Institute of Oceanology, Chinese Academy of Sciences
Article Information
- WANG Yueyun(王跃云), LI Xinzheng(李新正)
- A new species of Phyllochaetopterus Grube, 1863 (Polychaeta: Chaetopteridae) from Hainan Island, South China Sea
- Chinese Journal of Oceanology and Limnology, 35(2): 360-366
- http://dx.doi.org/
Article History
- Received Sep. 13, 2015
- accepted in principle Nov. 24, 2015
- accepted for publication Jan. 25, 2016
2 University of Chinese Academy of Sciences, Beijing 100049, China
The family Chaetopteridae is widely distributed from subtidal to deep waters, including hydrothermal vents and whale falls (Nishi and Rouse, 2014). Most chaetopterids are tube-dwelling worms. However, Chaetopterus pugaporcinus Osborn, Rouse, Goffredi & Robison, 2007 was described as a holopelagic species inhabiting deep mesopelagic waters off Monterey Bay, California (Osborn et al., 2007). Chaetopterids have three well-defined body regions, anterior (A), middle (B) and posterior (C); and one or more specialized enlarged chaetae in the fourth chaetiger of region A. The latter are a robust taxonomic character (e.g., in Bhaud (1998, 2003) for Spiochaetopterus), as well as a useful tool to identify species in planktonic stages (Bhaud, 1983).
Phyllochaetopterus Grube, 1863 differs remarkably from the other genera of the family in having species with a pair of so-called ‘tentacular cirri’ lying on each side of the prostomium. Their ‘tentacular cirri’ are probably nonhomologous to the tentacles of other polychaetes (Fauchald and Rouse, 1997). As they are supported by thin capillary chaetae (Potts, 1914), we here consider them to be reduced and modified peristomial notopodia.
When we studied the polychaete collections at the Marine Biological Museum of the Chinese Academy of Sciences, we discovered some fragments of an unidentified species of Phyllochaetopterus. These are fully described and illustrated here as a new species. The World Register of Marine Species (WoRMS) lists 20 species of Phyllochaetopterus (Read and Fauchald, 2015) and, of these, 12 species are described as inhabiting the Pacific Ocean, including the new species (Nishi and Rouse, 2007, 2014). We provide an identification key to distinguish among species from the Pacific Ocean.
2 MATERIAL AND METHODSpecimens and tubes were collected on 7 April 1958 in Lingao Harbor, north-west Hainan Island, and preserved in 72% alcohol. The specimens are deposited in the Marine Biological Museum at the Institute of Oceanology, Chinese Academy of Sciences (IOCAS), Qingdao, China. The samples were examined with a Zeiss Stemi 2000-C stereo microscope, and photographed and measured using an AxioCamMRc 5 camera. Detailed structures of the modified chaetae on the fourth chaetiger and notopodia of region C were observed with a scanning electron microscope.
3 RESULT AND DISCUSSION 3.1 SystematicsFamily Chaetopteridae Audouin & Milne Edwards, 1833
Genus Phyllochaetopterus Grube, 1863
Phyllochaetopterus hainanensis n. sp. (Figs. 1-4)
|
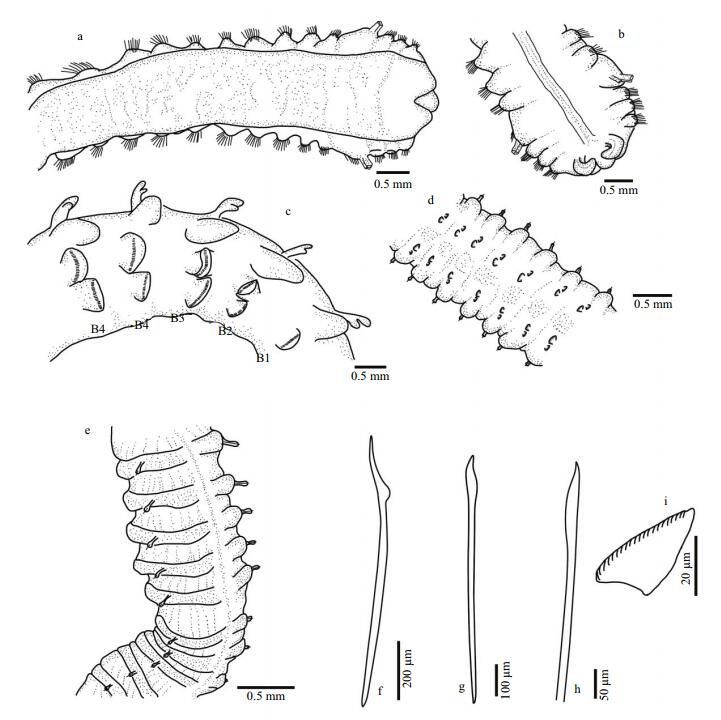
| Figure 1 Phyllochaetopterus hainanensis n. sp. paratype MBM 190829 a-b. ventral and dorsal view of region A, respectively; c. lateral view of region B; d-e. ventral and dorsal view of posterior part of region C, respectively; f-h. notochaetae of region A; i. uncini from B1 neuropodia. Scale bars: a-e: 0.5 mm; f: 200 μm; g: 100 μm; h:50 μm; i: 20 μm. |
|
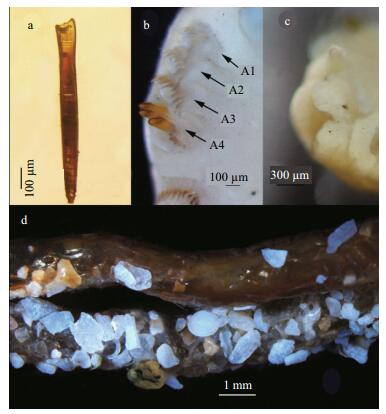
| Figure 2 Phyllochaetopterus hainanensis n. sp. a. modified chaeta from paratype MBM 283027; b. dorsal view of first four chaetigers (A1-A4) of paratype MBM 283027, showing notopodia (arrows); c. eyespots of paratype MBM 283027; d. tubes from paratype MBM 190829. |
|
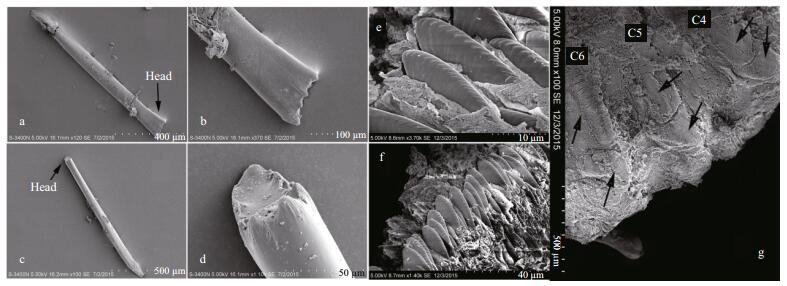
| Figure 3 Scanning electron micrographs of A4 modified chaetae and uncini from region C of Phyllochaetopterus hainanensis n. sp. from paratypes MBM 283029 and 283031 a. lateral view of A4 modified chaeta; b. lateral view of the head of A4 modified chaeta showing small teeth on lateral edge; c. ventral view of A4 modified chaeta; d. ventral view of the head of A4 modified chaeta; e. uncini from region C; f. row of uncini; g. ventral view of C4-C6, showing bilobed neuropodia of region C. |
|
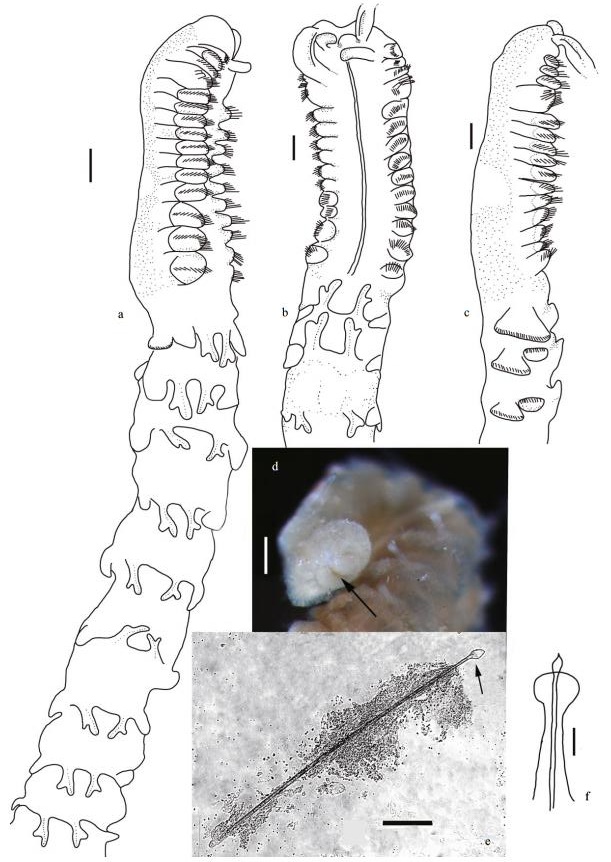
| Figure 4 Phyllochaetopterus hainanensis n. sp. a. holotype. Lateral view of regions A and B. Paratype MBM 283031; b. dorsal view; c. lateral view. Paratype MBM 283032; d. pygidium, arrow shows anus; e. notochaetae; f. notopodium. Scale bars: a-c: 0.5 mm; d: 200 μm; e: 100 μm; f: 50 μm. |
Material examined: Holotype, MBM283026, incomplete, palps and pygidium missing, 14 chaetigers in region A, 12 chaetigers in region B, six chaetigers in region C, with a section of tube, sandy beach, Lingao Harbor, Hainan Island, South China Sea, coll. Wu Baoling, 7 April 1958.
Paratypes, collected in the same manner as holotypes on the same day. MBM 190829, incomplete, middle part missing; MBM 283028, incomplete, one anterior part; MBM 283027, three anterior parts and four middle region parts; MBM 283029, one anterior part; MBM 283031, incomplete, one anterior part; MBM 283032, incomplete, anterior part missing.
Diagnosis: Middle peristomium recurved. Prostomium spherical, with one pair of conspicuous lateral eyespots. Peristomial notopodia beneath palps, stout, semicircular. Up to 13-14 chaetigers in region A, first four chaetigers slightly stouter than posterior ones. Usually three pairs of large modified chaetae on fourth parapodia. Ventral part of region A entirely covered by glandular shields (ventral shields), whitish in alcohol-preserved specimens. Twelve chaetigers in region B; neuropodia of B1 unilobed, others bilobed. About 52 chaetigers in region C; notopodia unilobed and neuropodia bilobed.
Description: Region A 4.3-9.0 mm long, ca 2.0 mm wide at widest point of fourth chaetiger excluding parapodia. In holotype, region A 4.3 mm long excluding palps, and region B 10.5 mm long. Body 1.0-2.0 mm wide at fourth chaetiger excluding parapodia. Region C of paratype MBM 283032 about 20 mm long.
Region A convex ventrally and flattened dorsally. Two grooved palps arising from lateral side of prostomium (Fig. 4b, c). Two cirri-like, conspicuous, stout peristomial notopodia on the posterolateral side of palps, semicircular or crescent (Fig. 1b), with inner chaetae. Prostomium spherical, extended forward. Two dark-brown eyespots, visible after removing cirri (Fig. 2c). Peristomium distinct with an obvious V-shaped incision ventrally (Fig. 1a). Region A with uniramous parapodia, usually with 14 chaetigers (13 in one of eight specimens examined). Holotype with 15 notopodia on the left side and 14 on the right side. First four chaetigers slightly wider than posterior ones (Figs. 1a-b, 2b). Chaetigers A1-A3 very short (Figs. 1a, 4a-c). Parapodia on A4 large, broadly rounded, with 2-3 golden-yellow modified chaetae and lanceolate companion chaetae. Plate-like cutting chaeta with obliquely truncated head, slightly inflated in lateral view (Fig. 3a, c), with 2-3 small teeth on both lateral edges (Figs. 2a, 3b, d). Remaining parapodia with lanceolate and paddle-shaped chaetae (Fig. 1f-h). Ventral shields (ventral glandular shields) from A3 to the posterior end of region A. A semicircular creamy white patch from A9 to A11 or from A10 to A12.
Region B slender, with 12 chaetigers similar in length. Notopodia bilobed, with Y-shaped inner lobe and triangular lateral branchial lobe (Figs. 1c, 4a-c); bases of paired inner lobes connected. Neuropodia of B1 unilobed, large, triangular; remaining ones bilobed. Neuropodial uncini triangular, with concave occipital face. Uncini from region B with 17-20 fine teeth (Fig. 1i).
Region C slender, fragile, with up to 52 chaetigers. Notopodia unilobed, digitate, with inflated head, with one slender lanceolate or paddle-like inner chaeta (Figs. 4e, f). Neuropodia bilobed (Figs. 1d, 3g), with small lateral lobe and larger ventral lobe (Fig. 3g), bearing rows of uncini (Fig. 3e, f). Uncini similar to those of region B, with ca 18 teeth. Pygidium simple (Fig. 4d), without any appendages.
Tube dark brown, with a thick wrinkled wall having attached sand, stones and shell fragments (Fig. 2d).
Color: Body pale yellow in alcohol-preserved specimens. Region B sometimes dark green.
Etymology: The specific name derives from Hainan Island (China), the type locality.
3.2 RemarksPhyllochaetopterus hainanensis n. sp. differs from all other species of the genus in having curved, inflated peristomial notopodia (slender, clavate in all other species). It resembles Phyllochaetopterus verrilli Treadwell, 1943 in having a V-shaped peristomium, but differs in having 14 (sometimes 13) chaetigers in region A (8-10 in P. verrilli) and 12 in region B (two in P. verrilli) (Treadwell and Verrill, 1943; Bhaud, 1983), as well as distinct eyespots (lacking in P. verrilli). The new species also resembles Phyllochaetopterus socialis Claparède, 1869 and P. sibogae Caullery, 1944 in numbers of chaetigers of region A and region B. Phyllochaetopterus socialis has 10-18 and 7-24 chaetigers in regions A and B, respectively (Day, 1967; Nishi and Rouse, 2007). Phyllochaetopterus sibogae has 13-14 and seven chaetigers in regions A and B, respectively (Caullery, 1944). In turn, neuropodia are all bilobed in P. socialis (neuropodia of B1 are unilobed in P. hainanensis n. sp.) and P. sibogae has a truncate peristomium, small peristomial notopodia and transparent tube (Caullery, 1944) (brown, thick, twisted in P. hainanensis n. sp.).
3.3 DiscussionChaetopterid worms are easily broken into fragments owing to careless collection and stripping them from their chitinous tubes (Gitay, 1969; Yang and Sun, 1988). Phyllochaetopterus can be distinguished from the other genera of chaetopteridae in having a pair of small ‘tentacular cirri’ on each side of the prostomium (Gitay, 1969; Fauchald, 1977; Nishi and Arai, 1996). We dissected one ‘tentacular cirrus’ of P. hainanensis n. sp. and observed the presence of an inner chaeta, in agreement with Potts (1914), who regarded the ‘tentacular cirri’ as reduced, modified notopodia of the peristomial segment. Despite the anatomical origin of these ‘cirri’ remaining unclear, it seems evident that they are not homologous to those of the other polychaetes (Fauchald and Rouse, 1997), so we are here referring to them as peristomial notopodia.
There are few discussions on the taxonomic and evolutionary significance of eyespots in Chaetopteridae and their presence is often unclear in some original descriptions. However, they are considered a robust taxonomic character. For instance, Nishi (1999) summarized the presence of eyespots in Mesochaetopterus and used it to differentiate M. capensis (McIntosh, 1885) and M. selangolus (Rullier, 1976); while Nishi et al.(2000, 2004) suggested they could be a diagnostic character in Chaetopterus, and analyzed them in eight species of Spiochaetopterus from the Indo-Pacific. Since Crossland (1903), who first described the eyespots of P. elioti Crossland, 1903 in detail, no other studies have been made. All the specimens we examined have brown eyespots despite being preserved in alcohol for decades, so we consider them a stable character of P. hainanensis n. sp. Among the 12 known species ofPhyllochaetopterus from the Pacific Ocean, the deep-sea P. lauensis Nishi and Rouse, 2007 and P. gigas Nishi and Rouse, 2014 lack eyespots. Therefore, we tentatively suggest that the eyespots in deep-sea species of Phyllochaetopterus may have a tendency to reduce (or disappear).
Accordingly, the presence of eyespots may be a useful character to distinguish among the Pacific species of Phyllochaetopterus as stated in the following dichotomous key.
Key to species of Phyllochaetopterus from the Pacific Ocean
1a. 1-6 A4 modified chaetae, ventral shields sometimes with four sections................................... 2
1b. > 6 A4 modified chaetae, ventral shield forming a large glandular cushion.............................. P. herdmani (Hornell in Willey, 1905)
2a (1). Region B with two chaetigers.................... 3
2b (1). Region B with three or more chaetigers.... 7
3a (2). Region C notopodia with 2-4 chaetae....... 4
3b (2). Region C notopodia with only one chaetae.................................................................................... 5
4a (3). Tube cylindrical, translucent, unbranched, non-annulated................. P. limicolus Hartman, 1960
4b (3). Tube cylindrical, semitransparent, forked, annulated..................... P. claparedii McIntosh, 1885
5a (3). Ventral shield with four sections with different colors, head of modified chaetae pear-shaped in frontal view................................. 6
5b (3). Ventral shield unknown, head of modified chaetae oval in frontal view.......................................... P. verrilli Treadwell, 1943
6a (5). 1-2 A4 modified chaetae.......................................... P. lauensis Nishi and Rouse, 2007
6b (5). 4-6 A4 modified chaetae.......................................... P. gigas Nishi and Rouse, 2014
7a (2). Peristomium V-shaped; peristomial notopodia large, curved............. P. hainanensis n. sp.
7b (2). Peristomium nearly truncated; peristomial notopodia club-like.................................................. 8
8a (7). White crescent ventral shield on region A..................................................................................... 9
8b (7). White crescent ventral shield on region A absent..................................................................... 10
9a (8). Head of A4 modified chaetae clearly inflated; neck more slender than shaft.................................... P. socialis Claparède, 1869
9b (8). Head of A4 modified chaetae slightly inflated; neck as wide as shaft......................................... P. prolifica Potts, 1914
10a (8). Head of modified chaetae obliquely truncated, slightly inflated...................................... 11
10b (8). Head of modified chaetae truncated and not inflated........................ P. sibogae Caullery, 1944
11a (10). Eyespots absent; ventral shield with a light brown anterior portion and a white posterior portion............... P. awasensis Nishi and Hsieh, 2009
11b (10). With one pair of eyespots; ventral shield milk-white........................... P. elioti Crossland, 1903
4 ACKNOWLEDGEMENTWe appreciate the assistance of Professor Dr. Eijiroh Nishi from Yokohama National University for providing relevant literature. We are grateful to three anonymous reviewers for their comments and suggestions, which allowed us to improve the manuscript. We also thank the managers of the Marine Biological Museum of the Chinese Academy of Sciences in the IOCAS for their help in sorting the studied material.
| Bhaud M R, 1983. Comparison of enlarged setae in larvae and adults of Phyllochaetopterus verrilli Treadwell (Polychaeta:Chaetopteridae). Ophelia, 22(2): 257–263. Doi: 10.1080/00785326.1983.10426600 |
| Bhaud M R, 1998. Species of Spiochaetopterus (Polychaeta, Chaetopteridae) in the Atlantic-Mediterranean biogeographic area. Sarsia, 83(3): 243–263. Doi: 10.1080/00364827.1998.10413685 |
| Bhaud M R, 2003. Identification of adults and larvae in Spiochaetopterus (Polychaeta, Chaetopteridae):consequences for larval transport and recruitment. Hydrobiologia, 496(1-3): 279–287. Doi: 10.1023/A:1026165419638 |
| Caullery M. 1944. Polychètes sédentaires de l'expédition du Siboga:Ariciidae, Spionidae, Chaetopteridae, Chlorhaemidae, Opheliidae, Oweniidae, Sabellariidae, Sternaspidae, Amphictenidae, Terebellidae. Siboga Expeditie. 204p. (in French) |
| Claparède É, 1869. Les Annélides Chétopodes du Golfe de Naples.Seconde partie. Annélides sédentaires. Mémoires de la Société de physique et d'histoire naturelle de Genève, 20(1): 1–225. |
| Crossland C, 1903. On the marine fauna of Zanzibar and British East Africa, from collections made by Cyril Crossland in the years 1901 and 1902.Polychaeta, Part I. Proceedings of the Zoological Society of London, 73(1): 169–176. |
| Day J H. 1967. A Monograph on the Polychaeta of Southern Africa. Part 2. Sedentaria. Trustees of the British Museum (Natural History), London. 440p. |
| Fauchald K, Rouse G, 1997. Polychaete systematics:past and present. Zoologica Scripta, 26(2): 71–138. Doi: 10.1111/zsc.1997.26.issue-2 |
| Fauchald K, 1977. The polychaete worms, definitions and keys to the orders, families and genera. Natural History Museum of Los Angeles County, Science Series, 28: 1–188. |
| Gitay A, 1969. A contribution to the revision ofSpiochaetopterus (Chaetopteridae, Polychaeta). Sarsia, 37(1): 9–20. Doi: 10.1080/00364827.1969.10411142 |
| Nishi E, Arai H, Sasanuma S I, 2000. A new species of Chaetopterus (Polychaeta:Chaetopteridae) from off Tokyo Bay, Central Japan, with comments on its bioluminescence. Actinia (Bulletin of the Manazuru Marine Laboratory for Science Education, Faculty of Education and Human Sciences, Yokohama National University), 13: 1–12. |
| Nishi E, Arai Y A, 1996. Chaetopterid polychaetes from Okinawa Island, Japan, with notes on the feeding behaviour of Spiochaetopterus costarum costarum. Publications of the Seto Marine Biological Laboratory, 37(1-2): 51–61. |
| Nishi E, Bhaud M R, Koh B S, 2004. Two new species of Spiochaetopterus (Annelida:Polychaeta) from Sagami Bay and Tokyo Bay, central Japan with a comparative table of species from Japanese and adjacent waters. Zoological Science, 21(4): 457–464. Doi: 10.2108/zsj.21.457 |
| Nishi E, Hsieh H L, 2009. Chaetopterid polychaetes from Taiwan and Okinawa Island (Japan), with descriptions of two new species. Zoological Studies, 48(3): 370–379. |
| Nishi E, Rouse G W, 2007. A new species of Phyllochaetopterus(Chaetopteridae:Annelida) from near hydrothermal vents in the Lau Basin, western Pacific Ocean. Zootaxa, 1621(1): 55–64. |
| Nishi E, Rouse G W, 2014. First whale fall chaetopterid; a gigantic new species of Phyllochaetopterus(Chaetopteridae:Annelida) from the deep sea off California. Proceedings of the Biological Society of Washington, 126(4): 287–298. Doi: 10.2988/0006-324X-126.4.287 |
| Nishi E, 1999. Redescription of Mesochaetopterus selangolus (Polychaeta:Chaetopteridae), based on type specimens and recently collected material from Morib Beach, Malaysia. Pacific Science, 53: 24–36. |
| Osborn K J, Rouse G W, Goffredi S K, Robison B H, 2007. Description and relationships of Chaetopterus pugaporcinus, an unusual pelagic polychaete (Annelida, Chaetopteridae). Biological Bulletin, 212(1): 40–54. Doi: 10.2307/25066579 |
| Polychaeta. 2008. Phyllochaetopterus Grube, 1863. In:Read G, Fauchald K ed. (2015) World Polychaeta database.http://www.marinespecies.org/polychaeta/aphia.php?p=taxdetails&id=129232. Accessed on 2015-11-28. |
| Potts F A, 1914. Polychaeta from the N.E. Pacific:the Chaetopteridae. With an account of the phenomenon of asexual reproduction in Phyllochaetopterus and the description of two new species of Chaetopteridae from the Atlantic. Proceedings of the Zoological Society of London, 67: 955–994. |
| Rullier F, 1976. Description d'un nouveau genre et d'une nouvelle espèce de Chaetopteridae Sasekumaria selangora (Annélides Polychètes) de Malaisie. Bulletin de la Société Zoologique de France, 101(2): 199–202. |
| Treadwell A L, 1943. New species of polychaetous annelids from Hawaii. American Museum Novitates(1233): 1–4. |
| Willey A. 1905. Report on the Polychaeta collected by professor Herdman, at Ceylon, in 1902. Report to the Government of Ceylon on the Pearl Oyster Fisheries of the Gulf of Manaar by W.A. Herdman, with Supplementary Reports Upon the Marine Biology of Ceylon, by Other Naturalists. Part Ⅳ. Supplementary Reports 30. p.243-324. |
| Yang D J, Sun R P. 1988. Polychaetous Annelids Commonly Seen from China Coastal Waters. China Agriculture Press, Beijing. p.224-226. (in Chinese) |
 2017, Vol. 35
2017, Vol. 35


