Institute of Oceanology, Chinese Academy of Sciences
Article Information
- DING Yi(丁奕), GAN Nanqin(甘南琴), LIU Jin(刘津), ZHENG Lingling(郑凌凌), LI Lin(李林), SONG Lirong(宋立荣)
- Survival, recovery and microcystin release of Microcystis aeruginosa in cold or dark condition
- Chinese Journal of Oceanology and Limnology, 35(2): 313-323
- http://dx.doi.org/
Article History
- Received Aug. 28, 2015
- accepted in principle Nov. 10, 2015
- accepted for publication Feb. 14, 2016
2 Key Laboratory of Plant Germplasm Enhancement and Speciality Agriculture, Wuhan Botanical Garden, Chinese Academy of Sciences, Wuhan 430074, China
Microcystis is one of the most common cyanobacterial species and causes water blooms in eutrophic lakes, ponds, and reservoirs all over the world (Paerl and Otten, 2013). Some strains of Microcystis produce cyanobacterial hepatotoxins called microcystins, which are a threat to human and environmental health (Babica et al., 2006). Microcystis species are recruited from the water bottom as unicellular entities or very small colonies in early spring, grow rapidly in the water column at the end of spring, develop blooms at the water surface during the summer, sink to the bottom sediment in autumn, and overwinter in a resting state on the sediment surface (Reynolds et al., 1981). Because they are present throughout the year indicates that Microcystis are often exposed to fluctuating environmental conditions. For instance, Microcystis have been found to undergo optimal growth and photosynthesis at 25℃ or above (Paerl and Huisman, 2009). However, the average water temperature does not reach the optimal temperature for Microcystis growth in Taihu Lake during the period of autumn and winter (Zhang et al., 2012b). In such circumstances, Microcystis are often stressed by low temperature and relatively weak illumination (Wu et al., 2008). Recent studies illustrated that cyanobacteria blooms can sustain in late autumn and winter in subtropical lakes, such as Taihu (Zhang et al., 2012a; Duan et al., 2014). Moreover, darkness may also be encountered in eutrophic lakes beneath thick surface scums formed by Microcystis themselves in summer (Bouchard and Purdie, 2011). Microcystis often accumulate during and after bloom formation, where little if any light penetrates (Jochem, 1999). Hence, it is important to investigate the acclimation strategies of Microcystis to low temperature or darkness.
Previous studies have been conducted to assess the responses of some phytoplankton to low temperature and darkness, but contradictory results were obtained. Oscillatoria was tolerant of relatively low temperatures (Jewson, 1976). Synechocystis sp. PCC 6803 had a strong tolerance to 4℃ in darkness, but if exposed to light at this temperature, cells completely lost viability within 10 days (Yin et al., 2007). Segovia et al. (2003) found that unicellular Dunaliella tertiolecta cells literally dissolved, and the culture turned from green to clear, within 8 days in darkness. Furusato et al. (2004) reported that Microcystis aeruginosa has a low tolerance to darkness. However, Wu et al. (2008) found that colonial Microcystis had greater endurance and adaptation ability to the stress of the combination of cold and dark condition than the Scenedesmus. Furthermore, programmed cell death (PCD), an irreversible and genetically controlled form of cell death, in cyanobacteria has been reported (Bidle, 2015). In recent years, cell death similar to PCD has been proposed to exist in M.aeruginosa induced by darkness or elevated temperatures (Bouchard and Purdie, 2011). Although many studies have focused on the effects of low temperature and darkness on phytoplankton (Davisa et al., 2009; Sabour et al., 2009; Le Blanc Renaud et al., 2011), the strategies by which the organisms withstand these unfavorable environmental factors are less well known. Successful adaptation to unfavorable environmental conditions is vital to the growth and survival of Microcystis (Dokulil and Teubner, 2000). When the adverse effects of environmental conditions exceed the tolerance limit of Microcystis, cells might die and disintegrate. The resulting sudden release of microcystin into the surrounding water may deteriorate water quality (White et al., 2005; Sakai et al., 2007). Recent experimental data indicated that microcystin plays an important role in microcystinproducing cyanobacteria under oxidative stress conditions (Dziallas and Grossart, 2011). For instance, Zilliges et al. (2011) found that under oxidative stress microcystin-producing Microcystis have a comparative advantage as microcystin acts as a protein-modulating metabolite and protectant, increasing the fitness of Microcystis. The experimental results of Ding et al. (2013) and Yang et al. (2015) are also supported that not only the mere presence of microcystin, but also its production is important for microcystin-mediated protection. Different physiological acclimation strategies to low temperature or darkness might contribute to the survival and the dominance of Microcystis in freshwaters where water bloom occurs annually. To test the hypothesis, we collected two toxic Microcystis aeruginosa strains, FACHB-905 and FACHB-915, and subjected them to low temperature (15℃, 4℃) or prolonged darkness to elucidate how the M.aeruginosa cells survived and recovered, and any associated microcystin release.
2 MATERIAL AND METHOD 2.1 Strains and growth conditionsTwo microcystin-producing strains M.aeruginosa FACHB-905 and FACHB-915, which are dominant species in cyanobacteria, were obtained from the Culture Collections of Freshwater Algae of the Institute of Hydrobiology (Wuhan, China). The two strains were grown in BG-11 medium at 25℃ under illumination with 20-25 μmol photons/(m2∙s) (LICOR, LI-185B, USA) with a 12 h: 12 h light:dark cycle. Laboratory cultures grown for 7-12 days were collected by centrifugation and washed three times with sterilized BG-11 medium and then the pellets were inoculated into 250 mL flasks. The inoculation concentration was about 8.5×106 cells/mL. The four culture treatments tested were as follows: (ⅰ) control conditions with cells incubated at 25℃ under illumination with 20-25 μmol photons/(m2∙s), (ⅱ) cells incubated at 15℃ with illumination at 20-25 μmol photons/(m2∙s) (12 h light: 12 h dark), (ⅲ) cells incubated at 4℃ with illumination at 20-25 μmol photons/(m2∙s) (12 h light: 12 h dark), (ⅳ) cells under darkness at 25℃. After 30 days, cultures were returned to the standard growth conditions (25℃, 20-25 μmol photons/(m2∙s) with a 12 h: 12 h light:dark cycle) to resume growth. Cells in the control treatment were maintained throughout the study period at standard conditions.
2.2 Measurements of growth and photosynthetic efficiencyGrowth in the cultures was measured by cell number and chlorophyll a and carotenoid pigment concentration. Cell number was determined with a hemocytometer using light microscopy (Magnification×400, Olympus CX41, Japan). Chlorophyll a and carotenoid were measured spectrophotometrically according to the method described by Richards and Thompson (1952) and Sta et al. (2012). After extraction of cells with 80% acetone at 4℃ in dark for 24 h, optical density was measured at 450, 645 and 663 nm and chlorophyll a and carotenoid content were determined according to the following equations:
Chl a (mg/L)=12.72OD663-2.7OD645=12.19OD663,
Carotenoids (mg/L)=4.1OD450-0.0435Chl a.
The photosynthetic efficiency of M.aeruginosa FACHB-905 and FACHB-915 were measured with a PHYTO-PAM fluorometer (Walz, Germany) (Ding et al., 2012). The value of the maximum effective quantum yield of photosystem Ⅱ (Fv/Fm), the maximum electron transport rate (ETRmax), and the saturating irradiance (Ik), were recorded. Each measurement was performed after dark adaptation for 15 min.
2.3 Cell viability assay using MTTThe viability of M.aeruginosa FACHB-905 and FACHB-915 cells after treatment was detected using the methylthiazolyldiphenyl-tetrazolium bromide (MTT) method. The MTT method is based on the ability of viable cells to reduce MTT to formazan and is a sensitive, simple, and reliable technique to assess temporal cell viability. The cell viability was measured by MTT method described by Ding et al. (2012). The harvested cells were washed and resuspended in 250-μL BG-11 medium before staining. The 250 μL samples were combined with 100-μL MTT (3-(4, 5-dimethylthiazol-2-yl)-2, 5-diphenyltetrazolium bromide; Sigma, USA) stock solution and incubated at 35±1℃ for 1.5 h. After staining, the MTT dye was removed by centrifugation at 8 000 r/min for 3 min. Then the cell pellets were resuspended in 250-μL distilled water and 8 μL of each suspension was examined with a hemocytometer using light microscopy (Olympus CX41, Japan). At least 300 cells were observed in each measurement.
2.4 Measurement of caspase-3-like activityThe caspase-3-like activity of M.aeruginosa FACHB-905 and FACHB-915 cells after treatments was determined by CaspGlow Fluorescein Active Caspase-3 Staining Kit (BioVision, USA) in accordance with Ding et al. (2012). The assay utilizes the caspase-3 inhibitor, DEVD-FMK, conjugated to fluorescein isothiocyanate (FITC) as a marker. FITCDEVD-FMK is cell permeable, non-toxic and irreversibly binds to the active enzyme. After labeling, cells were washed twice in buffer and resuspend in 100 μL wash buffer. Then the cell suspension was transferred to each well in the black microtiter plate. The appearance of fluorescent was assayed using a fluorescence microplate reader (Excitation: 485 nm, Emission: 535 nm). The M.aeruginosa cells under standard growth conditions without treatment were used as the control. Protein content of M.aeruginosa cells was estimated by the Bradford method (Bradford, 1976). The caspase-3-like activity was expressed as the percentage enzyme activity compared to the control group.
2.5 Terminal deoxynucleotidyl transferase labeling (TUNEL) assayTerminal deoxynucleotidyl transferase labelling (TUNEL) assays preferentially labels DNA strand breaks generated during apoptosis by Terminal deoxynucleotidyl transferase (TdT). This TUNEL assay was assessed using the In Situ Cell Death Detection Kit, Fluorescein (Roche Diagnostics, Cat. No.11 684 795 910, Mannheim, Germany). The detailed procedure was conducted as our previous description in Ding et al. (2013). The M.aeruginosa cells under standard condition, low temperature or darkness were harvested after 30 days incubation and were fixed at room temperature for 1.5 h in PBS (0.1 mol/L, pH 7.4, freshly prepared) supplemented with 2% paraformaldehyde. Then the mixture was washed once with PBS and permeabilized for 20 min at 4℃ in a solution containing 0.1% Triton X-100 and 0.1% sodium citrate. Subsequently cells were washed twice with PBS and labeled with TUNEL reaction mixture following the manufacturers’ instructions. The M.aeruginosa cells without the addition of terminal deoxynucleotidyl transferase enzyme were used as negative controls. The M.aeruginosa cells pretreated with DNase I (Invitrogen, Cat. No.18047-019) for 20 min prior to labeling were used as positive controls. Finally, samples were resuspended in PBS, and the green fluorescence was observed under a fluorescence microscope (Olympus BX51, Japan) using an excitation wavelength in the range 450-500 nm. The results were displayed using Image-Pro Express 6.0 software. Representative images were taken after analysis of at least 500 cells per sample.
2.6 Toxin analysisMicrocystin is a family of monocyclic heptapetides. Microcystin release was measured during the experimental period. M.aeruginosa control cells and cells after treatments were centrifuged at 7 000 r/min for 3 min. Supernatants were used for the determination of extracellular microcystin concentrations with an Enzyme linked immunosorbent assay (ELISA) kit (Institute of Hydrobiology, Chinese Academy of Sciences). ELISA is sensitive and simple method for the detection of microcystin (Dai et al., 2012). The ELISA test was carried out according to Lei’s method and this kit has a detection limit of 0.1 ng/mL (Lei et al., 2004).
2.7 Analysis of dataAll experiments were performed in triplicate. Data in the study were presented as means±standard deviations (SD) and analyzed using Microcal Origin Software (Version 8.0, Microcal Software Inc. Northampton, MA, USA). Differences between treatments were tested by one-way ANOVA followed by a Tukey’s test. Differences were considered to be significant when P < 0.05.
3 RESULT 3.1 Growth and photosynthetic efficiencyThe growth patterns of M.aeruginosa FACHB-905 and FACHB-915 cells exposed to low temperature or darkness were very similar (Fig. 1). The cell number of both strains increased gradually in control (standard) conditions. Cells started to grow after a time lag of 8 days at 15℃. However, the cell number of M.aeruginosa FACHB-905 cells exposed to 4℃ began to decline after 5 days of treatment, and decreased by 20% compared with initial value after the 29 days of treatment. The cell number of M. aeruginosa FACHB-915 exposed to darkness decreased by 60% after the 29-day darkness treatment. When M.aeruginosa FACHB-905 and FACHB-915 cells were then transferred from the low temperature or darkness conditions to grow in the standard conditions (25℃ with illumination), the cell numbers rose quickly. Recovery from 4℃ treatment was slower than from the other treatments (Fig. 1b). During the treatment period, the ratio of carotenoid content to chlorophyll a content of the cells exposed to 15℃ was higher than that in control cells and the other treatment groups (P < 0.05). When cells were transferred from low temperature or darkness to grow in standard conditions, the ratios of carotenoid to chlorophyll a content of the treatment groups became relatively stable and consistent (Fig. 2b).
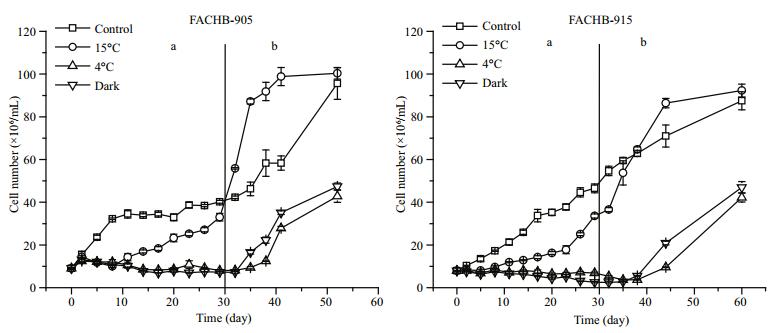
|
| Figure 1 Changes in cell numbers of M. aeruginosa FACHB-905 and FACHB-915 a. 30 days of low temperature or dark incubation; b. re-growth in standard conditions (25℃ with illumination) after 30 days of low temperature or darktreatment. |

|
| Figure 2 Ratio of carotenoid content to chlorophyll a content in M. aeruginosa FACHB-905 and FACHB-915 a. 30 days of low temperature or dark incubation; b. re-growth in standard conditions after 30 days of low temperature or dark-treatment. |
On 15℃ treatment, the Fv/Fm and ETRmax values of the cells first decreased and then began to increase. However, the photosynthetic efficiency of cells under 4℃ treatment decreased rapidly. The Fv/Fm and ETRmax values of cells in darkness also declined; the Fv/Fm value decreased to 0.06 and the ETRmax value to 8.83 in M.aeruginosa FACHB-905 after the 29-day dark treatment. The Ik values of 15℃ and dark-treated cells showed no significant change (P > 0.05)., but the Ik values of 4℃-treated cells decreased dramatically within 5 days. When these cells were transferred to grow in standard conditions, the Ik values rose quickly. During the recovery process, the change trends of Fv/Fm and ETRmax values were similar and the photosynthetic efficiency of M.aeruginosa cells could recover to the normal (control) level. The recovery rate of the dark-treated group was faster than that of the 4℃ treatment group.
3.2 MTT analysisAs Fig. 4 shows, the viability of control cells did not change significantly during incubation for the first 30 days. More than 60% of the cells treated at 15℃ were alive (MTT positive) after 29 days. The viability of cells treated at 4℃ or in darkness decreased gradually, and the rate of decline was fastest in cells under darkness for both M.aeruginosa FACHB-905 and FACHB-915. The proportions of MTT positive cells of M.aeruginosa FACHB-905 and FACHB-915 under darkness were only 5% and 19%, respectively, after 29 days. When cells were transferred from low temperature or darkness to grow in standard conditions (day 30), the viability of cells increased gradually, but there was a significant difference (P < 0.05) between the initial value (day 0) and final value after re-growth (day 60) for M.aeruginosa FACHB-905 cells treated at 4℃ (Fig. 4b).
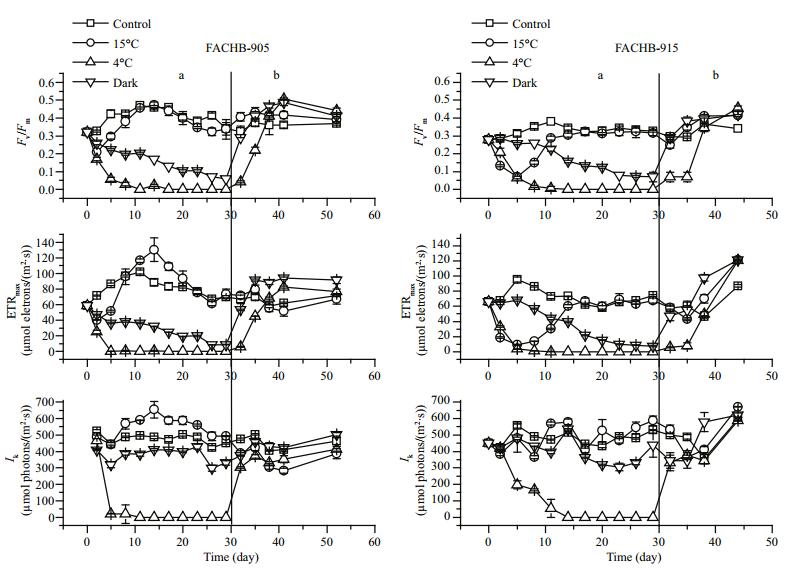
|
| Figure 3 Changes in photosynthetic efficiency (Fv/Fm, ETRmax, Ik) in M. aeruginosa FACHB-905 and FACHB-915 a. 30 days of low temperature or dark incubation; b. re-growth in standard conditions after 30 days of low temperature or dark-treatment. |
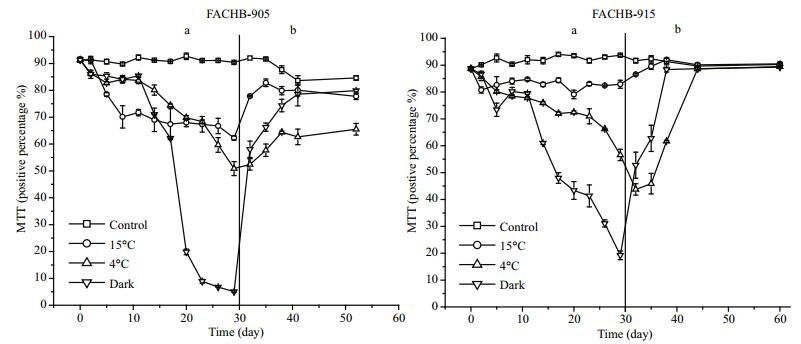
|
| Figure 4 Changes in percentage of MTT positive M. aeruginosa FACHB-905 and FACHB-915 cells a. 30 days of low temperature or dark incubation; b. re-growth in standard conditions after 30 days of low temperature or dark-treatment. |
Caspase-3-like activity was detected when M. aeruginosa FACHB-905 cells or FACHB-915 cells were incubated at low temperature or in darkness for 23 days (Fig. 5). Caspase-3-like activity of M.aeruginosa FACHB 905 and FACHB-915 cells treated at 4℃ showed a significant increase (P < 0.05), 2-fold and 3-fold, respectively, compared with control cells. Moreover, the DNA fragmentations in cells of M. aeruginosa occurred in the TUNEL assay (Fig. 6). In negative controls without the terminal deoxynucleotidyl transferase enzyme, no TUNELpositive (green fluorescence) cells were observed (Fig. 6a). In positive controls pretreated with DNase I, almost all cells were TUNEL-positive (green) (Fig. 6b). Among the experimental samples, the TUNEL-positive signal was found in cells incubated only at low-temperature or in darkness, while the control cells (standard growth conditions) showed no positive signal (Fig. 6c), apparently because of the absence of free DNA ends that allowed the incorporation of fluorescein labeled dUTP (Fig. 6). The highest percentage of DNA damaged cells was observed for M.aeruginosa FACHB 905 cells treated at 15℃, reaching almost 10% TUNEL-positive after 30 days (Fig. 6d). When cells were treated with 4℃ incubation and darkness (Fig. 6e, f), the percentage of TUNEL-positive cells after 30 days was lower than 5%.
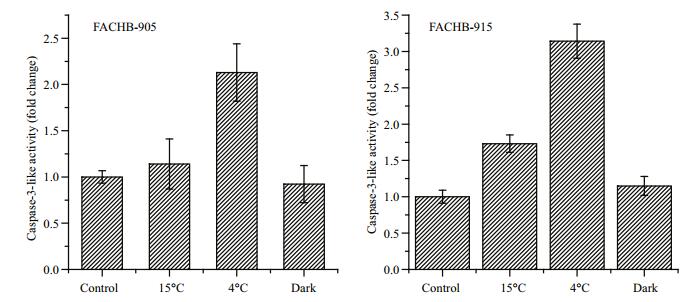
|
| Figure 5 Caspase-3-like activity in M. aeruginosa FACHB-905 and FACHB-915 cells after 23 days Control: cells incubated at 25℃ under illumination with 20-25 μmol photons/(m2∙s); 15℃: cells incubated at 15℃ with illumination at 20-25 μmol photons/ (m2∙s); 4℃: cells incubated at 4℃ with illumination at 20-25 μmol photons/(m2∙s); dark: cells under darkness at 25℃. |

|
| Figure 6 In situ detection of DNA fragmentation in M. aeruginosa FACHB-905 cells using the TUNEL assay after 30 days of incubation at low-temperature or in darkness TUNEL-positive cells=green; others=red; a. the negative control shows cells stained only with the label solution (without terminal transferase, red); b. the positive control shows TUNEL-stained cells (green) that were pretreated with DNase I; c. control cells (incubated at 25℃ with illumination); d. 15℃ incubation; e. 4℃ incubation; f. dark incubation. Representative images were taken after analysis of at least 300 cells per sample. Scale bars represent 5 μm in all panels. |
In the control samples, the extracellular microcystin concentrations in M.aeruginosa FACHB-905 and FACHB-915 cultures both increased during the incubation (Fig. 7). For the 4℃ and darkness treatments, the increases in extracellular microcystin concentration were much smaller than that of control group. However, the extracellular microcystin concentrations of cells grown at 15℃ were higher than those for control cells from Day 14 onwards (P < 0.05). The extracellular microcystin concentrations for M.aeruginosa FACHB-905 and FACHB-915 at 15℃ reached 332 and 93 ng/mL, respectively. When cells were transferred from low temperature or darkness to grow in the standard conditions, their extracellular microcystin concentrations increased (P < 0.05).

|
| Figure 7 Extracellular microcystin release from M. aeruginosa FACHB-905 and FACHB-915 a. 30 days of low temperature or dark incubation; b. re-growth in standard conditions after 30 days of low temperature or dark-treatment. |
In temperate and subtropical regions, cyanobacteria experience a decrease of temperature in autumn. A temperature of~15℃ is usually used for cold stress in the literature on cold acclimation in cyanobacteria. For Synechococcus sp. PCC 6301, cell growth stopped at 15℃ (Sakamoto and Bryant, 1999). Previous studies reported that preconditioning at 15℃ for 2 days greatly enhanced the chill-light tolerance (tolerance to chill while under illumination) of Synechocystis sp. strain PCC 6803 (Yang et al., 2008; Tan et al., 2011). A similar phenomenon was found for Microcystis sp. strain PCC 7806 (Yang et al., 2008). However, there is insufficient understanding of what happens when Microcystis are forced to exist at 15℃ for longer periods. In the present study, we found that M.aeruginosa FACHB-905 and FACHB-915 became chlorotic (unpublished data) after 30 days but were able to proliferate at 15℃ (Fig. 1). Photosynthetic parameters measured via PAM fluorescence showed that the Microcystis cells exposed to 15℃ were relatively healthy. Moreover, the relatively higher ratios of carotenoid/chlorophyll a indicated that the cells modulated their carotenoid content. Similarly, low temperatures have been found to increase the carotenoid content in diatoms (Anning et al., 2001). Hence, our results demonstrate that M.aeruginosa FACHB-905 and FACHB-915 grown at 15℃ are able to cope with this temperature and survive. Interestingly, conspicuously high extracellular microcystin production by M.aeruginosa FACHB-905 and FACHB-915 at 15℃ was observed after 14 days (Fig. 7), although the cell numbers were lower than in control samples (25℃). This remarkably high microcystin release may result from cell death because cell viability declined (Fig. 4). A reasonable explanation of the apparent link between high microcystin release and high resistance to 15℃ could lie in the possible advantages of microcystin for surviving cells. This result is coincided with previous report that microcystin release from dead cells may enhance the fitness of surviving cells, serving as an infochemical, and it has been suggested that toxins protect the cell against stressors (Schatz et al., 2007; Zilliges et al., 2011; Ding et al., 2013).
The combination of cold and light may have different effects on the cell activities. Yin et al. (2007) reported that the cyanobacterium Synechocystis sp. strain PCC 6803 was insensitive to chill in the dark but completely lost viability and the ability to reinitiate growth when exposed to chill (5℃) in the light (100 μmol photons/(m2∙s)). However, overwintering cyanobacteria are stressed by chill and relatively weak illumination, rather than excessive illumination. In our experimental conditions, we found that although transferring cultures of M.aeruginosa FACHB-905 and FACHB-915 from 25℃ (20-25 μmol photons/(m2∙s)) to 4℃ (20-25 μmol photons/ (m2∙s)) was adverse to the Microcystis cells, both strains were able to grow gradually when returned to 25℃ with the same illumination. The decline in Fv/Fm, ETRmax and Ik values observed at 4℃ (Fig. 3) reflect the harmful effect of low temperature on the structure of photosystem Ⅱ. We found the viability of cells (MTT positive) at 4℃ decreased gradually. This means that Microcystis cells could reduce their metabolic activity in order to cope with the stress of chill. On re-growth, the change trends of Fv/Fm and ETRmax values were similar to each other and the photosynthetic efficiency of M.aeruginosa cells could recover to the normal level. This suggests that the cells underwent a number of photosynthetic adjustments that allowed resumption of growth. The first decrease of photosynthetic efficiency under stress was perhaps indicative of “protective photoinhibition” occurring at a lower temperature, rather than permanent photodamage to PSⅡ (Allakhverdiev et al., 2008). These findings suggested that M.aeruginosa were only able to grow within a limited illumination range in winter. Microcystis may develop complex adaptive or acclimating responses to changes of temperature and light in winter.
Studies performed with phytoplankton kept in darkness have reported a range of different results. Zhang et al. (2011) showed that M.aeruginosa could retain its starting density for up to 8 days in the dark at 25℃. In contrast, Furusato et al. (2004) found that cell numbers of M.aeruginosa decreased markedly over 7 days in darkness. Darkness resulting in cell death of Amphidinium carterae and Dunaliella tertiolecta has also been reported (Berges and Falkowski, 1998). In our study, the cell number, Fv/Fm and ETRmax values of M.aeruginosa FACHB-915 exposed to darkness decreased. Moreover, the reducing proportion of MTT positive M.aeruginosa FACHB-905 and FACHB-915 cells under darkness indicated that most of the cells lost redox dehydrogenase activity. These results showed that long exposure to darkness resulted in a deteriorated physiological state of M.aeruginosa. On re-growth, the cell numbers were shown to rise quickly. Moreover, the viability of cells initially treated under darkness increased rapidly after re-growth. Similarly, Aureococcus anophagefferens could maintain its photosynthetic activity for two weeks in darkness, and was able to grow rapidly when put back into light (Popels et al., 2007).
Another important finding of this work is the occurrence of a type of cell death similar to PCD in M. aeruginosa FACHB-905 and FACHB-915 under cold stress. Indeed, PCD in M.aeruginosa was also detected when cells were exposed to oxidants (Ross et al., 2006; Mikula et al., 2012). Environmental factors that induce reactive oxygen species (ROS) formation are thought to lead to PCD (Guo et al., 2012; Moon et al., 2012; Franklin, 2014). In the present study, significant increases in caspase-3-like activity and DNA fragmentation of the cells were detected at low temperatures (Figs. 5, 6). These methods have already been adopted successfully to pursue apoptotic likePCD in Microcystis species (Ding et al., 2012, 2013). When FACHB-905 cells exposed to 4℃ were transferred to grow in the standard conditions, the viability increased but was still significantly lower than the initial value. This indicated that the some cells was totally dead and would not have been able to survive. A small fraction of cells died, leaving the rest of the population in a dormant state. Among the hypotheses to explain the drivers of PCD, one can speculate that PCD is an altruistic adaptation designed to benefit a population (Bidle and Falkowski, 2004). PCD is a process that is triggered in cells unable to repair inflicted damage. The removal of such cells from bloom forming Microcystis results in increased fitness of the surviving population and an additional nutrient source in heavily cyanobacterial blooming environments (Ding et al., 2013). The presence of PCD in Microcystis cells might increase their survival under cold stress.
5 CONCLUSIONIn this study, it is remarkable that both toxic M. aeruginosa FACHB-905 and FACHB-915 not only survived but also resumed active growth after long periods of incubation at low temperatures or in darkness. At 15℃, the cells modulated carotenoid content and microcystin release. Moreover, M. aeruginosa cells exposed to cold 4℃ (20-25 μmol photons/(m2∙s)) stress did not completely lose viability but retained the ability to reinitiate growth. In darkness, the Fv/Fm and ETRmax values and cell viability of M.aeruginosa cells gradually decreased. Additionally, our observations suggest the occurrence of a type of cell death similar to PCD under cold stress. Overall, our findings could help explain why M. aeruginosa can exist at low temperatures or in dark conditions encountered in an annual cycle. These results also indicate that low temperatures or darkness may play subtle roles affecting Microcystis blooms and microcystin release in natural waters.
| Allakhverdiev S I, Kreslavski V D, Klimov V V, Los D A, Carpentier R, Mohanty P, 2008. Heat stress:an overview of molecular responses in photosynthesis. Photosynthesis Research, 98(1-3): 541–550. Doi: 10.1007/s11120-008-9331-0 |
| Anning T, Harris G, Geider R, 2001. Thermal acclimation in the marine diatom Chaetoceros calcitrans (Bacillariophyceae). European Journal of Phycology, 36(3): 233–241. Doi: 10.1080/09670260110001735388 |
| Babica P, Bláha L, Maršálek B, 2006. Exploring the natural role of microcystins-a review of effects on photoautotrophic organisms. Journal of Phycology, 42(1): 9–20. Doi: 10.1111/jpy.2006.42.issue-1 |
| Berges J A, Falkowski P G, 1998. Physiological stress and cell death in marine phytoplankton:induction of proteases in response to nitrogen or light limitation. Limnology and Oceanography, 43(1): 129–135. Doi: 10.4319/lo.1998.43.1.0129 |
| Bidle K D, Falkowski P G, 2004. Cell death in planktonic, photosynthetic microorganisms. Nature Reviews Microbiology, 2(8): 643–655. Doi: 10.1038/nrmicro956 |
| Bidle K D, 2015. The Molecular ecophysiology of programmed cell death in marine phytoplankton. Annual Review of Marine Science, 7: 341–375. Doi: 10.1146/annurev-marine-010213-135014 |
| Bouchard J N, Purdie D A, 2011. Effect of elevated temperature, darkness, and hydrogen peroxide treatment on oxidative stress and cell death in the bloom-forming toxic cyanobacterium Microcystis aeruginosa. Journal of Phycology, 47(6): 1 316–1 325. Doi: 10.1111/jpy.2011.47.issue-6 |
| Bradford M M, 1976. Rapid and sensitive method for quantitation of microgram quantities of protein utilizing principle of protein-dye binding. Analytical Biochemistry, 72(1-2): 248–254. Doi: 10.1016/0003-2697(76)90527-3 |
| Dai G F, Quan C Y, Zhang X Z, Liu J, Song L R, Gan N Q, 2012. Fast removal of cyanobacterial toxin microcystinLR by a low-cytotoxic microgel-Fe (Ⅲ) complex. Water Research, 46(5): 1 482–1 489. Doi: 10.1016/j.watres.2011.11.010 |
| Davis T W, Berry D L, Boyer G L, Gobler C J, 2009. The effects of temperature and nutrients on the growth and dynamics of toxic and non-toxic strains of Microcystis during cyanobacteria blooms. Harmful Algae, 8(5): 715–725. Doi: 10.1016/j.hal.2009.02.004 |
| Ding Y, Gan N Q, Li J, Sedmak B, Song L R, 2012. Hydrogen peroxide induces apoptotic-like cell death in Microcystis aeruginosa (Chroococcales, Cyanobacteria) in a dosedependent manner. Phycologia, 51(5): 567–575. Doi: 10.2216/11-107.1 |
| Ding Y, Song L R, Sedmak B. 2013. UVB radiation as a potential selective factor favoring microcystin producing bloom forming cyanobacteria. PLoS One, 8(9):e73919, http://dx.doi.org/10.1371/journal.pone.0073919, [electronically published:September 2013]. |
| Dokulil M T, Teubner K, 2000. Cyanobacterial dominance in lakes. Hydrobiologia, 438(1-3): 1–12. |
| Duan H T, Ma R H, Zhang Y C, Loiselle S A, 2014. Are algal blooms occurring later in Lake Taihu? Climate local effects outcompete mitigation prevention. Journal of Plankton Research, 36(3): 866–871. Doi: 10.1093/plankt/fbt132 |
| Dziallas C, Grossart H P. 2011. Increasing oxygen radicals and water temperature select for toxic Microcystis sp. PLoS One, 6(9):e25569, http://dx.doi.org/10.1371/journal.pone.0025569. |
| Franklin D J, 2014. Explaining the causes of cell death in cyanobacteria:what role for asymmetric division?. Journal of Plankton Research, 36(1): 11–17. Doi: 10.1093/plankt/fbt114 |
| Furusato E, Asaeda T, Manatunge J, 2004. Tolerance for prolonged darkness of three phytoplankton species, Microcystis aeruginosa (Cyanophyceae), Scenedesmus quadricauda (Chlorophyceae), and Melosira ambigua (Bacillariophyceae). Hydrobiologia, 527(1): 153–162. Doi: 10.1023/B:HYDR.0000043198.08168.d3 |
| Guo L S, Zhang J, Wu J, et al, 2012. Morphological and biochemical changes of Microcystis aeruginosa PCC7806 subjected to dark and oxygen limitation. Acta Microbiologica Sinica, 52(2): 228–235. |
| Jewson D H, 1976. The interaction of components controlling net phytoplankton photosynthesis in a well-mixed lake (Lough Neagh, Northern Ireland). Freshwater Biology, 6(6): 551–576. Doi: 10.1111/fwb.1976.6.issue-6 |
| Jochem F J, 1999. Dark survival strategies in marine phytoplankton assessed by cytometric measurement of metabolic activity with fluorescein diacetate. Marine Biology, 135(4): 721–728. Doi: 10.1007/s002270050673 |
| Le Blanc Renaud S, Pick F R, Fortin N, 2011. Effect of light intensity on the relative dominance of toxigenic and nontoxigenic strains of Microcystis aeruginosa. Applied and Environmental Microbiology, 77(19): 7 016–7 022. Doi: 10.1128/AEM.05246-11 |
| Lei L M, Wu Y S, Gan N Q, Song L R, 2004. An ELISA-like time-resolved fluorescence immunoassay for microcystin detection. Clinica Chimica Acta, 348(1-2): 177–180. Doi: 10.1016/j.cccn.2004.05.019 |
| Mikula P, Zezulka S, Jancula D, et al, 2012. Metabolic activity and membrane integrity changes in Microcystis aeruginosa-new findings on hydrogen peroxide toxicity in cyanobacteria. European Journal of Phycology, 47(3): 195–206. Doi: 10.1080/09670262.2012.687144 |
| Moon Y J, Kim S I, Chung Y H, 2012. Sensing and responding to UV-A in cyanobacteria. International Journal of Molecular Sciences, 13(12): 16 303–16 332. Doi: 10.3390/ijms131216303 |
| Paerl H W, Huisman J, 2009. Climate change:a catalyst for global expansion of harmful cyanobacterial blooms. Environmental Microbiology Reports, 1(1): 27–37. Doi: 10.1111/emi4.2009.1.issue-1 |
| Paerl H W, Otten T G, 2013. Harmful cyanobacterial blooms:causes, consequences, and controls. Microbial Ecology, 65(4): 995–1 010. Doi: 10.1007/s00248-012-0159-y |
| Popels L C, MacIntyre H L, Warner M E, Zhang Y H, Hutchins D A, 2007. Physiological responses during dark survival and recovery in Aureococcus anophagefferens (Pelagophyceae). Journal of Phycology, 43(1): 32–42. Doi: 10.1111/jpy.2007.43.issue-1 |
| Reynolds C S, Jaworski G H M, Cmiech H A, Leedale G F, 1981. On the annual cycle of the blue-green alga Microcystis aeruginosa Kütz.Emend. Elenkin.Philosophical Transactions of the Royal Society of London. Series B, Biological Sciences, 293(1068): 419–477. Doi: 10.1098/rstb.1981.0081 |
| Richards F A, Thompson T G, 1952. The estimation and characterization of plankton populations by pigment analyses. Ⅱ. A spectrophotometric method for the estimation of plankton pigments. Journal of Marine Research, 11: 156–172. |
| Ross C, Santiago-Vázquez L, Paul V, 2006. Toxin release in response to oxidative stress and programmed cell death in the cyanobacterium Microcystis aeruginosa. Aquatic Toxicology, 78(1): 66–73. Doi: 10.1016/j.aquatox.2006.02.007 |
| Sabour B, Sbiyyaa B, Loudiki M, Oudra B, Belkoura M, Vasconcelos V, 2009. Effect of light and temperature on the population dynamics of two toxic bloom forming Cyanobacteria-Microcystis ichthyoblabe and Anabaena aphanizomenoides. Chemistry and Ecology, 25(4): 277–284. Doi: 10.1080/02757540903062525 |
| Sakai H, Oguma K, Katayama H, Ohgaki S, 2007. Effects of low or medium-pressure UV irradiation on the release of intracellular microcystin. Water Research, 41(15): 3 458–3 464. Doi: 10.1016/j.watres.2007.04.031 |
| Sakamoto T, Bryant D A, 1999. Nitrate transport and not photoinhibition limits growth of the freshwater cyanobacterium Synechococcus species PCC 6301 at low temperature. Plant Physiology, 119(2): 785–794. Doi: 10.1104/pp.119.2.785 |
| Schatz D, Keren Y, Vardi A, Sukenik A, Carmeli S, Börner T, Dittmann E, Kaplan A, 2007. Towards clarification of the biological role of microcystins, a family of cyanobacterial toxins. Environmental Microbiology, 9(4): 965–970. Doi: 10.1111/emi.2007.9.issue-4 |
| Segovia M, Haramaty L, Berges J A, Falkowski P G, 2003. Cell death in the unicellular chlorophyte Dunaliella tertiolecta.A hypothesis on the evolution of apoptosis in higher plants and metazoans. Plant Physiology, 132(1): 99–105. Doi: 10.1104/pp.102.017129 |
| Sta C, Ledoigt S, Ferjani E, Goupil P, 2012. Exposure of Vicia faba to sulcotrione pesticide induced genotoxicity. Pesticide Biochemistry and Physiology, 103(1): 9–14. Doi: 10.1016/j.pestbp.2012.02.002 |
| Tan X M, Zhu T, Shen S, Yin C T, Gao H, Xu X D, 2011. The role of Rbp1 in the acquired chill-light tolerance of cyanobacteria. Journal of Bacteriology, 193(11): 2 675–2 683. Doi: 10.1128/JB.01454-10 |
| White S H, Duivenvoorden L J, Fabbro L D, 2005. A decisionmaking framework for ecological impacts associated with the accumulation of cyanotoxins (cylindrospermopsin and microcystin). Lakes & Reservoirs:Research & Management, 10(1): 25–37. |
| Wu Z X, Song L R, Li R H, 2008. Different tolerances and responses to low temperature and darkness between waterbloom forming cyanobacterium Microcystis and a green alga Scenedesmus. Hydrobiologia, 596(1): 47–55. Doi: 10.1007/s10750-007-9056-7 |
| Yang Y, Yin C T, Li W Z, Xu X D, 2008. α-Tocopherol is essential for acquired chill-light tolerance in the cyanobacterium Synechocystis sp.Strain PCC 6803. Journal of Bacteriology, 190(5): 1 554–1 560. Doi: 10.1128/JB.01577-07 |
| Yang Z, Kong F X, Shi X L, Yu Y, Zhang M, 2015. Effects of UV-B radiation on microcystin production of a toxic strain of Microcystis aeruginosa and its competitiveness against a non-toxic strain. Journal of Hazardous Materials, 283: 447–453. Doi: 10.1016/j.jhazmat.2014.09.053 |
| Yin C T, Li W Z, Du Y, Kong R Q, Xu X D, 2007. Identification of a gene, ccr-1 (sll1242), required for chill-light tolerance and growth at 15℃ in Synechocystis sp. PCC 6803.Microbiology, 153(Pt 4): 1 261–1 267. |
| Zhang M, Duan H T, Shi X L, Yu Y, Kong F X, 2012a. Contributions of meteorology to the phenology of cyanobacterial blooms:implications for future climate change. Water Research, 46(2): 442–452. Doi: 10.1016/j.watres.2011.11.013 |
| Zhang M, Shi X L, Yu Y, Kong F X, 2011. The acclimative changes in photochemistry after colony formation of the cyanobacteria Microcystis aeruginosa. Journal of Phycology, 47(3): 524–532. Doi: 10.1111/jpy.2011.47.issue-3 |
| Zhang M, Yu Y, Yang Z, Kong F X, 2012b. Photochemical responses of phytoplankton to rapid increasingtemperature process. Phycological Research, 60(3): 199–207. Doi: 10.1111/pre.2012.60.issue-3 |
| Zilliges Y, Kehr J C, Meissner S, Ishida K, Mikkat S, Hagemann M, Kaplan A, Börner T, Dittmann E. 2011. The cyanobacterial hepatotoxin microcystin binds to proteins and increases the fitness of Microcystis under oxidative stress conditions. PLoS One, 6(3):e17615, http://dx.doi.org/10.1371/journal.pone.0017615. |
 2017, Vol. 35
2017, Vol. 35


