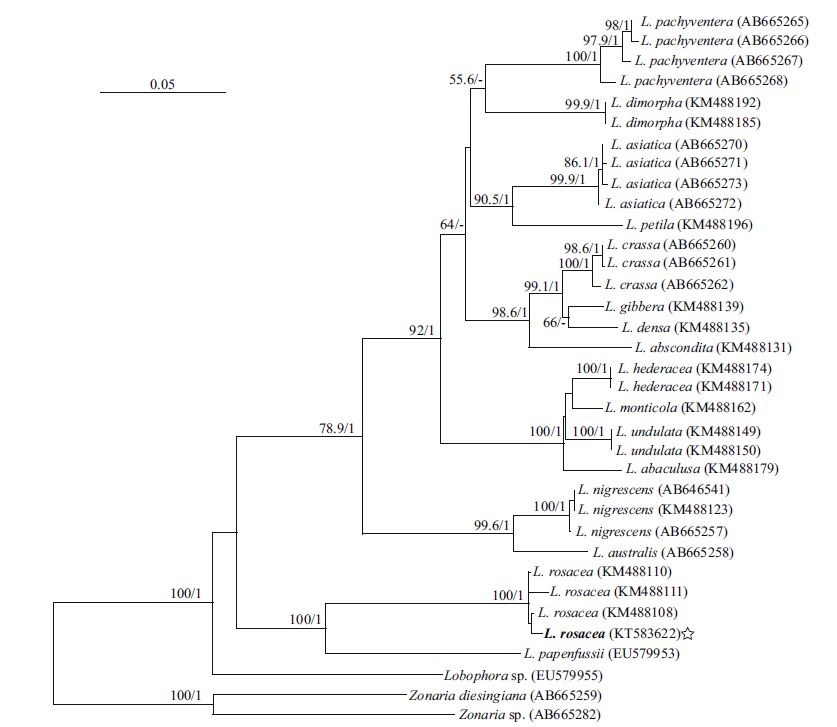
|
| Figure 2 Maximum-likelihood tree based on rbcL gene sequences The star indicates the new sequence. The bootstrap values are shown at each node ML/BI (Bayesian analysis); ML>50%, BI>0.8. |
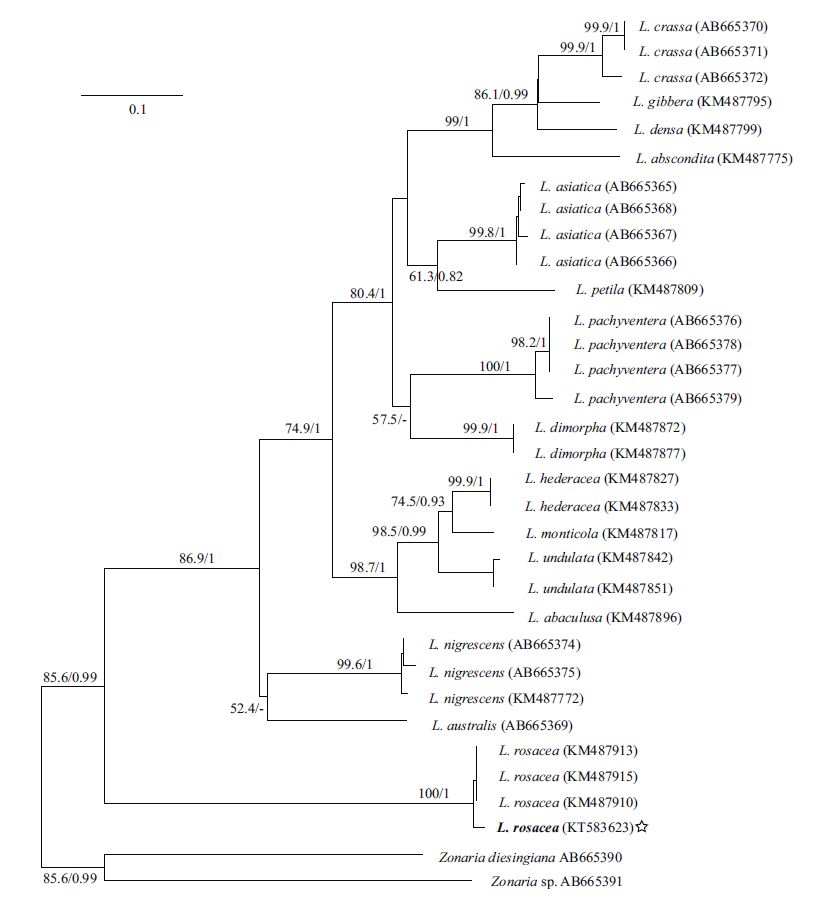
|
| Figure 3 Maximum-likelihood tree based on cox3 gene sequences The star indicates the new sequence. The bootstrap values are shown at each node ML/BI (Bayesian analysis), ML>50%, BI>0.8. |
Habitat : Hard substrates in the subtidal zone 2-5 m deep in moderately protected areas.
3.2.1 Specimens examinedChangpo, Qionghai, Hainan, 4th May, 2012, J. Yao & Z. Sun (MBM510-520, MBMD616-619); Changjiang, Changhua, Hainan, 13th April, 2014, Y. Wang & Z. Sun (MBM730-740, MBMD1501-1512); Changjiang, Changhua, Hainan, 18th March, 2015, J. Yao & Z. Sun (MBM870-880, MBMD1590-1599).
3.2.2 MorphologyThe plant grows erectly in the subtidal zone, attaching to the substrate by a basal holdfast. The fanshaped thallus is predominantly erect, up to 9 cm wide and 8 cm high, yellow to brown in color. The larger thallus is spirally arranged and forms a dense rosette (Fig. 4a). Dried specimens become dark brown in color (Fig. 4b). The middle part of the thallus is 140-165 μm thick, composed of a single layer of large medullary cells and four layers of cortical cells on both sides of the medulla (Fig. 4c, d). The basal part of thallus is composed of three layers of cortical cells on both sides of the medulla, with tubular rhizoids issuing from the ventral surface (Fig. 4e). Sporangial sori are mainly scattered on the ventral surface of the upper part of the mature thallus. The sporangia are sessile and ovate without paraphyses (Fig. 4f), and developed ones are 60-75 μm in diameter at surface view.
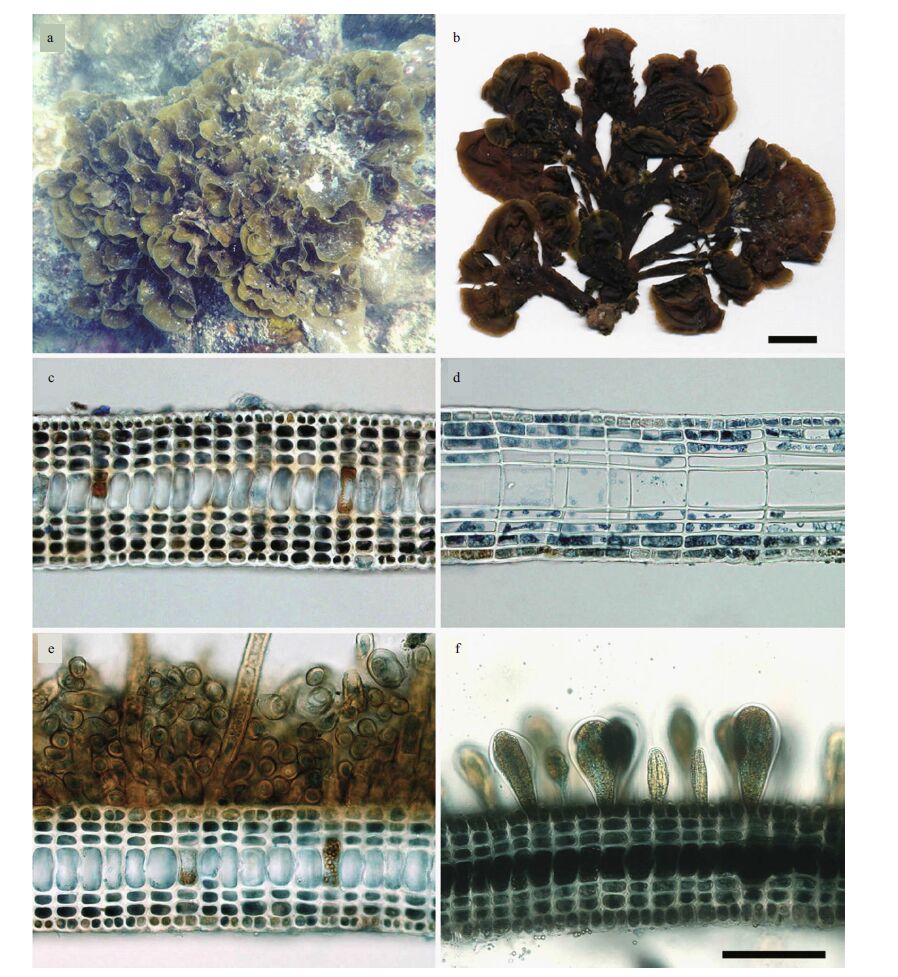
|
| Figure 4 Morphology of Lobophora rosacea from the South China Sea a. plant growing under water (Changjiang, Changhua, Hainan, 18th March, 2015); b. herbarium of L. rosacea, scale bar=1 cm; c. transverse section of the middle portion of the thallus, showing an obvious medullary layer and a four layered cortex on both sides; d. longitudinal section of the middle portion, showing nine layers of thallus; e. transverse section of the basal portion, showing rhizoids and a three-layered cortex; f. transverse section of the middle portion of a mature thallus, showing 3 a three layered cortex and sporangia without paraphyses. Scale bar=100 μm, applies to c-f. |
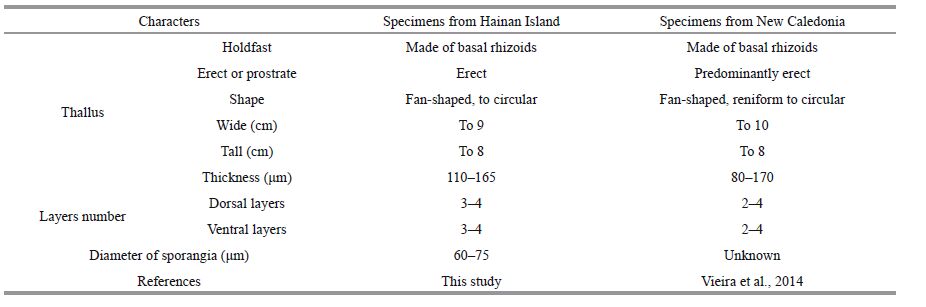
Morphologically, Chinese specimens are very similar to Lobophora rosacea described from New Caledonia with an erect, fan-shaped thallus and a basal mound of rhizoids. However, the thallus of some specimens from New Caledonia is thinner than that from the South China Sea, because the former has a two-cell-layered cortex (Table 1). Vieira et al.(2014) examined numerous specimens and described two L. rosacea morphotypes, the thinner type has less than seven-cell-layers in the thallus and the thicker type has nine cell-layers, but no reproductive structure was found. We found sporangial sori scattered on both surfaces of the mature thallus, and the thinner thallus tended to produce more sporangial sori than the thicker one. We did not detect any gametophyte, though numerous specimens have been collected.
|
Our new sequences differ from those of L. rosacea from New Caledonia by a few bp substitutions. Based on the combination of morphological and genetic data, we propose a new record from the South China Sea to be named L. rosacea C.W. Vieira, Payri et De Clerck. L. rosacea was located at the basal portion in the Lobophora clade in the phylogenetic tree, suggesting that the ancestral Lobophora had erect Zonaria -like thalli, and the crustose and prostrate species probably originated from the erect ones (Sun et al., 2012 ; Vieira et al., 2014).
That only one haplotype was detected in this study suggests the Hainan L. rosacea population originated from southern regions and Hainan Island is close to its northernmost boundary. Many dictyotalean species have wide biogeographic distributions, e.g., some Padina species are widely distributed in the Indo- Pacific (Silberfeld et al., 2013). However, to date, L. rosacea has not been found in the Ryukyu Islands where the water temperature is similar to that of the South China Sea.
Some L. rosacea specimens deposited in MBMCAS had been misidentified as Zonaria sp., probably because they possess erect Zonaria -like thalli. Luan et al.(2013) described Lobophora variegata (Lamouroux) Womersley et E.C. Oliveira and L. lubaoreniana Luan et Ding from the South China Sea. However, L. variegata has been confirmed to be an endemic species from the West Indies (Schultz et al., 2015), and we suspect that L. lubaoreniana corresponds to L. asiatica Z. Sun, J. Tanaka et H. Kawai based on morphological comparisons, but molecular analysis is required for correct taxonomic assignment.
5 CONCLUSIONLobophora rosacea is described from the South China Sea for the first time. To date, four species have been confirmed from the South China Sea based on morphological and phylogenetic analysis, including L. asiatica, L. crassa Z. Sun, P. E. Lim et H. Kawai, L. pachyventera Z. Sun, P. E. Lim, J. Tanaka et H. Kawai and L. rosacea. The four species are distributed in New Caledonia, three of which, L. rosacea being the exception, are distributed in Ryukyu Islands.
| Akaike H, 1974. A new look at the statistical model identification. IEEE Transactions on Automatic Control, 19 (6) : 716 –723. Doi: 10.1109/TAC.1974.1100705 |
| Bittner L, Payri C E, Couloux A, Cruaud C, de Reviers B, Rousseau F, 2008. Molecular phylogeny of the Dictyotales and their position within the brown algae, based on nuclear, plastidial and mitochondrial sequence data. Molecular Phylogenetics and Evolution, 49 : 211 –226. Doi: 10.1016/j.ympev.2008.06.018 |
| Felsenstein J, 1985. Confidence limits on phylogenies: an approach using the bootstrap. Evolution, 39 (4) : 783 –791. Doi: 10.2307/2408678 |
| Guiry M D, Guiry G M. 2015. Algaebase. Worldwide electronic publication. National University of Ireland, Galway, http://www.algaebase.org. Accessed on 2015-08-15. |
| Jobb G, von Haeseler A, Strimmer K, 2004. TREEFINDER: a powerful graphical analysis environment for molecular phylogenetics. BMC Evolutionary Biology, 4 (1) : 18 . Doi: 10.1186/1471-2148-4-18 |
| Luan R X, Ding L P, Lu B R, Tseng C K. 2013. Flora Algarum Marinarum Sinicarum Tomus Ⅲ Phaeophyta No. I (1):Ectocarpales Ralfsiales Sphaceariales Dictyotales.Science Press, Beijing. |
| Rambaut A, Suchard M A, Xie D, Drummond A J. 2014. Tracer.http://beast.bio.ed.ac.uk/tracer. Accessed on 2015-07-25. |
| Ronquist F, Huelsenbeck J P, 2003. MrBayes 3: Bayesian phylogenetic inference under mixed models. Bioinformatics, 19 (12) : 1572 –1574. Doi: 10.1093/bioinformatics/btg180 |
| Schultz N E, Lane C E, Le Gall L, Gey D, Bigney A R, de Reviers B, Rousseau F, Schneider C W, 2015. A barcode analysis of the genus Lobophora (Dictyotales, Phaeophyceae) in the western Atlantic Ocean with four novel species and the epitypification of L. variegata (J.V.Lamouroux) E. C. Oliveira. European Journal of Phycology, 50 (4) : 481 –500. |
| Schwarz G, 1978. Estimating the dimension of a model. The Annals of Statistics, 6 (2) : 461 –464. Doi: 10.1214/aos/1176344136 |
| Silberfeld T, Bittner L, Fernández García C, Cruaud C, Rousseau F, de Reviers B, Leliaert F, Payri C E, de Clerck, O, 2013. Species diversity, phylogeny and large scale biogeographic patterns of the genus Padina(Phaeophyceae, Dictyotales). Journal of Phycology, 49 (1) : 130 –142. Doi: 10.1111/jpy.2013.49.issue-1 |
| Sun Z, Hanyuda T, Lim P E, Tanaka J, Gurgel C F D, Kawai H, 2012. Taxonomic revision of the genus Lobophora(Dictyotales, Phaeophyceae) based on morphological evidence and analyses rbcL and cox3 gene sequences. Phycologia, 51 (5) : 500 –512. Doi: 10.2216/11-85.1 |
| Tanabe A S, 2007. KAKUSAN: a computer program to automate the selection of a nucleotide substitution model and the configuration of a mixed model on multilocus data. Molecular Ecology Notes, 7 (6) : 962 –964. Doi: 10.1111/men.2007.7.issue-6 |
| Tanabe A S. 2010. Phylogears Version 1.5. Software distributed by the author. http://www.fifthdimension.jp/. Accessed on 2015-8-15. |
| Thompson J D, Gibson T J, Plewniak F, Jeanmougin F, Higgins D G, 1997. The CLUSTAL_X windows interface: flexible strategies for multiple sequence alignment aided by quality analysis tools. Nucleic Acids Research, 25 (24) : 4876 –4882. Doi: 10.1093/nar/25.24.4876 |
| Vieira C, D'Hondt S, de Clerck O, Payri C E, 2014. Toward an inordinate fondness for stars, beetles and Lobophora? Species diversity of the genus Lobophora (Dictyotales, Phaeophyceae) in New Caledonia. Journal of Phycology, 50 (6) : 1101 –1119. Doi: 10.1111/jpy.12243 |
| Vos R A, 2003. Accelerated likelihood surface exploration: the likelihood ratchet. Systematic Biology, 52 (3) : 368 –373. |
| Womersley H B S, 1967. A critical survey of the marine algae of southern Australia. Ⅱ. Phaeophyta. Australian Journal of Botany, 15 (2) : 189 –270. Doi: 10.1071/BT9670189 |
| Womersley H B S. 1987. The Marine Benthic Flora of Southern Australia. Part Ⅱ. Australian Biological Resources Study, Australia. 484p. |
 2017, Vol. 35
2017, Vol. 35


