Institute of Oceanology, Chinese Academy of Sciences
Article Information
- LU Lin(鹿琳), WANG Jun(王军), YANG Guanpin(杨官品), ZHU Baohua(朱葆华), PAN Kehou(潘克厚)
- Biomass and nutrient productivities of Tetraselmis chuii under mixotrophic culture conditions with various C: N ratios
- Chinese Journal of Oceanology and Limnology, 35(2): 303-312
- http://dx.doi.org/
Article History
- Received Oct. 23, 2015
- accepted in principle Dec. 31, 2015
- accepted for publication Feb. 23, 2016
2 College of Marine Life Sciences, Ocean University of China, Qingdao 266003, China;
3 Function Laboratory for Marine Fisheries Science and Food Production Processes, Qingdao National Laboratory for Marine Science and Technology, Qingdao 266071, China
Microalgae are rich in nutrients, including proteins, lipids, polysaccharides and vitamins (Becker, 2013), and these nutrient components can be readily consumed by young aquatic animals with a high conversion efficiency (Benemann, 1992). Therefore, they are widely used as larval stage feed, or nutrient supplements in feeds (Hemaiswarya et al., 2011). Furthermore, the species and the quantity of the microalgae can directly affect the hatching, survival, and growth of aquatic animals (Liu et al., 2007). Thus, mass-cultivation of microalgae is necessary for the development of aquaculture. To date, approximately 20 bait-microalgae, especially Chlorella, Tetraselmis, Isochrysis, and Nannochloropsis, have been used in mariculture (Hemaiswarya et al., 2011). Microalgal mass culture has been carried out mainly under autotrophic conditions in many countries; however, this mode is vulnerable to restrictions of low cell density, harvest problem, and high costs (PerezGarcia et al., 2011). In contrast, mixotrophic cultivation of microalgae can effectively improve the growth rate and biomass productivity, and is considered an effective approach to achieve high cell density and effective production of high value-added products (Chen and Zhang, 1997). For example, the biomass of Chlamydomonas globosa, Chlorella minutissima, and Scenedesmus bijuga cultured under mixotrophic conditions was determined to be 3-to 10-fold higher than that under phototrophic conditions (Bhatnagar et al., 2011). Nevertheless, only a few microalgal species have been cultured mixotrophically; these include Chlamydomonas reinhardtii, Chlorella sp., and Haematococcus pluvialis, mostly Chlorella (Heifetz et al., 2000; Alcántara et al., 2015; Alkhamis and Qin, 2016). To meet the high demand for baitmicroalgae and to enrich the bait-microalgal resource, it is necessary to collect more mixotrophically cultivable microalgae.
Species of Tetraselmis are important microalgae used in rearing larval mollusks, crustaceans, and several marine fish species (Muller-Feuga et al., 2003; Tredici et al., 2009). In addition, Tetraselmis suspension and extract have been demonstrated to possess antibacterial activity against many pathogens (Austin et al., 1992), highlighting the potential use of this microalgae as probiotics in aquaculture (Irianto and Austin, 2002; Tredici et al., 2009). Moreover, the species of Tetraselmis are tolerant to extreme acidity, salinity, and temperature, and are able to adapt to different environments. These characteristics make them excellent bait-microalgae (Garofalo, 2009). However, the growth of Tetraselmis is very slow under autotrophic culture conditions. They have a doubling time of 24-48 h (Sun et al., 2000), which severely restricts its mass culture.
In the present study, a strain of Tetraselmis chuii was isolated from south Yellow Sea and was found to grow efficiently under mixotrophic culture conditions. We determined its nutritional components under different carbon:nitrogen (C:N) ratios with an aim of developing it as a mass cultivable bait-microalga.
2 MATERIAL AND METHOD 2.1 Microalgal isolation and identificationThe microalgal strain (named H20) was collected from south Yellow Sea (120°39.631′E, 34°30.485′N) in June 2010. It was purified by micromanipulation and streak plating (Guillard, 2005). Pure enrichment cultivation was carried out in f/2 medium (Guillard and Ryther, 1962). Stock cultures were prepared under aseptic conditions without aeration. Microalgal genomic DNA was extracted with CTAB method (Murray and Thompson, 1980). PCR primers were designed based on 18S rRNA and rbcL gene sequences retrieved from GenBank. 18S rRNA gene was amplified using the primers 5′-CTTATACTGTGAAACTGCGAATGGC-3′ (forward) and 5′-ACCTTGTTACGACTTCTCCTTCCTC-3′ (reverse). The reaction was carried out in a 50-μL volume containing 1×buffer, 10 nmol dNTP (each), 2.5 ng template DNA, 1 nmol primer (each), and 2.0 U Taq DNA polymerase. The PCR conditions were as follows: initial denaturation at 94℃ for 5 min, followed by 35 cycles of denaturation at 94℃ for 30 s, annealing at 55℃ for 30 s, and extension at 72℃ for 1 min, and a final extension at 72℃ for 10 min. The rbcL was amplified under the same conditions except that the annealing temperature was 58℃ and the number of cycles was reduced to 30; the primers used were 5′-GGTCGTGGTTTATTAGGGTG-3′ (forward primer) and 5′-AGCTGTACGGATGATGTCTC-3′ (reverse primer).
The amplified sequences were aligned with those of other green algae, retrieved from GenBank, using BLAST (NCBI). Genetic distance was calculated using the Kimura 2-parameter model with 1 000 bootstrap replicates in Mega 5.10 (http://www.megasoftware.net). The neighbor-joining (NJ) phylogenetic tree was constructed using Mega 5.10 software.
2.2 Medium formulation and experimental designThe microalga was cultured in f/2 medium. To avoid any possible effects caused by nutrient deficiency, all the components of f/2 medium (except sodium nitrate) were added in double the amount. The molar ratio of carbon and nitrogen was set at 24, 20, 16, 12, 8, 4 by adding different concentrations of sodium nitrate in relative to 10 g/L glucose. The values were chosen based on reports involving Tetraselmis sp. and other microalgal species (Hassan, 1996; Jiang and Chen, 2000; Azma et al., 2011). f/2 medium without external carbon source was used as control. Inoculation was done with cells in the exponential growth phase. The culture was done in triplicate by subculturing in 500-mL shake flasks containing 350 mL medium supplemented with 10 g/L glucose. The flasks were incubated on an orbital shaker (200 r/min, 25℃; ZQZY-A, China). All the cultures were kept under a light intensity of 150 μE/(m2∙s), and the photoperiod was 14:10 (L:D).
2.3 Measurement of growth parameters and pigment contentThe growth curve was made based on the optical density (OD) of the culture at 750 nm. For determining the dry weight (g/L) of the cells, a measured volume of algal culture was centrifuged at 8 000×g for 10 min, the obtained pellet was washed twice with 0.5 mol/L NH4HCO3 (Zhu and Lee, 1997), dried for 12 h in a vacuum freeze drier at 0.03 atm, and weighed. Chlorophyll a was determined spectrophotometrically according to the method described by Arnon (1949). In brief, 7 mL homogenized algal cells were centrifuged and washed with distilled water. The pellet was suspended in the same volume of 80% (v/v) aqueous acetone. After overnight extraction at 4℃, the suspension was centrifuged and the supernatant was used for the measurement of absorbance at 663 (A663) and 645 nm (A645). The formula used for determining the chlorophyll a content (Ca) was as follows: Ca=12.21A663-2.81A645.
2.4 Determination of total soluble polysaccharide and total proteinTotal polysaccharide was determined with the anthrone colorimetric method (Chaplin and Kennedy, 1994) which involved hydrolysis by sulfuric acid in the presence of anthrone, in a boiling water bath. The absorbance of the sample solution was measured spectrophotometrically at 620 nm and the concentration was calculated by referring to a calibration curve prepared using glucose. Total protein content was assayed by Coomassie Brilliant Blue G-250 method (Bradford, 1976). Briefly, cell pellet resuspended in PBS solution was ultrasonicated and the absorbance of the suspension was measured at 575 nm using bovine serum albumin as a standard.
2.5 Analysis of total lipid and fatty acid compositionTotal lipid was extracted from approximately 50 mg dried algal biomass using a gravimetric method (Bligh and Dyer, 1959). Fatty acids were determined in approximately 30 mg biomass using a modification of the method described by Lepage and Roy (1984). The fatty acid methyl esters were detected on an Agilent 7820A gas chromatograph using an Agilent J and WGC columns (Agilent Technologies, USA). The column temperature was programmed at 50℃ for 1 min, followed by an increase to 175℃ at 25℃/ min, and finally to 250℃ at 4℃/min, the column being held at this temperature for 5 min. High-purity nitrogen was used as the carrier gas. The injector and detector were held at 250℃ and 280℃, respectively. The injection volume was 1 μL.
2.6 Statistical analysisAll data are presented as mean±standard deviation, and the difference between the control and treatment was assessed by one-way analysis of variance followed by a Tukey’s test. Significance was accepted at P < 0.05. All statistical tests were conducted using SPSS 19.0 software (SPSS Inc., USA).
3 RESULT 3.1 Microalgal isolation and identificationThe purified green alga, designated as H20, was broadly oval and slightly asymmetric in shape. The cell was wide at its anterior end and relatively narrow at the opposite end. The four flagella emerged from the base of apical trough. The large chromatophore in the cell was cup-shaped. On the basis of these morphological characteristics, the strain was preliminarily identified as Tetraselmis. The phylogenetic trees constructed based on 18S rRNA and rbcL gene sequences revealed that H20 had a close relationship with Tetraselmis chuii with 100% (Fig. 1a) and 85% (Fig. 1b) of high bootstrap value, respectively, indicating that the strain was T. chuii.

|
| Figure 1 Neighbor-joining phylogenetic trees based on 18S rRNA (a) and rbcL gene sequences (b) of H20 and other green algae Bootstrap values were calculated with 1 000 repeats. |
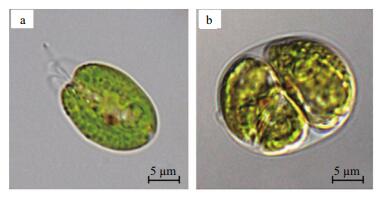
T. chuii was greenish under autotrophic, and blackish-green under mixotrophic culture conditions. Microscopically, T. chuii cells cultured under autotrophic conditions were oval with several clear flagellums on one side (Fig. 2a) whereas they were rounded and flagellum-less under mixotrophic conditions. Moreover, almost all T. chuii cells observed were in splitting state (Fig. 2b).
|
| Figure 2 Cell morphology of T. chuii under autotrophic (a) and mixotrophic (b) culture conditions |
T. chuii showed sustained growth under mixotrophic culture conditions with different C:N ratios using glucose as a carbon source (initial concentration, 10 g/L) and sodium nitrate as nitrogen source. However, the growth rate at different ratios differed greatly (Fig. 3). At a C:N ratio of 24, the highest growth rate was observed in the first 24 days. The growth rate declined later on and the cell density was lower than that at C:N ratios of 16 and 12. After one month of culture, the highest OD (1.111±0.020) was observed at a C:N ratio of 16, which was followed by that obtained at ratio of 12 (1.073±0.011). Under autotrophic culture conditions, the growth of T. chuii attained an OD of 0.298±0.008 in 12 days, which was significantly lower than that observed under mixotrophic conditions (P < 0.05).

|
| Figure 3 Growth curve of T. chuii under mixotrophic culture conditions with different C:N ratios |
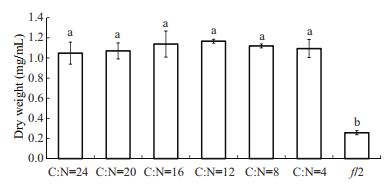
The biomass of T. chuii under mixotrophic conditions with different C:N ratios did show significant difference (Fig. 4). At the C:N ratio of 12, the biomass was as high as 1.17±0.02 mg/mL, which was almost 4-fold higher than that of T. chuii cultured under autotrophic conditions. The average biomass of T. chuii at ratios of 16, 8, 4, 20, and 24 was 1.138, 1.116, 1.093, 1.070, and 1.048 mg/mL, respectively, which was significantly higher than that obtained under autotrophic conditions (P < 0.05).
|
| Figure 4 Dry biomass weights of T. chuii clutured under mixotrophic conditions Different letters indicate significant differences (P < 0.05). |
Polysaccharides were present in the highest amounts in T. chuii under mixotrophic culture conditions followed by proteins and total lipids (Fig. 5). Chlorophyll a was present in the least amounts. At a C:N ratio of 4, polysaccharides accounted for 48.60% of the total weight, which was 2.5-fold of that obtained under the autotrophic culture conditions. The polysaccharide content at different C:N ratios was significantly higher than that obtained under autotrophic culture conditions (P < 0.05). With the increase in C:N ratio, the total lipid content increased continuously while the protein content decreased steadily. Such trend varied significantly at different C:N ratios (P < 0.05). The total lipid content at C:N ratio of 24 was the highest in percentage (13.30%), which was 2-fold higher than that obtained under autotrophic culture conditions. However, the protein content of T. chuii under mixotrophic culture conditions was lower than that of the control (P < 0.05). When C:N ratio was 4, the protein content reached the maximum (19.16%).

|
| Figure 5 Concentrations of lipid, protein, polysaccharide, and chlorophyll a in T. chuii under mixotrophic culture conditions Different letters in the same group (for example, lipid) indicate a significant difference (P < 0.05). |
Under mixotrophic conditions (C:N ratio between 4 and 20), T. chuii produced many new fatty acids (C16:3, C18:3, and C20:0), which were not synthesized under the autotrophic conditions. Of all fatty acids, C16:0 and C18:1 fatty acids accounted for a comparatively higher percentage, and their highest proportions (30.08% and 24.65%, respectively) were observed at the C:N ratio of 16 (Table 1). Small amount of EPA was found under mixotrophic and autotrophic culture conditions; however, its content under mixotrophic conditions was higher than that under autotrophic conditions (P < 0.05).

|
In the present study, T. chuii isolated and purified from south Yellow Sea was found to be capable of growing quickly and synthesizing more nutritional components under mixotrophic culture conditions. 18S rRNA gene, having a highly conserved sequence, has frequently been used in molecular phylogenetic analysis of microalgae (Luo et al., 2006). rbcL, a conserved chloroplast gene, does not contain introns and has also been used for algal identification in recent years (Nozaki et al., 1997; Wan et al., 2011). The purified algal strain had morphological characteristics similar to Tetraselmis. Phylogenetic trees of 18S rRNA and rbcL genes confirmed that the alga was T. chuii.
T. chuii showed obviously distinct morphological characteristics under autotrophic and mixotrophic culture conditions. The cells were rounded and almost all of them were at the splicing state under the autotrophic culture conditions. Moreover, the growth curve of T. chuii plateaued in 12 days under the autotrophic culture conditions while T. chuii cultured under mixotrophic conditions continued to grow until 30 days and attained an OD, 3.7-fold higher than that obtained under autotrophic conditions, indicating that T. chuii grew efficiently under mixotrophic culture conditions. Other commonly used bait microalgae such as Chlorella sp. and Phaeodactylum tricornutum can also grow faster under mixotrophic culture conditions using glucose as the carbon source than under autotrophic culture conditions (Cerón et al., 2006; Liang et al., 2009). Previously, large-scale heterotrophic cultivation of Tetraselmis was attempted but was finally ceased due to inhibitory costs arising from the use of organic carbon sources (Gladue, 1991; Laing and Verdugo, 1991). Subsequently, researchers determined that glucose enzymatically hydrolyzed from raw starch could be used for microalgal culture (Wu et al., 1994; Miao and Wu, 2006). The glucose from such sources reduced the production cost of culturing T. chuii.
The dry weight of T. chuii biomass under mixotrophic culture conditions was significantly higher than that under autotrophic conditions, but varied with the different C:N ratios used. When the C:N ratio was 12, the biomass of mixotrophically cultured T. chuii was 5-fold higher than that obtained under autotrophic culture conditions. The low biomass in autotrophic group maybe caused by carbon limitation as no CO2 was added into the medium. Most of the previous studies focused on the influence of C:N ratio on the total lipid content and fatty acid composition (Jiang et al., 2012; Sathish et al., 2015). In the present study, the use of different C:N ratios was evaluated for increasing the productivity of microalgae. Theoretically, adequate nutrition in the medium maintains microalgae in a good growth state, but the abundant carbon or nitrogen does not guarantee an increased growth rate. In the present study, T. chuii cultured in a medium with C:N ratio of 16 grew at the highest rate while at a ratio of 12, the culture accumulated the highest biomass. However, the biomass decreased at other C:N ratios, which was in conformity to the results obtained in Tetraselmis suecica, as reported in a previous study (Azma et al., 2011). Thus, Tetraselmis may be suitably cultured under mixotrophic conditions within a narrow range of low nitrogen concentrations. Microalgae cultured under mixotrophic conditions can use organic compounds and their growth is not strictly dependent on photosynthesis, which makes mixotrophic cultures independent of light intensity (Andrate and Costa, 2007; Fernández et al., 2013). Thus, mixotrophic cultivation of microalgae can increase its growth rate, shorten the culture time, and increase the biological productivity (Andrate and Costa, 2007; Park et al., 2012). The mixotrophic cultivation of T. chuii could provide substantial nutrition for aquaculture.
This study further analyzed the nutritional components, including proteins, polysaccharides, and fatty acids in T. chuii cultured under mixotrophic conditions at different C:N ratios. Microalgae typically contain 30%-40% protein, 10%-20% lipid, and 5%-15% polysaccharide (Fujii et al., 2010). In the present study, polysaccharides accounted for the largest proportion of the total weight of T. chuii, especially at a C:N ratio of 4, its percentage was as high as 48.60%, which was 2.5-fold higher than that obtained under the autotrophic conditions. Polysaccharides play an important role in the growth and development of aquatic animals. Bait with higher polysaccharide content could significantly promote the growth of turbot (Psetta maxima) and European flat oyster (Ostrea edulis) larvae (Mahious et al., 2006; Ponis et al., 2006). Thus, high polysaccharide content of mixotrophically cultured T. chuii made it an optional bait microalga. Moreover, T. chuii cultured under mixotrophic conditions at C:N ratios between 4 and 20 could produce some fatty acids that were absent under autotrophic cultivation; these fatty acids included C16:3, C18:3, C20:0, and C20:1 among others. When the C:N ratio was 16, the proportion of C16:0 and C18:1 was as high as 30.08% and 24.65%, respectively. These two fatty acids were reported to help Atlantic salmon (Salmo salar) in assimilating astaxanthin (Olsen et al., 2005). Moreover, T. chuii cultured under mixotrophic conditions produced more EPA, an essential fatty acid for many fish and shrimp larvae, than under autotrophic conditions. The diets supplemented with EPA could increase the growth rate and survival of aquatic animals (Floreto et al., 1996) while its lack seriously affected the growth of aquatic animals (Ruyter et al., 2000; Glencross, 2009). In the present study, T. chuii produced the highest percentage of EPA at a C:N ratio of 24, which was almost 1.8-fold higher than the production under autotrophic cultivation. However, the protein content was decreased under mixotrophic culture conditions, and the proportion of protein decreased with the increase of C:N ratio. Organic compounds added to medium could aid the production of more polysaccharides and lipids, but hindered the protein synthesis in Chlorella vulgaris (Kong et al., 2013). Eriksen (2008) considered that the initial C:N ratio affected the biochemical components by regulating the switchingon of protein, polysaccharide, and lipid synthesis. Under the optimal cultivation conditions, the protein synthesized was sufficient to sustain the growth of microalgae, and the polysaccharides and fatty acids were maintained at a relatively low level. The medium with C:N ratios higher than 10 was recognized as nitrogen deficient (Hillebrand and Sommer, 1999), and the metabolic processes in the cells cultured in such medium would change from protein synthesis to polysaccharide storage (Thomas et al., 1983, 1984). For example, nitrogen deficiency decreased the protein percentage from 46% to 10% and increased the polysaccharide proportion from 15% to 50% (Bondioli et al., 2012). In the present study, a similar phenomenon was observed when C:N ratio was increased from 12 to 24. The protein content was reduced and the polysaccharide content was increased from 29.32% to 48.14%. The content of fatty acids, proteins, and polysaccharides varied greatly in different microalgae (Brown, 1991). Chen and Johns (1991) reported that C:N ratio of 20 was the turning point for carbon and nitrogen restrictions. The proportion of total fatty acids was lowest at this ratio, but it increased at any other C:N ratios. In the present study, the total lipid content increased and the protein content continued to decline when the C:N ratio was increased from 4 to 24, but no clear inflection point was observed. However, the polysaccharide content was lowest at a C:N ratio of 12. Presumably, a C:N ratio of 12 may be a turning point for polysaccharide synthesis.
5 CONCLUSIONT. chuii could grow efficiently and accumulate nutrients under mixotrophic culture conditions. The nutritional components were modifiable by changing the C:N ratio, and might be sufficient to meet the different nutritional needs of the aquatic animals. Our findings are expected not only to enrich the microalgal resource, but also illustrate the possibility of mass (large-scale) mixotrophic culture of T. chuii.
6 ACKNOWLEDGEMENTThe authors thank our laboratory members for their helpful advice.
| Alcántara C, Fernández C, García-Encina P A, Muñoz R, 2015. Mixotrophic metabolism of Chlorella sorokiniana and algal-bacterial consortia under extended dark-light periods and nutrient starvation. Applied Microbiology and Biotechnology, 99(5): 2 393–2 404. Doi: 10.1007/s00253-014-6125-5 |
| Alkhamis Y, Qin J G, 2016. Comparison of pigment and proximate compositions of Tisochrysis lutea in phototrophic and mixotrophic cultures. Journal of Applied Phycology, 28(1): 35–42. Doi: 10.1007/s10811-015-0599-0 |
| Andrade M R, Costa J A V, 2007. Mixotrophic cultivation of microalga Spirulina platensis using molasses as organic substrate. Aquaculture, 264(1-4): 130–134. Doi: 10.1016/j.aquaculture.2006.11.021 |
| Arnon D I, 1949. Copper enzymes in isolated chloroplasts.Polyphenoloxidase in Beta vulgaris. Plant Physiology, 24(1): 1–15. Doi: 10.1104/pp.24.1.1 |
| Austin B, Baudet E, Stobie M, 1992. Inhibition of bacterial fish pathogens by Tetraselmis suecica. Journal of Fish Diseases, 15(1): 55–61. Doi: 10.1111/jfd.1992.15.issue-1 |
| Azma M, Mohamed M S, Mohamad R, Rahim R A, Ariff A B, 2011. Improvement of medium composition for heterotrophic cultivation of green microalgae, Tetraselmis suecica, using response surface methodology. Biochemical Engineering Journal, 53(2): 187–195. Doi: 10.1016/j.bej.2010.10.010 |
| Becker E W. 2013. Microalgae for aquaculture:nutritional aspects. In:Richmond A, Hu Q eds. Handbook of Microalgal Culture:Applied Phycology and Biotechnology. 2nd edn. John Wiley & Sons, Ltd, New York. p.671-691. |
| Benemann J R, 1992. Microalgae aquaculture feeds. Journal of Applied Phycology, 4(3): 233–245. Doi: 10.1007/BF02161209 |
| Bhatnagar A, Chinnasamy S, Singh M, Das K C, 2011. Renewable biomass production by mixotrophic algae in the presence of various carbon sources and wastewaters. Applied Energy, 88(10): 3 425–3 431. |
| Bligh E G, Dyer W J, 1959. A rapid method of total lipid extraction and purification. Canadian Journal of Biochemistry and Physiology, 37(8): 911–917. Doi: 10.1139/o59-099 |
| Bondioli P, Bella L D, Rivolta G, Zittelli G C, Bassi N, Rodolfi L, Casini D, Prussi M, Chiaramonti D, Tredici M R, 2012. Oil production by the marine microalgae Nannochloropsis sp. F & M-M24 and Tetraselmis suecica F & M-M33. Bioresource Technology, 114: 567–572. Doi: 10.1016/j.biortech.2012.02.123 |
| Bradford M M, 1976. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Analytical Biochemistry, 72(1-2): 248–254. Doi: 10.1016/0003-2697(76)90527-3 |
| Brown M R, 1991. The amino-acid and sugar composition of 16 species of microalgae used in mariculture. Journal of Experimental Marine Biology and Ecology, 145(1): 79–99. Doi: 10.1016/0022-0981(91)90007-J |
| Cerón G M C, Camacho F G, Mirón A S, Sevilla J M F, Chisti Y, Grima E M, 2006. Mixotrophic production of marine microalga Phaeodactylum tricornutum on various carbon sources. Journal of Microbiology and Biotechnology, 16(5): 689–694. |
| Chaplin M F, Kennedy J F. 1994. Carbohydrate A nalysis:A Practical Approach. 2nd edn. Oxford University Press, Oxford. |
| Chen F, Johns M R, 1991. Effect of C/N ratio and aeration on the fatty acid composition of heterotrophic Chlorella sorokiniana. Journal of Applied Phycology, 3(3): 203–209. Doi: 10.1007/BF00003578 |
| Chen F, Zhang Y M, 1997. High cell density mixotrophic culture of Spirulina platensis on glucose for phycocyanin production using a fed-batch system. Enzyme and Microbial Technology, 20(3): 221–224. Doi: 10.1016/S0141-0229(96)00116-0 |
| Eriksen N T, 2008. The technology of microalgal culturing. Biotechnology Letters, 30(9): 1 525–1 536. Doi: 10.1007/s10529-008-9740-3 |
| Fernández F G A, Sevilla J M F, Grima E M, 2013. Photobioreactors for the production of microalgae. Reviews in Environmental Science and Bio/Technology, 12(2): 131–151. Doi: 10.1007/s11157-012-9307-6 |
| Floreto E A T, Teshima S, Ishikawa M, 1996. The effects of seaweed diets on the growth, lipid and fatty acids of juveniles of the White Sea Urchin Tripneustes gratilla. Fisheries Science, 62(4): 589–593. |
| Fujii K, Nakashima H, Hashidzume Y, Uchiyama T, Mishiro K, Kadota Y, 2010. Potential use of the astaxanthinproducing microalga, Monoraphidium sp.GK12, as a functional aquafeed for prawns. Journal of Applied Phycology, 22(3): 363–369. Doi: 10.1007/s10811-009-9468-z |
| Garofalo R. 2009. Algae and aquatic biomass for a sustainable production of 2nd generation biofuels. FP7-ENERGY-2009-1. AquaFUELs-Taxonomy, Biology and Biotechnology. p.1-258. |
| Gladue R M. 1991. Heterotrophic microalgae production:potential for application to aquaculture feeds. In:Fulks W, Main K L eds. Rotifer and Microalgae Culture Systems.Proceedings of a US-Asia Workshop, HI. p.275-286. |
| Glencross B D, 2009. Exploring the nutritional demand for essential fatty acids by aquaculture species. Reviews in Aquaculture, 1(2): 71–124. Doi: 10.1111/raq.2009.1.issue-2 |
| Guillard R R L, Ryther J H, 1962. Studies of marine planktonic diatoms.I. Cyclotella nana Hustedt, and Detonula confervacea (cleve) gran. Canadian Journal of Microbiology, 8(2): 229–239. Doi: 10.1139/m62-029 |
| Guillard R R. 2005. Purification methods for microalgae. In:Andersen R A ed. Algal Culturing Techniques. Elsevier, New York. p.117-132. |
| Hassan M, Blanc P J, Granger L M, Pareilleux A, Goma G, 1996. Influence of nitrogen and iron limitations on lipid production by Cryptococcus curvatus grown in batch and fed-batch culture. Process Biochemistry, 31(4): 355–361. Doi: 10.1016/0032-9592(95)00077-1 |
| Heifetz P B, Förster B, Osmond C B, Giles L J, Boynton J E, 2000. Effects of acetate on facultative autotrophy in Chlamydomonas reinhardtii assessed by photosynthetic measurements and stable isotope analyses. Plant Physiology, 122(4): 1 439–1 446. Doi: 10.1104/pp.122.4.1439 |
| Hemaiswarya S, Raja R, Kumar R R, Ganesan V, Anbazhagan C, 2011. Microalgae:a sustainable feed source for aquaculture. World Journal of Microbiology and Biotechnology, 27(8): 1 737–1 746. Doi: 10.1007/s11274-010-0632-z |
| Hillebrand H, Sommer U, 1999. The nutrient stoichiometry of benthic microalgal growth:redfield proportions are optimal. Limnology and Oceanography, 44(2): 440–446. Doi: 10.4319/lo.1999.44.2.0440 |
| Irianto A, Austin B, 2002. Probiotics in aquaculture. Journal of Fish Diseases, 25(11): 633–642. Doi: 10.1046/j.1365-2761.2002.00422.x |
| Jiang Y L, Yoshida T, Quigg A, 2012. Photosynthetic performance, lipid production and biomass composition in response to nitrogen limitation in marine microalgae. Plant Physiology and Biochemistry, 54: 70–77. |
| Jiang Y, Chen F, 2000. Effects of temperature and temperature shift on docosahexaenoic acid production by the marine microalge Crypthecodinium cohnii. Journal of the American Oil Chemists' Society, 77(6): 613–617. Doi: 10.1007/s11746-000-0099-0 |
| Kong W B, Yang H, Cao Y T, Song H, Hua S F, Xia C G, 2013. Effect of glycerol and glucose on the enhancement of biomass, lipid and soluble carbohydrate production by Chlorella vulgaris in mixotrophic culture. Food Technology and Biotechnology, 51(1): 62–69. |
| Laing I, Verdugo C G, 1991. Nutritional value of spray-dried Tetraselmis suecica for juvenile bivalves. Aquaculture, 92: 207–218. Doi: 10.1016/0044-8486(91)90022-Y |
| Lepage G, Roy C C, 1984. Improved recovery of fatty acid through direct transesterification without prior extraction or purification. Journal of Lipid Research, 25(12): 1 391–1 396. |
| Liang Y N, Sarkany N, Cui Y, 2009. Biomass and lipid productivities of Chlorella vulgaris under autotrophic, heterotrophic and mixotrophic growth conditions. Biotechnology Letters, 31(7): 1 043–1 049. Doi: 10.1007/s10529-009-9975-7 |
| Liu J G, Yin M Y, Zhang J P, Li B Q, Meng Z C, 2007. Application of Nannochloropsis salina as feedstuff in aquaculture. Marine Sciences, 31(5): 4–9. |
| Luo L M, Hu H J, Li Y G, Qi Y Z, Lv S H, Geng Y H, Deng G, 2006. Molecular identification on Prorocentrum donghaiense. Acta Oceanologica Sinica, 28(1): 127–131. |
| Mahious A S, Gatesoupe F J, Hervi M, Metailler R, Ollevier F, 2006. Effect of dietary inulin and oligosaccharides as prebiotics for weaning turbot, Psetta maxima (Linnaeus, C.1758). Aquaculture International, 14(3): 219–229. Doi: 10.1007/s10499-005-9003-4 |
| Miao X L, Wu Q Y, 2006. Biodiesel production from heterotrophic microalgal oil. Bioresource Technology, 97(6): 841–846. Doi: 10.1016/j.biortech.2005.04.008 |
| Muller-Feuga A, Robert R, Cahu C, Robin J, Divanach P. 2003. Uses of microalgae in aquaculture. In:Støttrup J G, McEvoy L A eds. Live Feeds in Marine Aquaculture.Blackwell Science Ltd, Oxford. p.253-299. |
| Murray M G, Thompson W F, 1980. Rapid isolation of high molecular weight plant DNA. Nucleic Acids Research, 8(19): 4 321–4 326. Doi: 10.1093/nar/8.19.4321 |
| Nozaki H, Ito M, Watanabe M M, Takano H, Kuroiwa T, 1997. Phylogenetic analysis of morphological species of Carteria (Volvocales, Chlorophyta) based on rbcL gene sequences. Journal of Phycology, 33(5): 864–867. Doi: 10.1111/j.0022-3646.1997.00864.x |
| Olsen R E, Kiessling A, Milley J E, Ross N W, Lall S P, 2005. Effect of lipid source and bile salts in diet of Atlantic salmon, Salmo salar L., on astaxanthin blood levels. Aquaculture, 250(3-4): 804–812. Doi: 10.1016/j.aquaculture.2005.03.013 |
| Park K C, Whitney C, McNichol J C, Dickinson K E, MacQuarrie S, Skrupski B P, Zou J T, Wilson K E, O'Leary S J B, McGinn P J, 2012. Mixotrophic and photoautotrophic cultivation of 14 microalgae isolates from Saskatchewan, Canada:potential applications for wastewater remediation for biofuel production. Journal of Applied Phycology, 24(3): 339–348. Doi: 10.1007/s10811-011-9772-2 |
| Perez-Garcia O, Escalante F M E, de-Bashan L E, Bashan Y, 2011. Heterotrophic cultures of microalgae:metabolism and potential products. Water Research, 45(1): 11–36. Doi: 10.1016/j.watres.2010.08.037 |
| Ponis E, Probert I, Véron B, Mathieu M, Robert R, 2006. New microalgae for the Pacific oyster Crassostrea gigas larvae. Aquaculture, 253(1-4): 618–627. Doi: 10.1016/j.aquaculture.2005.09.011 |
| Ruyter B, Røsjø C, Einen O, Thomassen M S, 2000. Essential fatty acids in Atlantic salmon:time course of changes in fatty acid composition of liver, blood and carcass induced by a diet deficient in n-3 and n-6 fatty acids. Aquaculture Nutrition, 6(2): 109–117. Doi: 10.1046/j.1365-2095.2000.00136.x |
| Sathish A, Marlar T, Sims R C, 2015. Optimization of a wet microalgal lipid extraction procedure for improved lipid recovery for biofuel and bioproduct production. Bioresource Technology, 193: 15–24. Doi: 10.1016/j.biortech.2015.06.052 |
| Sun Y M, Shi Y, Hao Y Z. 2000. Technical manual for cultivation of aquatic organism. Agriculture Press, Beijing, China. p.1-17. (in Chinese) |
| Thomas W H, Seibert D L R, Alden M, Eldridge P, Neori A. 1983. Yields, photosynthetic efficiencies, and proximate chemical composition of dense cultures of marine microalgae. Technical Report. Scripps Institution of Oceanography, San Diego, CA. |
| Thomas W H, Seibert D L R, Alden M, Neori A, Eldridge P, 1984. Yields, photosynthetic efficiencies and proximate composition of dense marine microalgal cultures.I.Introduction and Phaeodactylum tricornutum experiments. Biomass, 5(3): 181–209. Doi: 10.1016/0144-4565(84)90022-2 |
| Tredici M R, Biondi N, Ponis E, Rodolfi L, Zittelli G C. 2009.Advances in microalgal culture for aquaculture feed and other uses. In:Burnell G, Allan G eds. New Technologies in Aquaculture:Improving Production Efficiency, Quality and Environmental Management. Woodhead Publishing, Cambridge, UK. p.610-676. |
| Wan M X, Rosenberg J N, Faruq J, Betenbaugh M J, Xia J L, 2011. An improved colony PCR procedure for genetic screening of Chlorella and related microalgae. Biotechnology Letters, 33(8): 1 615–1 619. Doi: 10.1007/s10529-011-0596-6 |
| Wu Q Y, Yin S, Sheng G Y, Fu J M, 1994. New discoveries in study on hydrocarbons from thermal degradation of heterotrophically yellowing algae. Science in China, Series B, Chemistry, Life Sciences and Earth Sciences, 37(3): 326–335. |
| Zhu C J, Lee Y K, 1997. Determination of biomass dry weight of marine microalgae. Journal of Applied Phycology, 9(2): 189–194. Doi: 10.1023/A:1007914806640 |
 2017, Vol. 35
2017, Vol. 35


