Institute of Oceanology, Chinese Academy of Sciences
Article Information
- LIU Yan(刘艳), ZHAO Weihong(赵卫红), LI Caiyan(李彩艳), MIAO Hui(苗辉)
- Free polyamine content during algal bloom succession in the East China Sea in spring 2010
- Chinese Journal of Oceanology and Limnology, 35(1): 215-223
- http://dx.doi.org/10.1007/s00343-016-5089-y
Article History
- Received Apr. 7, 2015
- accepted for publication Aug. 6, 2015
- accepted in principle Oct. 30, 2015
2 University of Chinese Academy of Sciences, Beijing 100049, China;
3 College of Resource and Environment, Linyi University, Linyi 276005, China
Polyamines are short-chained aliphatic amines present in prokaryotic and eukaryotic cells. Spermine (Spm), putrescine (Put) and spermidine (Spd) are common polyamines and have been detected in seaweeds (Scoccianti et al., 1995 ; Bachrach, 2010 ; Fuell et al., 2010), phytoplankton (Nishibori and Nishio, 1997), bacteria (Hamana and Matsuzaki, 1992), and vertebrates (Nishibori et al., 2006). Polyamines are essential for normal plant growth, and play important roles in protein synthesis and differentiation by binding to DNA and RNA (Bouchereau et al., 1999 ; Igarashi and Kashiwagi, 2000 ; Hwang et al., 2003 ; Bachrach, 2010).
Some polyamines function similar to or interact with plant hormones and have even been reported to be a new kind of plant hormone (Altman, 1982). As biologically active organic compounds, polyamines stimulate the growth of bloom-forming phytoplankton (Iwasaki, 1984). Additionally, polyamines alter the silicatization of diatoms, and respond to the physiological stress experienced by phytoplankton (Maestrini et al., 1999). Putrescine concentrations are positively related with primary production, suggesting they are a direct nitrogen source for algae (Lu and Hwang, 2002). Polyamines are also significant components of dissolved organic nitrogen (DON) in seawater (Nishibori et al., 2001), and play an important role in the growth process of Chattonella antiqua and Heterosigma akashiwo(Nishibori and Nishijima, 2004 ; Nishibori et al., 2006). Marine phytoplankton use amines and amino acids as nitrogen sources, with a cell surface amine oxidase (Palenik and Morel, 1991). A previous study reported the oxidation of fluorescent-labelled cadaverine and lysine by phytoplankton culture, and suggesting the importance of this nitrogen-uptake pathway in the marine nitrogen cycle (Pantoja et al., 1993). The components of DON pools, such as urea and amino acids, can be significant players in nitrogen flux (Berman and Bronk, 2003). Polyamines may stimulate and regulate harmful algal blooms (Johnsen et al., 1999 ; Legrand et al., 2003), and it has been suggested that harmful algal blooms in the Ofotfjord-Vestford Bay of Norway and fish farms in Scotland's coastal areas may be related to polyamines (Gentien, 1998).
Harmful algal blooms have been happening frequently and widely in the East China Sea in recent years (Zhou et al., 2003 ; Ji et al., 2011), including succession of diatom blooms and dinoflagellate blooms (Zhou and Zhu, 2006). Algal bloom occurrence and succession in the East China Sea have been of particular interest. In this study we analyzed polyamine content of impacted seawater during two successive algal blooms. First, in early April 2010, a diatom bloom dominated by Skeletonema costatum occurred. Then, when S. costatum dispersed in late April, the dinoflagellate Prorocentrum donghaiense became the dominant species developing into a large scale P. donghaiense bloom in early May. Concentrations and distributions of free spermine, putrescine, and spermidine were measured during the course of algal bloom succession, and their distribution and relationship with the success of each algal bloom was analyzed.
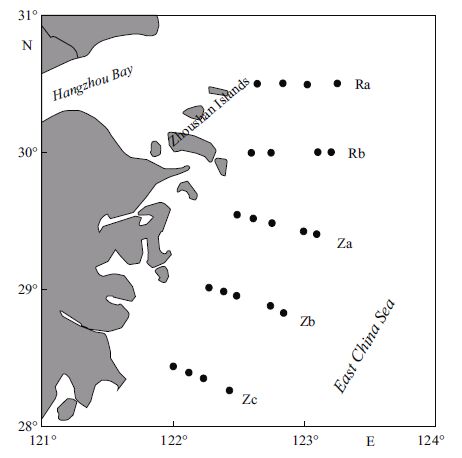
2 MATERIAL AND METHOD 2.1 Sample collectionData collection was performed offZhejiang Province (28.0°-31.0°N, 121°-124°E), April 17-24 and May 7-14, 2010. Five transects were sampled and labeled for each transect: Ra, Rb, Za, Zb and Zc (Fig. 1). On transect Za, four 24-h continuous sampling events were conducted on April 10, April 25, May 8, and May 11. Water samples from the surface layer (1 m below sea level), middle layer (the layer with largest amount of chlorophyll a), and bottom layer (2-2.5 m above seafloor) were collected using a Niskin sampler (Agassiz trawl, KC-Denmark, Silkeborg, Denmark). Water samples were filtered with a Whatman GF/F filter (Whatman, GEWhatman, Maidstone, UK) Φ =25 mm, pre-combusted at 450℃ for 4 h). The filtrates were collected in 60 mL browntinted glass bottles and then frozen at -20℃ in the dark for later analysis.
|
| Figure 1 Location of polyamine sampling during diatom and dinoflagellate blooms in the East China Sea in spring 2010 |
Filtrates were thawed before analysis. For each field sample tested, a 1 mL filtrate sample was mixed with 1, 6-diaminohexane as an internal standard, for a final concentration of 1.0×10-7 mol/L. Next, 12 μL 70% perchloric acid was added and the sample was maintained at 4℃ for 30 min. The sample was then mixed with 90 μL 2 mol/L sodium hydroxide and 70 μL borate buffer, resulting in a pH of 9.18. The mixture was vortexed for 30 s, then mixed with 1 mL of 6 mg/mL acetone solution of dansyl chloride, and then incubated in the dark for 45 min at 40℃. The reaction was terminated by adding 40 μL of 25% ammonia in water; the sample was then incubated again in the dark for 30 min. Finally, 60 μL of acetonitrile was added to the sample, and the mixture was analyzed using high performance liquid chromatograph.
Samples were run on a Waters e2695 HPLC with a Waters e2475 Fluorescence Detector and a C18 column (150 mm×4.6 mm i.d., 5 μm particle size, Agilent, Santa Clara, CA, USA). The fluorescence of 12 conjugates of each polyamine was measured at an excitation wavelength of 340 nm and an emission wavelength of 515 nm. The mobile phase solution A was 0.1 mol/L NH 4 Ac; the mobile phase solution B was acetonitrile. Prior to use, mobile phase solutions were filtered with a Whatman GF/F filter (Whatman, GEWhatman, Maidstone, UK, Φ =25 mm, pre-combusted at 450℃ for 4.5 h). The elution gradient started at 35% A and 65% B and changed as follows: 0-10 min, 35% A to 60% A; 10-15 min, 60% A to 80% A; 15-20 min, 80% A to 100% A; 20-30 min, 100% A to 35% A. Column temperature was kept at 40℃, the flow rate was 1.0 mL/min, and the sample injection volume was 10 μL.
At a range of 1.0×10-9 -1.0×10-7 mol/L, the peak high ratio between polyamines and the internal standard showed good linearity (R 2 >0.99), and recovery of all three polyamines was 90.1%-110.9%. Determining dissolved free polyamines (putrescine, spermidine, and spermine) in seawater were based on methods of Fu et al.(2010).
Boat temperature (T) and salinity (S) were recorded using a Seabird 911-plus Conductivity, Temperature and Depth (CTD) recorder (CTD SBE25, Qingdao, China); and dissolved inorganic nitrogen (DIN) was measured using a QuAAtrO Continuous-flow Analyzer (Bran+Luebbe GmbH, Norderstedt, Germany). Nitrate and nitrite were determined using the standard pink azodye method, and ammonium was determined using the hypobromateoxidation pink azo dye method. DIN is considered to be the sum of nitrate, nitrite, and ammonium concentrations (Wu et al., 2013). Dissolved organic carbon (DOC) and total dissolved nitrogen (TDN) were analyzed using high temperature catalytic oxidation, and thawed samples were directly tested using a Shimadzu TOC-V total organic carbon analyzer. DON was calculated as TDN minus DIN (Zhang et al., 2013). Chlorophyll a concentrations were measured with fluorescence method. Chlorophyll a samples were extracted for 12 h with 90% acetone at a low temperature (-20℃) in dry and dark conditions, and analyzed with a Turner-II fluophotometer (F-4500, HITACHI, Tokyo, Japan)(Wang et al., 2009).
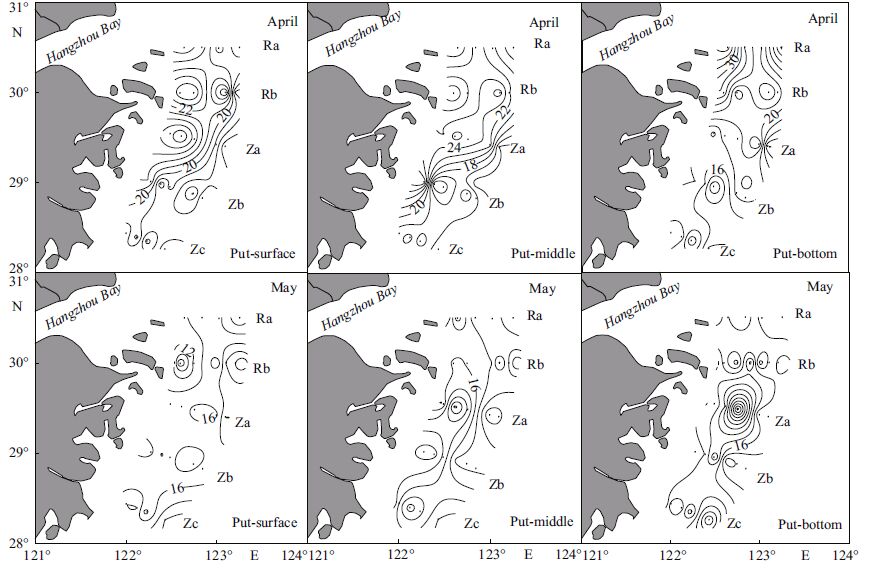
3 RESULT 3.1 Distribution patterns of polyamines in late April and early May, 2010Spermine, putrescine, and spermidine were the major free polyamines identified in East China Sea seawater during the spring 2010 algal blooms. In late April, spermine, putrescine, and spermidine concentrations ranged from 5-64, 7-81, and 0-19 nmol/L, with overall average concentrations of 43, 20, and 3 nmol/L. Across the study area, spermine, putrescine, and spermidine were present in proportions of 64%, 31%, and 5%. Spermine distribution was similar and patchy in all three layers; high concentrations in the form of a patch center surrounded by areas with lower concentrations were observed near several stations. The patch center with the highest spermine concentration was at the northwest part of the survey area (Fig. 2). The highest concentrations of putrescine occurred in the western part of the survey area along the coast of the Zhejiang Province, and concentrations decreased gradually with distance from the shore. High putrescine concentrations were also seen in surface and middle layers at Section Za nearshore, and in the bottom layer in the northwest part of the survey area near Hangzhou Bay (Fig. 3). Spermidine concentrations were an order of magnitude lower than spermine, and did not show a systematic regularity. The higher value in the surface layer occurred at Section Za offshore; the highest value in the middle layer was at Section Rb and Zb (Fig. 4). Overall, polyamine concentrations increased toward the northwest in late April, the time period when the S. costatum -dominated diatom bloom dispersed. This large-scale bloom had occurred in the northern part of the survey area in early to mid-April, thus the increase in polyamine concentrations observed may have resulted from diatom decomposition during the dispersal process.

|
| Figure 2 Horizontal distribution of spermine (Spm) during a diatom bloom in late April and during a dinoflagellate bloom in early May 2010 |
|
| Figure 3 Horizontal distribution of putrescine (Put) during a diatom bloom in late April and during a dinoflagellate bloom in early May 2010 |

|
| Figure 4 Horizontal distribution of spermidine (Spd) during a diatom bloom in late April and during a dinoflagellate bloom in early May 2010 |
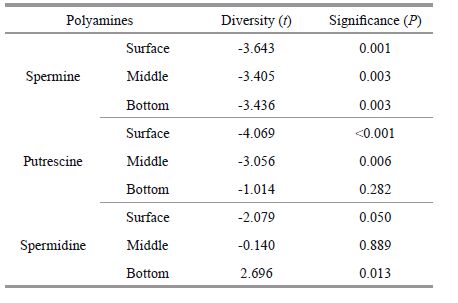
In early May, spermine, putrescine, and spermidine ranged from 1-51, 7-35, and 0-12 nmol/L. The average composition of polyamines (i.e., proportion of each of the three types) were similar between late April and early May, while the average concentrations of each polyamine dropped. Pairwise testing of polyamines concentrations were performed across locations and layers between late April and early May (Table 1). Results showed that spermine concentrations differed in surface, middle, and bottom layers over this time frame, as did putrescine concentrations in the surface and middle layers. Between April and May, there was no significant change in polyamine concentrations. At the study area, the average polyamine concentrations decreased from late April to early May. The spermine concentration was high in the surface layer in the central and southern parts of the survey area, but high in the middle and bottom layers in the southwest area. The chlorophyll a value was also relatively high in this area, with an average of 4.30 μg/L (Fig. 2). For putrescine, the highest concentration area was along Section Zc in the surface layer; in the western survey area, the highest concentrations were in the middle layer; and in the southern offshore survey area, concentrations were highest in the bottom layer. The highest putrescine concentrations in all three layers occurred at Section Za (Fig. 3); spermidine was evenly distributed at low concentrations across all layers of the survey area (Fig. 4).
|
Polyamines were generally higher in the south and lower in the north in early May, in contrast with the distribution observed in late April. The large-scale dinoflagellate bloom dominated by P. donghaiense occurred in the south region of the survey area in early May, and polyamine concentrations were relatively higher in areas where this bloom had occurred.
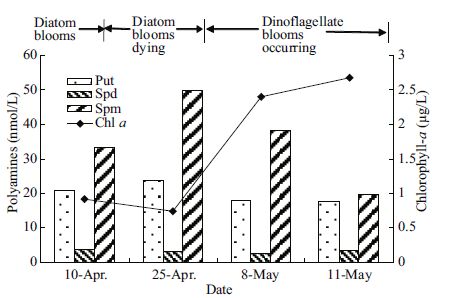
3.2 Variation of polyamines in Za sectionThe most substantial change in polyamine concentrations over the period of the algal bloom succession was recorded in the Za section (Fig. 6). April 10 was during the diatom bloom; April 25 was during the diatom bloom dispersion period; the dinoflagellate bloom dominated by P. donghaiense occurred after May 8. While the diatom bloom was dispersing on April 25, concentrations of spermine and putrescine increased slightly (Fig. 5), and the ratio of (Spd+Spm)/Put increased during the same period (Fig. 6). At the same time, the chlorophyll a level, which was mainly contributed by the diatom, declined (Figs. 5-6). On May 8, spermine, putrescine and spermidine concentrations began to decrease as the dinoflagellate bloom emerged; chlorophyll a, mainly from the dinoflagellate, also increased. On May 11, the dinoflagellate population bloomed on a large area in all the study area, spermine and the ratio of (Spd+Spm)/Put concentrations markedly declined, while chlorophyll a continued to increase.
|
| Figure 5 Variation in polyamine concentrations over time in the Za section |

|
| Figure 6 Ratio variation of (Spd+Spm)/Put and chlorophyll a in the Za section |
Correlations of polyamines with temperature (T), salinity (S), dissolved organic carbon (DOC), total nitrogen (TN), dissolved organic nitrogen (DON) and chlorophyll a, suggest that environmental factors may have influenced on the polyamine content in the survey area (Tables 2-3).

|
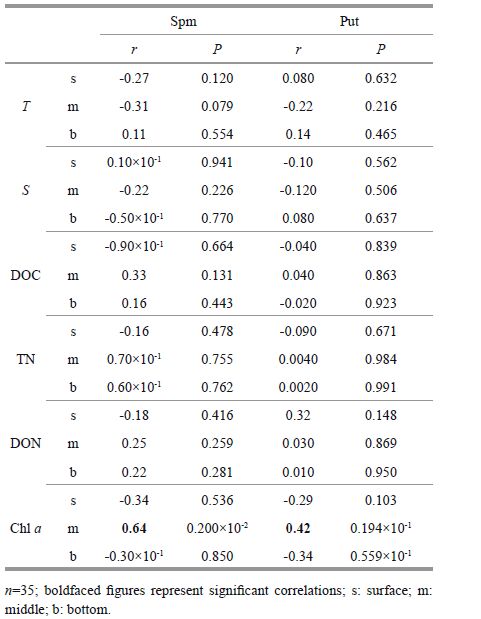
|
In the study area, temperature and salinity are influenced mainly by the Changjiang (Yangtze) River water, the Yellow Sea cold water mass, Taiwan warm current, and other open seawater masses. During the study period, polyamine levels showed no significant correlations with temperature and salinity, and polyamine contents were not significantly affected by the water current.
In late April, spermine in the middle layer was positively correlated with DON; putrescine in surface and middle layers was positively correlated with TN and DON. Positive correlations were also seen between putrescine in the middle layer with DOC. There was no correlation between spermine, putrescine and contemporaneous chlorophyll a, and polyamine concentrations of where the diatom bloom had occurred were relatively higher (Table 2).
In early May, spermine and putrescine in the middle layer were positively correlated with chlorophyll a ; no other correlations with DOC, TN, or DON were observed (Table 3). Polyamine concentrations had decreased compared with concentrations in late April; however, localized polyamine concentrations in areas where the P. donghaiense bloom had occurred were higher than areas outside the bloom.
4 DISCUSSIONSignificant algal blooms occurred in the East China Sea in the spring of 2010. During these blooms, the primary free polyamines found in the seawater where the blooms occurred were spermine, putrescine and spermidine. The average concentration of spermine was the highest, followed by putrescine and spermidine. In contrast, Nishibori et al.(2001) reported that during a summer bloom period in the Seto Inland Sea of Japan, putrescine and spermidine were the major polyamines in the coastal seawater, fluctuating from 2.0-32.6 and 1.0-14.1 nmol/L. Spermine was presented at
Polyamine types and concentration ranges in the East China Sea differed from that in the Seto Inland Sea of Japan due to the composition of algal species. Polyamine concentrations in East China Sea seawater were much higher. Polyamines have been reported to be more abundant in other types of natural waters compared with seawater. For example, in a eutrophic salt pond, putrescine ranged from undetectable to 200 nmol/L (Lee and Jørgensen, 1995); in a lagoon, the concentrations of putrescine, spermidine and spermine were 0-570, 0-920, and 0-20 nmol/L (Badini et al., 1994). The different compositions and concentrations of polyamines in diverse water bodies appear to be related to eutrophication levels.
Polyamine concentrations in areas of highfrequency algal bloom were much higher than areas that did not experience blooms, suggesting that polyamines may have close contact with algal bloom organisms. Other researchers have reported high level polyamines caused by diatom bloom-dispersing processes. For example,Gentien (1998) reported that putrescine concentrations reached 100 nmol/L, which was attributed to a diatom bloom decomposition and dispersal, after a spring bloom offthe coast of France. As a second example, in the Uranouchi Inlet in Japan, putrescine and spermidine concentrations increased after a summertime Chaetoceros bloom (Nishibori et al., 2003).
We previously reported that through experimentally culturing S. costatum, polyamine concentrations increased suddenly during a decline phase, and putrescine concentrations in the culture solution increased to 367 nmol/L (Zhao et al., 2014). In addition, polyamine concentrations in cells were at a high level when entering an exponential growth phase during S. costatum culture experiments. In particular, spermine levels reached 3×10-16 mol/cell (Zhu et al., 2015). This suggests that when a large volume of polyamines are decomposed and released by the diatom bloom, the bloom dispersal process may increase polyamine concentrations in seawater, specifically spermine levels.
Except during algae decomposition and release, live algal cells metabolize and secrete polyamines. This was supported by our previous S. costatum culture experiments, wherein polyamine concentrations increased when algae were transitioning into an exponential growth phase and secreted polyamines during the growth process (Zhao et al., 2014). These findings are supported by correlations between polyamine concentrations and algal blooms. For example, Höfle (1984) found that polyamine concentrations were rich in the euphotic zone where a phytoplankton bloom broke out in a marine environment. Similarly, in the present study, polyamine distribution in the East China Sea in early May 2010 correlated with phytoplankton when a P. donghaiense bloom occurred; this implies that P.donghaiense release polyamines into seawater through metabolic and secretion activities.
Other studies have found that some exogenous polyamines can stimulate the growth of some marine algae. Iwasaki (1984) found that different types and levels of exogenous polyamines affected different microalgae growth differently. Putrescine could promote Alexandrium minutum growth at low levels (0.1 μmol/L), while spermidine could restrain A. minutum growth. Putrescine concentrations could stimulate Karenia mikimotoi growth at levels of 0.1- 5.0 μmol/L. Putrescine and cadaverine concentrations less than 110 μmol/L promoted Chrysochromulina leadbeateri growth (Gentien, 1998). Adding polyamines, especially putrescine, to natural phytoplankton assemblages of Microcystis aeruginosa increased in vivo fluorescence (Maestrini et al., 1999).
We previously found that adding polyamines could affect algal growth in the laboratory. Spermine, putrescine and spermidine concentrations from 5 to 100 nmol/L could facilitate P. donghaiense growth, and spermine addition could result in the most significant action (Liang et al., 2013). Based on this, it is hypothesized that, with the S. costatum bloom transitioning into a dispersion period in late April 2010, polyamine concentrations dominated by spermine continuously increased. This may have stimulated P. donghaiense growth, gradually boosting it to be more competitive in the survey area.
In early May in the East China Sea, the average seawater temperature was 18℃, the average salinity was 30 and the average concentrations of NO3 -N and PO4 -P were 11.85 μmol/L and 0.8 μmol/L. Nutrient levels were relatively adequate, and under this temperature and salinity, P. donghaiense proliferated rapidly, developing into a bloom. The variation recorded for the polyamines, that variation in polyamines decreased and variation in chlorophyll a increased in the Za study section, could validate this point.
The (Spd+Spm)/Put ratio has been a standard indicator of cell growth (division) in plants (Liu et al., 2004). When the P. d onghaiense bloom occurred, the ratio of (Spd+Spm)/Put in seawater decreased in the Za section, suggesting that these polyamines were absorbed and metabolized by P. d onghaiense. Similar observations have been reported in other regions. In the summer, putrescine and spermidine concentrations increased in Uranouchi Inlet, Japan after the Chaetoceros bloom occurred and stimulated a Gymnodinium mikimotoi bloom (Nishibori et al., 2003). Gentien (1998) reported that a K. mikimotoi bloom occurred after a springtime diatom bloom offthe coast of France; putrescine decomposing and production in the diatom bloom promoted K. mikimotoi growth. This indicates that polyamines may have played a role in P. donghaiense dominance in the algal bloom succession in the East China Sea in the spring of 2010.
5 CONCLUSIONThe dominant free polyamines found in East China Sea seawater during significant 2010 algal blooms were spermine, putrescine and spermidine. Spermine was the most abundant polyamine, followed by putrescine and spermidine. In late April, a diatom bloom dominated by S. costatum decomposed and released a large amount of polyamines. The high polyamine content in the water, dominated by spermine, may have stimulated the growth of P. donghaiense, providing this dinoflagellate with a competitive advantage and may have resulted in the large-scale P. donghaiense bloom seen in early May.
6 ACKNOWLEDGMENTThe authors appreciate the help of Prof. WANG Jiangtao, Prof. SHI Xiaoyong, Prof. ZHU Dedi, Prof. LÜ Songhui and Mr. ZHANG Shuwei for providing data for temperature, salinity, chlorophyll a, TN, DON and DOC.
| Altman A, 1982. Retardation of radish leaf senescence by polyamines. Physiologia Plantarum, 54 (2) : 189 –193. Doi: 10.1111/ppl.1982.54.issue-2 |
| Bachrach U, 2010. The early history of polyamine research. Plant Physiology and Biochemistry, 48 (7) : 490 –495. Doi: 10.1016/j.plaphy.2010.02.003 |
| Badini L, Pistocchi R, Bagni N, 1994. Polyamine transport in the seaweed Ulva rigida (Chlorophyta). Journal of Phycology, 30 (4) : 599 –605. Doi: 10.1111/j.0022-3646.1994.00599.x |
| Berman T, Bronk D A, 2003. Dissolved organic nitrogen: a dynamic participant in aquatic ecosystems. Aquatic Microbial Ecology, 31 (3) : 279 –305. |
| Bouchereau A, Aziz A, Larher F, et al, 1999. Polyamines and environmental challenges: recent development. Plant Science, 140 (2) : 103 –125. Doi: 10.1016/S0168-9452(98)00218-0 |
| Fu M, Zhao W H, Miao H, et al, 2010. Determination of dissolved free putrescine, spermidine and spermine in seawater by high performance liquid chromatography. Chinese Journal of Analytical Chemistry, 38 (10) : 1445 –1449. |
| Fuell C, Elliott K A, Hanfrey C C, et al, 2010. Polyamine biosynthetic diversity in plants and algae. Plant Physiology and Biochemistry, 48 (7) : 513 –520. Doi: 10.1016/j.plaphy.2010.02.008 |
| Gentien P, 1998. Bloom dynamics and ecophysiology of the Gymnodinium mikimotoi species complex. Physiological Ecology of Harmful Algal Bloom, 41 : 155 –173. |
| Hamana K, Matsuzaki S, 1992. Polyamines as a chemotaxonomic marker in bacterial systematics. Critical Reviews in Microbiology, 18 (4) : 261 –283. Doi: 10.3109/10408419209113518 |
| Höfle M G, 1984. Degradation of putrescine and cadaverine in seawater cultures by marine bacteria. Appl. Environ.Microbiol., 47 (4) : 843 –849. |
| Hwang D F, Lu Y H, Noguchi T, 2003. Effects of exogenous polyamines on growth, toxicity, and toxin profile of dinoflagellate Alexandrium minutum. Journal of the Food Hygienic Society of Japan, 44 (1) : 49 –53. Doi: 10.3358/shokueishi.44.49 |
| Igarashi K, Kashiwagi K, 2000. Polyamines: mysterious modulators of cellular functions. Biochem. Biophys. Res.Commun., 271 (3) : 559 –564. Doi: 10.1006/bbrc.2000.2601 |
| Iwasaki H, 1984. Growth physiology of red-tide microorganisms. Microbiology Science, 1 (7) : 179 –182. |
| Ji X Q, Han X T, Bai J, Zheng L, Yu Z M, 2011. Effects of allelopathy and allelochemicals in algal succession. Marine Sciences, 35 (2) : 92 –98. |
| Johnsen G, Dalløkken R, Eikrem W, Legrand C, Aure J, Skjoldal H R, 1999. Eco-physiology, Bio-optics and toxicity of the ichthyotoxic Chrysochromulina leadbeateri(Prymnesiophyceae). Journal of Phycology, 35 (6) : 1465 –1476. Doi: 10.1046/j.1529-8817.1999.3561465.x |
| Lee C, Jørgensen N O G, 1995. Seasonal cycling of putrescine and amino acids in relation to biological production in a stratified coastal salt pond. Biogeochemistry, 29 (2) : 131 –157. |
| Legrand C, Rengefors K, Fistarol G O, Granéli E, 2003. Allelopathy in phytoplankton-biochemical, ecological and evolutionary aspects. Phycologia, 42 (4) : 406 –419. Doi: 10.2216/i0031-8884-42-4-406.1 |
| Liang C C, Zhao W H, Miao H, 2013. How the biogenic amines affect HAB algae's growth: a preliminary exploration. Oceanologia et Limnologia Sinica, 44 (3) : 709 –716. |
| Liu H P, Dong B H, Zhang Y Y, Liu Z P, LiuY L, 2004. Relationship between osmotic stress and the levels of free, conjugated and bound polyamines in leaves of wheat seedlings. Plant Science, 166 (5) : 1261 –1267. Doi: 10.1016/j.plantsci.2003.12.039 |
| Lu Y H, Hwang D F, 2002. Polyamine profile in the paralytic shellfish poison-producing alga Alexandrium minutum. Journal of Plankton Research, 24 (3) : 275 –279. Doi: 10.1093/plankt/24.3.275 |
| Maestrini S Y, Balode M, Béchemin C, Purina I, 1999. Nitrogenous organic substances as potential nitrogen sources, for summer phytoplankton in the Gulf of Riga, eastern Baltic Sea. Plankton Biology and Ecology, 46 (1) : 8 –17. |
| Nishibori N, Fujihara S, Nishijima T, 2006. Changes in intracellular polyamine concentration during growth of Heterosigma akashiwo (Raphidophyceae). Fisheries Science, 72 (2) : 350 –355. Doi: 10.1111/fis.2006.72.issue-2 |
| Nishibori N, Matuyama Y, Uchida T, et al, 2003. Spatial and temporal variations in free polyamine distributions in Uranouchi Inlet, Japan. Marine Chemistry, 82 (3-4) : 307 –314. Doi: 10.1016/S0304-4203(03)00076-8 |
| Nishibori N, Nishijima T, 2004. Changes in polyamine levels during growth of a red-tide causing phytoplankton Chattonella antiqua (Raphidophyceae). Eur. J. Phycol., 39 (1) : 51 –55. Doi: 10.1080/09670260310001636677 |
| Nishibori N, Nishio S, 1997. Occurrence of polyamines in bloom forming toxic dinoflagellate Alexandrium tamarense. Fisheries Science, 63 : 319 –320. |
| Nishibori N, Yuasa A, Sakai M, et al, 2001. Free polyamine concentrations in coastal seawater during phytoplankton bloom. Fisheries Science, 67 (1) : 79 –83. Doi: 10.1046/j.1444-2906.2001.00202.x |
| Palenik B, Morel F M M, 1991. Amine oxidases of marine phytoplankton. Appl. Environ. Microbiol., 57 (8) : 2440 –2443. |
| Pantoja S, Lee C, Marecek J F, Palenik B P, 1993. Synthesis and use of fluorescent molecular probes for measuring cell-surface enzymatic oxidation of amino acids and amines in seawater. Analytical Biochemistry, 211 (2) : 210 –218. Doi: 10.1006/abio.1993.1259 |
| Scoccianti V, Penna A, Penna N, Magnani M, 1995. Effect of heat stress on polyamine content and protein pattern in Skeletonema costatum. Marine Biology, 121 (3) : 549 –554. Doi: 10.1007/BF00349465 |
| Wang Z H, Shi X Y, Zhang C S, Liang S K, Wang Li S, 2009. Primary study on characteristics of distribution of chlorophyll a in the Yellow Sea and East China Sea. Progress in Fishery Sciences, 30 (2) : 120 –126. |
| Wu T, Han X R, Zhao Q, Shi X Y, 2013. Effect of red tide on nitrogen in typical section of frequent HAB area, East China Sea. Marine Environmental Science, 32 (2) : 196 –200. |
| Zhang S W, Wang J T, Li N, Yan X J, 2013. Dissolved organic carbon and nitrogen in Changjiang Estuary and adjacent sea areas in spring. Marine Environmental Science, 32 (1) : 33 –37. |
| Zhao W H, Wang J, Li CY, Miao H, 2014. The free polyamine in the body and the culture medium during the growth cycle of Skeletonema Costatum. Journal of Unicersity of Jinan (Science & Technology), 28 (3) : 165 –169. |
| Zhou M J, Yan T, Zou J Z, 2003. Preliminary analysis of the characteristics of red tide areas in Changjiang River estuary and its adjacent sea. Chinese Journal of Applied Ecology, 14 (7) : 1031 –1038. |
| Zhou M J, Zhu M Y, 2006. Progress of the project "ecology and oceanography of harmful algal blooms in China". Advances in Earth Science, 21 (7) : 673 –679. |
| Zhu X W, Zhao W H, Miao H, 2015. Relationship between growth and variation of endogenous polymine content under salinity stress in Skeletonema costatum S. L. and Prorocentrum donghaiense. Oceanologia et Limnologia Sinica, 46 (1) : 50 –57. |
 2017, Vol. 35
2017, Vol. 35


