Institute of Oceanology, Chinese Academy of Sciences
Article Information
- SHEN Linnan(沈林南), CHEN Min(陈敏), LAN Binbin(兰彬斌), QI Hongshuai(戚洪帅), ZHANG Aimei(张爱梅), LAN Dongzhao(蓝东兆), FANG Qi(方琦)
- Diatom distribution as an environmental indicator in surface sediments of the West Philippine Basin
- Chinese Journal of Oceanology and Limnology, 35(2): 431-443
- http://dx.doi.org/10.1007/s00343-016-5306-8
Article History
- Received Oct. 29, 2015
- accepted in principle Jan. 4, 2016
- accepted for publication Feb. 25, 2016
2 Geology Laboratory of Marine and Coastal Environment, Third Institute of Oceanography, State Oceanic Administration, Xiamen 361005, China
The tropical western Pacific has some of the warmest waters in the world; these waters regulate global climate through their heat exchange with the tropics and higher latitudes (Meyers et al., 1986; Barlow et al., 2002; Sun et al., 2003). The Philippine Basin is the largest marginal basin in the West Pacific (Scott et al., 1981). The West Philippine Basin (WPB) is an ideal location for reconstructing palaeoenvironments and palaeoproductivity, because it is located near an area of linked ocean-atmosphere circulation over the Pacific and Indian Ocean, where major climate and environmental variability is generated (Wan et al., 2012). Various studies have been carried out in the WPB using foraminifera (Yamasaki et al., 2008; Qiu et al., 2014), stable isotopes (Tang et al., 2013), clay minerals (Xu et al., 2012) and calcareous nannofossils (Sun et al., 2011). However, to date, few studies have been conducted on diatoms (Zhai et al., 2009, 2012).
Diatoms are microscopic algae found in almost all aquatic habitats (Round et al., 1990). Each diatom habitat has its own characteristic diatom fl ora, developed under distinct environmental variables (Hendey, 1964). Thus, distinct ocean currents in a given region may be recognized through the diatom assemblage in the sediment record. In areas where they are diverse and abundant, diatoms can thus be used for studies of marine palaeoecology and palaeoceanography (Smol and Stoermer, 2010). However, uncertainties arise when attempting to reconstruct the palaeoenvironment based on diatom records, because the diatom assemblage preserved in the sediments does not correspond precisely to the diatom fl ora in the seawater above (Jiang et al., 2004). Therefore, prior to carrying out palaeoceanographical reconstructions using fossil diatom records, studies on diatoms from the surface sediments and their relationships with local environments are necessary (Sancetta, 1982; Karpuz and Schrader, 1990; Jiang et al., 2005).
Ethmodiscus rex (Wallich) Hendey is a widelydistributed warm-water diatom, characteristic of oligotrophic open ocean water (Villareal, 1993; Villareal et al., 1999). Ethmodiscus has been particularly observed in equatorial areas, such as the eastern Atlantic Ocean (Gardner and Burckle, 1975; Stabell, 1986) and the eastern Indian Ocean (De Deckker and Gingele, 2002). In addition, some authors have reported that Ethmodiscus ooze is present in southwestern North Pacific sediments (Belyayeva, 1968). Ethmodiscus contains the largest known diatoms, with diameters of 2-3 mm reported (Round et al., 1990). The species are capable of buoyancy regulation, migrating vertically within the water column to exploit nutrients in the sub-photic zone (Villareal, 1993). Owing to its high abundance in the diatom ooze, Ethmodiscus rex plays a significant role in global carbon and silicon cycles (Kemp et al., 2006). However, little is known of the distribution of Ethmodiscus rex in the WPB.
Thus, the objectives of this paper were to (1) explore diatom distribution in surface sediments of the WPB, particularly Ethmodiscus rex; (2) distinguish diatom assemblages in different areas and discuss the factors aff ecting diatom preservation in the WPB.
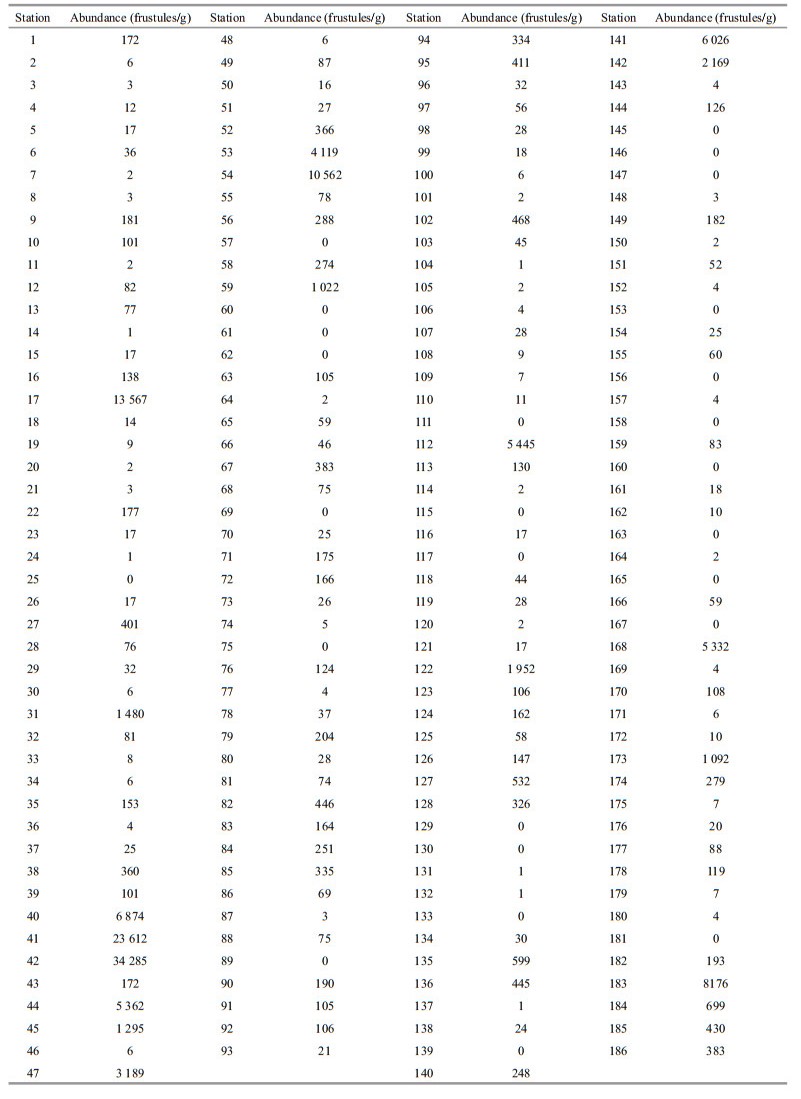
2 REGIONAL SETTINGThe Philippine Sea, located in the northwestern part of the Pacific Ocean, consists of several small basins, ridges and troughs with various seafloor ages (Isse et al., 2010). The geological setting of the WPB is unique, with a complex crustal structure, located between the Pacific, Eurasian and Australian plates. It is the world’s largest marginal basin plate, and surrounded by subduction boundaries. It is not possible to link the WPB’s motion to the global plate circuit through time, but it is known that the age of the seafloor in the WPB is 35-50 Ma (Scott et al., 1981; Sdrolias et al., 2004; Taylor and Goodliffe, 2004). The West Pacific Basin is dominated by the sub-tropical East Asian monsoonal climate (Liu et al., 2009). The annual sea surface temperature (SST) in the WPB is 28.5℃ and the sea-surface salinity (SST) is 34.43 (Schlitzer, 2007). The North Equatorial Current (NEC) and the Kuroshio Current (KC) are the most important oceanographic currents in the WPB (Xu et al., 2012). The NEC provides significant pathways for zonal heat and water mass exchange across the tropical Pacific Ocean (Qiu et al., 2014). Along the Philippine coast in the western Pacific, the NEC bifurcates into the northward flowing Kuroshio and the southward flowing Mindanao Current (MC) at about 14.5°N (Fig. 1) (Qiu and Lukas, 1996; Qu and Lukas, 2003; Kim et al., 2004). The latitude of bifurcation is strongly correlated with monsoon and El Niño/Southern Oscillation (ENSO) events; during the El Niño years, bifurcation of the NEC takes place at higher latitudes and vice versa, with the bifurcation always occurring south of the WPB (Wang and Hu, 2006).
|
| Figure 1 Maps showing (a) the regional setting of the WPB and (b) the distribution of diatom samples used in this study (modified from Zhai et al., 2009; Li et al., 2010) a. general location of the study area (black square) and current systems in the WPB. The dashed black rectangle is where Ethmodiscus rex occurs in the northwest of the Parece Vela Basin in the eastern Philippine Sea (after Zhai et al., 2009). Arrows represent major currents in the area; NEC is the North Equatorial Current, KC is the Kuroshio Current, and MC is the Mindanao Current; b. the black dots are the 186 diatom sample sites. |
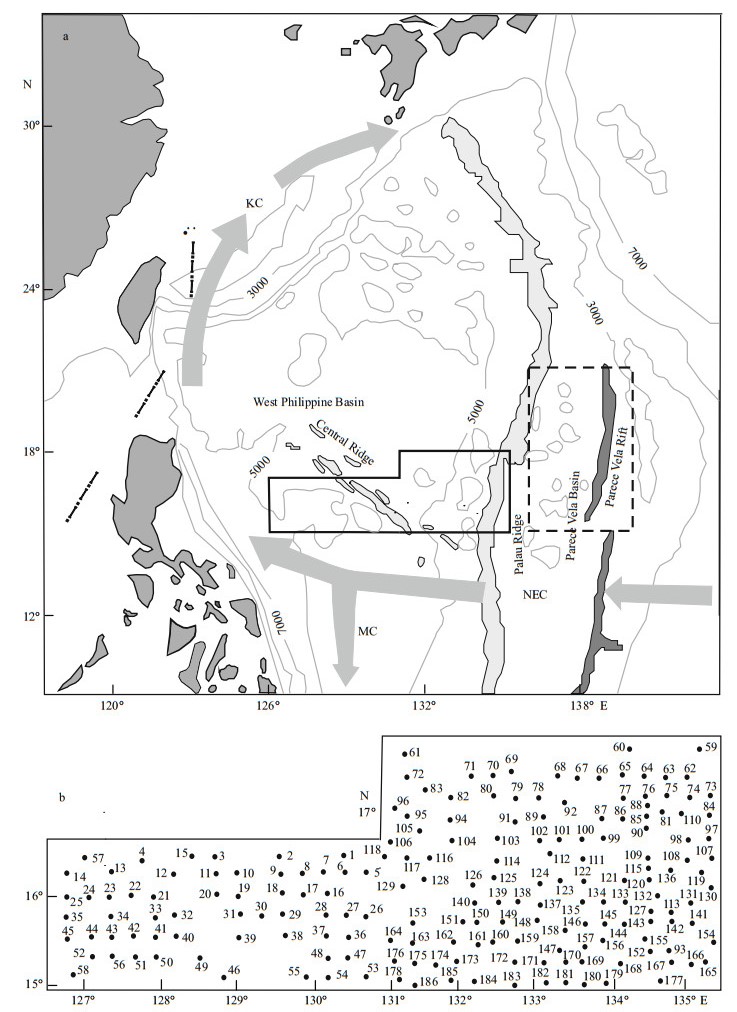
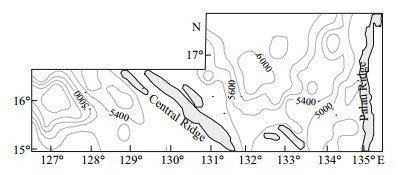
The study area (15°-18°N, 125°-136°E) was located in the WPB at the margin of the West Pacific Warm Pool, where water depth ranges from 2 976 to 6 214 m (Fig. 2). Diatom analyses were carried out on the uppermost 1 cm of 186 sediment samples obtained by either a grab or a box corer in 2004-2005.
|
| Figure 2 Depth and bathymetry in the WPB |
All samples were processed at the diatom analysis laboratory in the Third Institute of Oceanography, China State Oceanic Administration. The samples were prepared according to Håkansson (1984). Carbonates and organic material were removed using 10% HCl and 30% H2O2, respectively; subsequent washings in distilled water were used to remove the chemicals from solution and produce 2 mL of concentrated diatom suspension. After complete homogenization, a subsample was transferred to a cover slip and air-dried. Permanent drop slides were made with Canada balsam (Håkansson, 1984).
3.2 Diatom identification and countingDiatom species present on the drop slides were observed and identified using an Olympus BX51 optical microscope (objective lens 40×, ocular lens 20×) (Manufactured by Olympus Corporation, Tokyo, Japan). In each sample, at least 300 diatom frustules were counted and identified in random transects in at least 3 slides (including Ethmodiscus rex). For incomplete frustules, more than half a frustule was treated as whole. Using illustrations in the literature (Jin et al., 1965, 1991; Round et al., 1990; Cheng et al., 1996; Guo and Qian, 2003; Qi and Li, 2004; Smol and Stoermer, 2010; Cheng and Gao, 2012), each specimen was identified to species or variety level, or when impossible, to genus level.
3.3 Data processingDiatom assemblages were differentiated using multivariate techniques and CANOCO (version 4.5) for numerical analysis (Ter Braak and Smilauer, 2002). Several statistical methods in CANOCO can be used for data analysis, based on the characteristics of the dataset (e.g. linear or non-linear methods, direct or indirect analyses) (Jiang et al., 2001).
If variations in the species data is within a wide range, non-linear ordination methods such as correspondence analysis (CA), detrended correspondence analysis (DCA) and canonical correspondence analysis (CCA) are appropriate (Ter Braak and Prentice, 1988). If the variation falls within a narrow range, the linear ordination methods such as principal component analysis (PCA) and redundancy analysis (RDA) are preferred (Ter Braak and Prentice, 1988). Before choosing linear or non-linear ordination methods, a DCA was used on the diatom data to determine which method is appropriate, with gradient length as the criterion (Ter Braak and Prentice, 1988). In our database, variation in the species data fell within a narrow range, and thus a linear method of principal components analysis (PCA) was employed to analyze statistically significant directions of variation within the samples. Species data were transformed before statistical analyses; only species with more than 2% relative abundance in at least one sample could be included in further statistical analyses (Lopes et al., 2006).
4 RESULT 4.1 Absolute diatom abundance in surface sedimentsDiatoms were abundant in most of the surface sediments from the WPB, with abundance varying spatially (Table 1). Diatoms were absent in the central and eastern area, whereas in the western region abundance was 3.4×104 frustules/g. In the western WPB, which is northeast of the Philippines, sediments were composed of brown clay. The results indicate that the highest diatom abundance occurred in the western sector of the study area. Abundance values in this region averaged 2.05×104 frustules/g and were commonly greater than 1×104 frustules/g. Areas off the western WPB are part of the Central Ridge, where water depth ranges from 4 276 to 5 436 m (Fig. 2). The abundance of diatoms was between 0.1×104 to 1×104 frustules/g in this region, and the average diatom abundance was 0.33×104 frustules/g. In the eastern WPB, The abundance of diatoms ranged from 0 to 0.82×104 frustules/g, with a low average abundance of 538 frustules/g. Diatom abundance was lower in the eastern WPB than the western WPB.
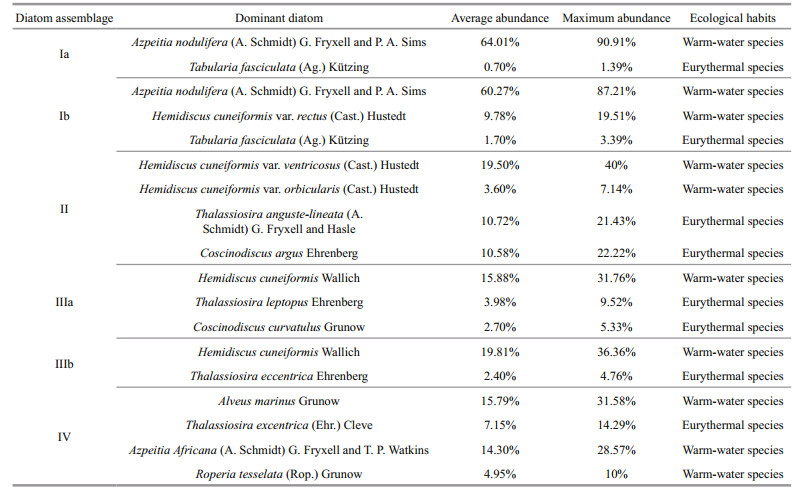
Altogether, 68 species and varieties of diatoms from 26 genera were identified in the WPB. According to Pokras and Molfi no, when the relative percentage of an individual species reaches 10%, or even 5% under certain environmental conditions, the species can be considered dominant (Pokras and Molfino, 1986). Therefore, the 10 most dominant taxa (relative percentage reaches 10%) in our study were Alveus marinus, Azpeitia africana, Azpeitia nodulifera, Thalassiosira leptopus, Cyclotella spp., Hemidiscus cuneiformis, Hemidiscus cuneiformis var. ventricosus, Roperia tesselata, Thalassiosira anguste -lineatus and Thalassiosira excentrica. Detailed descriptions of these species can be found in Chen et al. (2014).
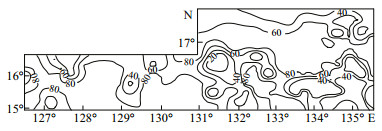
4.3 Distribution of tropical pelagic species in the WPBIn the WPB, 7 species were abundant: A. a fricana (0-33.33%), A. nodulifera (0-87.21%), H. cuneiformis (0-57.14%), H. cuneiformis var. ventricosus (0-40%), A. marinus (0-33.33%), R. tesselata (0-13.79%), and Rhizosolenia bergonii (0-1.89%). These species are mainly found in warm-water areas (Hasle and Syvertsen, 1997), and they are typical oceanic, planktonic species in surface sediments throughout the tropic and low-latitude Pacific Ocean (Jousé et al., 1971; Muhina, 1971). In this study, these species were present (relative abundance >20%) at most stations (Fig. 3).
|
| Figure 3 The distribution of tropical pelagic species in the WPB |
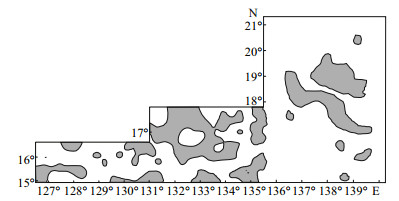
Ethmodiscus rex was found at 156 stations in the WPB (Fig. 4). The WPB water depth ranged from 2 976 m to 6 200 m; average depth was 5 174 m, and most of the stations were below the Carbonate Compensation Depth (CCD). The CCD in the Philippine Sea is located at 3 300-3 800 m (Yan et al., 2007). In the western WPB, Ethmodiscus rex was concentrated in the area 15°-15.7°N, 126.5°-128.4°E, and 15.7°-16.5°N, 129.5°-131.4°E. In the eastern WPB, Ethmodiscus rex was present at most stations. The distribution area was larger in the eastern WPB than in the western WPB. In the WPB, Ethmodiscus rex comprised the main part of the diatomaceous ooze, and thus formed Ethmodiscus ooze.
|
| Figure 4 The distribution of Ethmodiscus rex in surface sediment of the WPB Relative abundance of Ethmodiscus rex was higher than 5%. |
In the northwest of the Parece Vela Basin of the Eastern Philippine Sea, Ethmodiscus rex was found at 32 stations, distributed in a NW-SE direction. They were mostly distributed in the deep water area with a flat seafloor, between 17°-20°N and at a water depth of 4 837-6 150 m, which was below the CCD (Zhai, 2009). As shown in Fig. 4, the distribution of Ethmodiscus rex was far broader in the WPB than in the Eastern Philippine Sea.
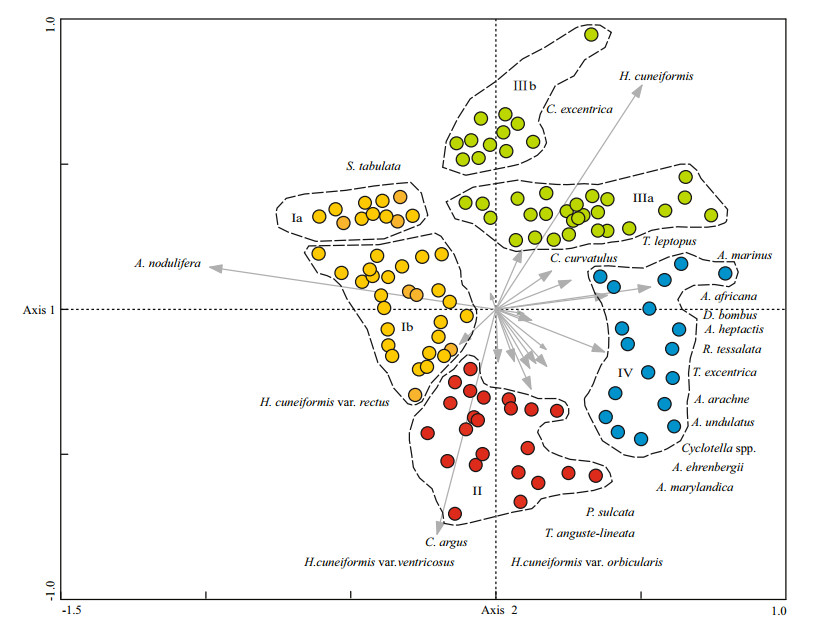
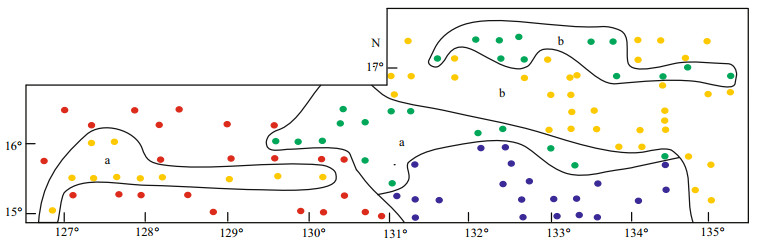
4.5 Characteristics of diatom assemblages in the WPBCorrelations between diatom taxa and samples are shown in Fig. 5, from a PCA biplot of taxa and samples. Species with less than 2% abundance in all samples were removed from the data set (Imbrie and Kipp, 1971). In this paper, 69 samples had less than 2% species abundance, and thus 117 samples were counted in the PCA. Samples located in the direction of vectors were mainly composed of taxa represented by these vectors; the longer the vectors, the more abundant the diatom taxa (Jiang et al., 2004). The four sample groups shown in Fig. 6 could be identified as separate diatom assemblages, based on their positions in the biplot, the relationships between the taxa and samples (Fig. 5), and the percentage and geographical distribution of the diatom samples in the WPB (Table 2).
|
| Figure 5 PCA biplot of diatom taxa and samples The eigenvalues for PCA axis 1 and 2 were 0.303 and 0.157, respectively. |
|
| Figure 6 Distribution of four diatom assemblages in the WPB The symbols for samples were: yellow circle (assemblage Ⅰa and b), red circle (assemblage Ⅱ), green circle (assemblage Ⅲa and b) and blue circle (assemblage Ⅳ). |
|
Diatom assemblage (Ⅰ) Azpeitia nodulifera -Hemidiscus cuneiformis var. rectus -Tabularia fasciculata (western and northeastern WPB) (Figs. 5, 6)
Azpeitia nodulifera was the most important species in diatom assemblage I. The assemblage could be further divided into two sub-assemblages Ia, and Ib. These sub-assemblages were based on the positions of the samples in the PCA biplot of taxa and samples, and decided by their spatial distribution in the WPB. Subassemblage Ia, located in the western part of the WPB, was represented by stations 22-23, 37-44 and 59 (Fig. 6). This assemblage contained 19 genera and 33 diatom species; the relative abundance of A. nodulifera accounted for 37.02%-90.91% of the assemblage, and T. fasciculata, a eurythermal species, for 0-1.39%. Furthermore, warm-water species, A. marinus, accounted for 0-19.48%;A. africana for 0-17.53%; and H. cuneiformis for 0-17.33%. The eurythermal species, including T. excentrica and T. leptopus comprised 0-10.34% and 0-10%, respectively.
Sub-assemblage Ib was located in the northeastern WPB (Fig. 6), and contained 35 stations. There were fewer diatom species than in Ib than Ia; only 12 genera and 29 diatom species were identified in Ib. In sub-assemblage Ib, the warm-water species included A. nodulifera accounting for 33.33%-87.21%, H. cuneiformis var. rectus for 0-19.51%, and eurythermal T. fasciculata for 0-3.39%.
The higher percentage of warm-water and eurythermal species in sub-assemblage Ia than Ib indicated that sub-assemblage Ia, located at the margin of the North Equatorial Current (Fig. 1), was affected by the warm current flowing into this region. Azpeitia nodulifera, an oceanic planktonic species, was the most abundant species in assemblage I. This species is generally found in the surface sediments of the equatorial and tropical Pacific (Jousé et al., 1971) and mainly recorded in warm-water areas (Hasle and Syvertsen, 1997). However, A. nodulifera is also associated with eurythermal and coastal species T. fasciculata, T. leptopus and T. excentrica, suggesting that some other unknown environmental factors might also have a strong impact on its distribution (Jiang et al., 2004). Thus, we must be careful when using A. nodulifera as an environmental indicator in late Quaternary palaeoceanographic reconstruction of the WPB.
Diatom assemblage (Ⅱ) Hemidiscus cuneiformis var. ventricosus -Hemidiscus cuneiformis var. orbicularis -Thalassiosira anguste-lineata -Coscinodiscus argus (western WPB) (Figs. 5, 6)
Diatom assemblage Ⅱ was located in the western WPB, except in the area that contained subassemblage Ia. There were more diatom species in assemblage Ⅱ than in Ⅰa; it contained 22 genera and 40 diatom species. Hemidiscus cuneiformis var. ventricosus was the dominant species throughout assemblage Ⅱ, comprising 40% of the assemblage at station 50, which was south of the Central Ridge at a depth of 5 522 m. The second most dominant species was H. cuneiformis var. orbicularis, accounting for 0-7.14%. The eurythermal and coastal species mainly included T. anguste -lineata (0-21.43%), and C. argus (0-22.22%). Furthermore, the warm-water species R. tesselata accounted for 0-13.79%; and coastal species Cyclotella stylorum Brightwell, Cyclotella striata (Kütz.) Grunow, and Paralia sulcata (Ehr.) Kützing for 0-37.50%, 0-7.14% and 0-3.28%, respectively. Station 34 had the highest proportion of coastal species, with freshwater species Diploneis crabro Ehrenberg accounting for 0-12.50%.
The chief components of the assemblage were warm-water species, suggesting the North Equatorial Current influenced the western WPB (Fig. 1). High percentages of eurythermal and coastal species indicated that the coastal areas of the Philippines could influence the western WPB, while the presence of the freshwater species D. crabro showed that the assemblage environment was affected by freshwater masses.
Diatom assemblage (Ⅲ) Hemidiscus cuneiformis -Thalassiosira eccentrica -Thalassiosira leptopus -Coscinodiscus curvatulus (central and northern WPB) (Figs. 5, 6)
Assemblage Ⅲ could be divided into two subassemblages, Ⅲa and b, according to the PCA biplot of taxa and samples and on their spatial distribution in the WPB. Sub-assemblage Ⅲa was located between sub-assemblage Ib and assemblage Ⅱ (Fig. 6), and was represented by 15 stations (1, 5-6, 16-18, 26, 118-119, 126-128, 138, 149 and 166). There were fewer diatom species in assemblage Ⅲ than in the western WPB; only 12 genera and 24 diatom species were identified in the former. The warm-water species, H. cuneiformis, accounted for 0-31.76%; the eurythermal species T. leptopus for 0-9.52%, and C. curvatulus for 0-5.33%. In addition, the warm-water species A. africana accounted for 0-8.24%, Asterolampra marylandica Ehrenberg for 0-2.50%, R. tesselata for 0-2.08%, and eurythermal and coastal species P. sulcata for 0-5%.
Sub-assemblage Ⅲb was located in the northern WPB (Fig. 6). There were only 9 genera and 25 diatom species detected. The dominant species were mainly warm-water species, for example, H. cuneiformis (3.25%-36.36%) and A. marinus (0-29.27%). The eurythermal species included T. leptopus (0-4.76%) and T. eccentrica (0-4.76%).
A decrease in the warm-water and eurythermal species in assemblage Ⅲ, indicated that the effect of the North Equatorial Current in the north WPB weakened, and was separated by the Central Ridge and the Palau Ridge. The Ridge was generally greater than 4 000 m, except for station 128 (15°56′41.60″N, 134°36′18.30″E) at 3 381 m deep, suggesting there was less incursion of the waters from the southern region of the WPB.
Diatom assemblage (Ⅳ) Alveus marinus -Thalassiosira eccentrica -Azpeitia africana -Roperia tesselata (southwestern WPB) (Figs. 5, 6)
There were 11 genera and 24 diatom species identified in assemblage Ⅳ; warm-water species were dominant, of which A. marinus, A. africana, R. tesselata accounted for 0-31.58%, 0-28.57% and 0-10%, respectively. The eurythermal species, T. excentrica and Actinocyclus ehrenbergii, accounted for 0-14.29% and 0-11.11%, respectively. The dominant warm-water species included A. marylandica, Asteromphalus arachne (Breb.) Ralfs, Coscinodiscus denarius A. Schmidt and R. bergonii.
The diatom assemblage Ⅳ indicated that warm water currents influence this area. Just like assemblage Ⅱ, assemblage Ⅳ was located at the margin of the North Equatorial Current. Oceanic water had the strongest effect on the assemblage and there was little impact of coastal waters and freshwater on the region.
5 DISCUSSIONIn this study, the diatom distribution in surface sediments from the WPB was examined. Changes in the abundance and types of diatoms indicated environmental variations exist in the WPB.
5.1 effect of seabed topography in the WPBResults showed that the distribution of diatom abundance was closely related to environmental variables, including water depth and bathymetry (Fig. 2). Stations 64 and 114 were located on seamounts, at water depths of 3 953 and 4 311 m, respectively, and yielded the lowest values of diatom abundance at 2 frustules/g. Station 42 (5 159-m basin) had the highest diatom abundance, of 34 285 frustules/g. Furthermore, most stations with the diatom sub-assemblages Ⅰb and Ⅲb on the western Palau Ridge, were located on seamounts at water depths of < 5 000 m (Fig. 2). The average abundance was 553 and 152 frustules/g, and the abundance of Ⅲb was the lowest of all the assemblages. The flat abyssal plain is conducive to deposition, because it is located in a low-lying area. Sediments from the top of the seamounts were easily washed away by currents, particularly the bottom currents, and deposited on the slopes and basins.
On the western Central Ridge, most of the region was composed of basins and flat abyssal plains at water depths less than 5 400 m. Diatom diversity was rich and the average abundance reached 7 099 frustules/g in sub-assemblage Ⅰa, the highest value in the study area. The average and maximum abundance of warm-water species, A. nodulifera, reached 64.01% and 90.91% (Table 2), respectively. It was the most abundant and widely distributed species in sub-assemblage Ia, where warm water from the North Equatorial Current flows into this area. The average abundance in assemblage Ⅱ was 1 000 frustules/g, lower than Ⅰa. Moreover, subassemblages Ⅲa and assemblage Ⅳ, located between the Central Ridge and the Palau Ridge at water depths ranging from 3 381 to 6 200 m, had average abundances of 1 020 and 869 frustules/g, respectively. The dominance of eurythermal species in assemblage Ⅲ, such as T. leptopus, C. curvatulus and T. eccentrica, indicated that the composition of the assemblage was quite different from those in neighboring areas, because it was separated by the Central Ridge and Palau Ridge. Bathymetry influenced the warm current incursion on the region, which suggested that the ocean currents are to a large degree affected by topography, and finally in turn determine the diatom species and abundance in the areas.
5.2 effects of primary production and biogenic silica in the WPB 5.2.1 effects of primary production and nutrient in the WPBThe low-latitude biological pump is believed to have a strong influence on oceanic biological production (Turk et al., 2001). In the WPB, primary productivity is largely affected by photosynthetic organisms, especially diatoms. Ethmodiscus rex is referred to as a ‘giant’ diatom, simply because of its unusually large size which can reach up to 1.8 mm in diameter. The cells are strongly buoyant and can move up to 3.4 m/h (De Deckker and Gingele, 2002). This buoyancy strategy is linked to vertical migration between the surface for photosynthesis, and the nutricline for nutrients (Villareal et al., 2007). Open sea waters represent favorable environments for Ethmodiscus rex to contribute to new production (Strutton et al., 2011). The biological and chemical characteristics of Ethmodiscus rex were different from the diatom assemblages. In the WPB, Ethmodiscus rex was recorded in large numbers (Fig. 4), indicating that the environment in the WPB was beneficial for Ethmodiscus rex growth.
In the low tropical Pacific, the tropical trade winds transport surface waters westward, lifting the thermocline along the equator in the central and eastern Pacific, but deepening the thermocline in the western Pacific (Tang et al., 2013). The relaxation of trade winds allows warm water to flow back westward, which results in a shallower thermocline in the tropical western Pacific. Such shoaling of the thermocline produces an increase in primary production (Beaufort et al., 2001; Turk et al., 2001). Furthermore, deep-accumulated nutrients might become accessible to Ethmodiscus cells, with the sudden and abrupt strengthening in thermohaline circulation (Romero and Schmieder, 2006). A stable, well-stratified and nutrient-enriched water column supports dense Ethmodiscus rex populations (De Deckker and Gingele, 2002). Earlier work indicated that Ethmodiscus was distributed across the Pacific Ocean (Villareal et al., 2007). Belyayeva (1970) noted that Ethmodiscus increased in abundance westward in the Pacific Ocean, with the maximum value recorded near the equator and decreasing to the north. Therefore, the highest abundances of Ethmodiscus rex were mainly distributed in the tropical Pacific, including in the WPB. Ethmodiscus rex is a major contributor to primary production in this region.
5.2.2 Distribution of biogenic silica in the WPB sedimentsBiogenic silica in sediments is an important component in Si cycling of the oceans (Shibamoto and Harada, 2010). Some researchers estimate that diatom species constituted 50%-70% of primary production in the oceans, and at least 50% of the silica produced by diatoms in the euphotic zone dissolves in the upper 50-100 m (Heath, 1974; Brzezinski and Nelson, 1989; Nelson et al., 1991). Shibamoto and Harada (2010), working in the Philippine Sea and mid-Pacific Basin, noted that there was a high biogenic silica content and the sediment was diatomaceous ooze composed of Ethmodiscus fragments. In the WPB, Ethmodiscus fragments were discovered at 159 stations. The abundant Ethmodiscus indicated that the WPB had high biogenic silica content, which was consistent with a former study (Shibamoto and Harada, 2010). In the northwest of the Parece Vela Basin of the Eastern Philippine Sea, 32 stations were found to have Ethmodiscus fragments in the area, most of them distributed in the region between 17° and 20°N (Zhai, 2009). Therefore, in the tropical Pacific, biogenic silica content was widely distributed, covering an area from the WPB to the Parece Vela Basin and the mid-Pacific Basin.
The distribution of biogenic silica in surface sediments in the tropical Pacific was closely related to primary production. In general, primary production in the surface water was the main source of biogenic particles in the sediments, and their abundance was greatly influenced by current patterns (Zabel et al., 1998). The distribution of biogenic silica in the tropical Pacific was widespread, linked to the high nutrient levels. In subtropical regions, biogenic silica was less than 5% of all sediments, due to low primary production. The distribution pattern of biogenic silica in the surface sediment reflects the trend for BSi flux; large BSi fluxes were found in the tropical Pacific, and smaller fluxes were observed in subtropical region (Shibamoto and Harada, 2010). It provides evidence that the different distribution of biogenic silica reflects primary production in the surface water.
5.3 effects of hydrography and current pattern in the WPBTiny diatom cells are easily transported by currents and deposited in the sediment. The high durability of diatom frustules is reflected in the preservation in accumulated sediments. Sediments are inclined to be washed away by bottom currents and deposited on the basins, concentrating diatom frustules in abyssal plains (Smol and Stoermer, 2010; Wu et al., 2013). In the WPB, the Northern Equatorial Current (NEC) and the Kuroshio Current (KC) are the most important oceanographic currents (Li et al., 2010). The NEC runs westward and supplies a large amount of heat along the Philippine coast and then northwards with the KC. According to Lan et al. (2002), a relative abundance of tropical pelagic species higher than 20% can be considered the backbone of KC flow through the area. In the WPB, 7 tropical pelagic diatoms were abundant (Fig. 3); the relative abundances were higher than 20% at most stations, particularly at latitudes of 15°-16°N, where the value was generally greater than 40%. The distribution was fairly consistent with the Kuroshio Current.
Diatom distribution reflected changes in the environment. Sub-assemblage Ⅰa, assemblage Ⅱ and IV were located in the southern part of the WPB, which is influenced by the warm North Equatorial Current flowing into the areas (Fig. 6). The warmwater species were abundant at the margin of the NEC, indicating the diatom assemblages were consistent with the tropic Pacific environment. Subassemblage Ⅰb and assemblage Ⅲ lied in the northeastern WPB, where water from the southern NEC was less incursive, and thus less warm-water species were part of the assemblages. Therefore, we can use diatom assemblages to reconstruct late Quaternary palaeoceanography of the WPB.
In summary, seabed topography, primary production, nutrients, biogenic silica, hydrography and currents influenced the abundance and variety of diatoms in the WPB. The combined effect of these factors determined the distribution of diatoms in these areas.
6 CONCLUSION(1) Diatom abundance varied greatly on a spatial scale in the WPB. The absolute abundance of diatoms in the WPB ranged from 0 to 3.4×104 frustules/g, with the value tending to decrease from the western to the eastern WPB. We identified 68 species and varieties of diatoms from 26 genera.
(2) The seven tropical pelagic diatoms were Alveus marinus, Azpeitia africana, Azpeitia nodulifera, Hemidiscus cuneiformis, Hemidiscus cuneiformis var. ventricosus, Roperia tesselata, and Rhizosolenia bergonii. Their relative abundance was higher than 20%, and the distribution was consistent with the path of the Kuroshio Current.
(3) Ethmodiscus rex was distributed at 159 stations in the WPB; it composed the main part of the diatomaceous ooze, and hence is referred to as the Ethmodiscus ooze. Ethmodiscus rex was a major contributor to primary production in the region. In the tropical Pacific, biogenic silica content was widely distributed, and the different distribution of biogenic silica reflected primary production in the surface water.
(4) Four diatom assemblages were distinguished, representing different oceanographic conditions; their spatial distributions were closely related to the current patterns of the region. Diatom assemblage Ⅰ (western and northeastern WPB) represented an environment affected by a warm current, the North Equatorial Current, which flows into this region. Diatom assemblage Ⅱ (western WPB) was also affected by a warm current, and the coastal masses of the Philippines. Diatom assemblage Ⅲ (central and northern WPB) was separated by the Central Ridge and the Palau Ridge; the warm-water mass had less effect on this southern region. Diatom assemblage Ⅳ (southwestern WPB) was subject to the strongest effect from warm currents, and there was little impact of coastal and freshwater on the region. Diatom assemblages could therefore be important in late Quaternary palaeoceanographic reconstructions of the WPB.
7 ACKNOWLEDGEMENTWe thank SHI Xuefa, the First Institute of Oceanography, State Oceanic Administration, China, for providing surface sediment samples. We would like to acknowledge LI Chao, Xiamen University, Xiamen, China, for providing helpful advice. We are also grateful to the reviewers and editors for their critical reviews and helpful suggestions.
| Barlow M, Cullen H, Lyon B, 2002. Drought in central and southwest Asia:La Niña, the warm pool, and Indian Ocean precipitation. Journal of Climate, 15(7): 697–700. Doi: 10.1175/1520-0442(2002)015<0697:DICASA>2.0.CO;2 |
| Beaufort L, de Garidel-Thoron T, Mix A C, Pisias N G, 2001. ENSO-like forcing on oceanic primary production during the Late Pleistocene. Science, 293(5539): 2440–2444. Doi: 10.1126/science.293.5539.2440 |
| Belyayeva T V. 1968. Range and numbers of diatoms in the genus Ethmodiscus Castr. In:Pacific Plankton and Sediments.Oceanology Academy of Sciences, USSR. p.79-85. |
| Belyayeva T V. 1970. Abundance of Ethmodiscus in Pacific plankton. Oceanology Academy of Sciences, USSR.p.672-675. |
| Brzezinski M A, Nelson D M, 1989. Seasonal changes in the silicon cycle within a Gulf Stream warm-core ring. Deep Sea Research Part A. Oceanographic Research Papers, 36(7): 1009–1030. Doi: 10.1016/0198-0149(89)90075-7 |
| Chen M, Lan B B, Shen L N, Lan D Z, Fang Q, Qi H S, 2014. Characteristics of diatom distribution in the surface sediments of the Western Philippine Basin. Acta Micropalaeontologica Sinica, 31(4): 321–334. |
| Cheng Z D, Gao Y H, Mike D. 1996. Color Atlas of Diatoms.China Ocean Press, Beijing, China. p.1-120. (in Chinese) |
| Cheng Z D, Gao Y H. 2012. Marine Bacillariophyta Pennatae (I) Flora of China Seas. Science Press, Beijing, China.p.1-137. (in Chinese) |
| De Deckker P, Gingele F X, 2002. On the occurrence of the giant diatom Ethmodiscus rex in an 80-ka record from a deep-sea core, southeast of Sumatra, Indonesia:implications for tropical palaeoceanography. Marine Geology, 183(1-4): 31–43. Doi: 10.1016/S0025-3227(01)00252-3 |
| Gardner J V, Burckle L H, 1975. Upper Pleistocene Ethmodiscus rex oozes from the eastern equatorial Atlantic. Micropaleontology, 21(2): 236–242. Doi: 10.2307/1485026 |
| Guo Y J, Qian S B. 2003. Marine Bacillariophyta Centricae Flora of China Seas. Science Press, Beijing, China. p.1-493. (in Chinese) |
| Håkansson H. 1984. The recent diatom succession of Lake Havgårdssjön, South Sweden. In:Mann D G ed.Proceedings of the 7th International Diatom Symposium.Otto Koeltz Science Publishers, Koenigstein. p.411-429. |
| Hasle G R, Syvertsen E E. 1997. Marine diatoms. In:Tomas C R ed. Identifying Marine Phytoplankton. Academic Press, California. p.5-85. |
| Heath G R. 1974. Dissolved silica and deep-sea sediments. In:Hay W W ed. Studies in Paleo-Oceanography. Society of Economic Paleontologists and Mineralogists, Tulsa, Okla.p.77-93. |
| Hendey N I. 1964. An Introductory Account of the Smaller Algae of British Coastal Waters. Fishery Investigations, Series IV Part V. Bacillariophyceae (Diatoms). H M S O.London Ottokoelz Science Publishers, West Germany. |
| Imbrie J, Kipp N G. 1971. A new micropaleontological method for quantitative paleoclimatology:application to a late Pleistocene Caribbean core. In:Turekian K K ed. Late Cenozoic Glacial Ages. Yale University Press, New Haven, CT. p.71-181. |
| Isse T, Shiobara H, Montagner J P, Sugiokac H, Ito A, Shitoc A, Kanazawa T, Yoshizawa K, 2010. Anisotropic structures of the upper mantle beneath the northern Philippine Sea region from Rayleigh and Love wave tomography. Physics of the Earth and Planetary Interiors, 183(1-2): 33–43. Doi: 10.1016/j.pepi.2010.04.006 |
| Jiang H, Eiríksson J, Schulz M, Knudsen K L, Seidenkrantz M S, 2005. Evidence for solar forcing of sea-surface temperature on the North Icelandic shelf during the late Holocene. Geology, 33(1): 73–76. Doi: 10.1130/G21130.1 |
| Jiang H, Seidenkrantz M S, Knudsen K L, Eirıksson J, 2001. Diatom surface sediment assemblages around Iceland and their relationships to oceanic environmental variables. Marine Micropaleontology, 41(1-2): 73–96. Doi: 10.1016/S0377-8398(00)00053-0 |
| Jiang H, Zheng Y L, Ran L H, Seidenkrantz M S, 2004. Diatoms from the surface sediments of the South China Sea and their relationships to modern hydrography. Marine Micropaleontology, 53(3-4): 279–292. Doi: 10.1016/j.marmicro.2004.06.005 |
| Jin D X, Chen J H, Huang K G. 1965. Planktonic Diatoms of China Seas. Shanghai Science and Technology Press, Shanghai, China. p.1-229. (in Chinese) |
| Jin D X, Cheng Z D, Lin J M et al. 1982. Benthic Diatoms of China Seas. China Ocean Press, Beijing, China. p.1-323.(in Chinese) |
| Jin D X, Cheng Z D, Liu S C et al. 1991. Benthic Diatoms of China Seas. China Ocean Press, Beijing, China. p.1-437.(in Chinese) |
| Jousé A P, Kozlova O G, Muhina V V. 1971. Distribution of diatoms in the surface layer of sediment from the Pacific Ocean. In:Funnell B M, Riedel W R eds. The Micropalaeontology of Oceans. Cambridge University Press, London. p.263-269. |
| Karpuz N K, Schrader H, 1990. Surface sediment diatom distribution and Holocene paleotemperature variations in the Greenland, Iceland and Norwegian Sea. Paleoceanography, 5(4): 557–580. Doi: 10.1029/PA005i004p00557 |
| Kemp A E S, Pearce R B, Grigorov I, Rance J, Lange C B, Quilty P, Salter I. 2006. Production of giant marine diatoms and their export at oceanic frontal zones:implications for Si and C flux from stratified oceans.Global Biogeochemical Cycles, 20(4):GB4S04, http://dx.doi.org/10.1029/2006GB002698. |
| Kim J H, Rimbu N, Lorenz S J, Lohmann G, Nam S I, Schouten S, Rühlemann C, Schneider R R, 2004. North Pacific and North Atlantic sea-surface temperature variability during the Holocene. Quaternary Science Reviews, 23(20-22): 2141–2154. Doi: 10.1016/j.quascirev.2004.08.010 |
| Lan D Z, Fang Q, Liao L Z, 2002. Surficial sedimentary diatoms in Okinawa Trough and its response to Kuroshio Current. Journal of Oceanography in Taiwan Strait, 21(1): 1–5. |
| Li T G, Zhao J T, Sun R T, Chang F M, Sun H J, 2010. The variation of upper ocean structure and paleoproductivity in the Kuroshio source region during the last 200 kyr. Marine Micropaleontology, 75(1-4): 50–61. Doi: 10.1016/j.marmicro.2010.02.005 |
| Liu Z F, Zhao Y L, Colin C, Siringan F P, Wu Q, 2009. Chemical weathering in Luzon, Philippines from clay mineralogy and major-element geochemistry of river sediments. Applied Geochemistry, 24(11): 2195–2205. Doi: 10.1016/j.apgeochem.2009.09.025 |
| Lopes C, Mix A C, Abrantes F, 2006. Diatoms in northeast Pacific surface sediments as paleoceanographic proxies. Marine Micropaleontology, 60(1): 45–65. Doi: 10.1016/j.marmicro.2006.02.010 |
| Meyers G, Donguy J R, Reed R K, 1986. Evaporative cooling of the western equatorial Pacific Ocean by anomalous winds. Nature, 323(6088): 523–526. Doi: 10.1038/323523a0 |
| Muhina V V. 1971. Problems of diatom and silicoflagellate Quaternary stratigraphy in the equatorial Pacific Ocean.In:Funnell B M, Riedel W R eds. The Micropalaeontology of Oceans. Cambridge University Press, London. p.423-431. |
| Nelson D M, Ahern J A, Herlihy L J, 1991. Cycling of biogenic silica within the upper water column of the Ross Sea. Marine Chemistry, 35(1-4): 461–476. Doi: 10.1016/S0304-4203(09)90037-8 |
| Pokras E M, Molfino B, 1986. Oceanographic control of diatom abundances and species distributions in surface sediments of the Tropical and Southeast Atlantic. Marine Micropaleontology, 10(1-3): 165–188. Doi: 10.1016/0377-8398(86)90028-9 |
| Qi Y Z, Li J Y. 2004. Flora Algarum Sinicarum Aquae Dulcis (Tomus X). Science Press, Beijing, China. (in Chinese) |
| Qiu B, Lukas R, 1996. Seasonal and interannual variability of the North Equatorial Current, the Mindanao Current, and the Kuroshio along the Pacific western boundary. Journal of Geophysical Research, 101(C5): 12315–12330. Doi: 10.1029/95JC03204 |
| Qiu X H, Li T G, Chang F M, Nan Q Y, Xiong Z F, Sun H J, 2014. Sea surface temperature and salinity reconstruction based on stable isotopes and Mg/Ca of planktonic foraminifera in the western Pacific Warm Pool during the last 155 ka. Chinese Journal of Oceanology and Limnology, 32(1): 187–200. Doi: 10.1007/s00343-014-3073-y |
| Qu T D, Lukas R, 2003. The bifurcation of the North Equatorial current in the Pacific. Journal of Physical Oceanography, 33(1): 5–18. Doi: 10.1175/1520-0485(2003)033<0005:TBOTNE>2.0.CO;2 |
| Romero O, Schmieder F, 2006. Occurrence of thick Ethmodiscus oozes associated with a terminal MidPleistocene Transition event in the oligotrophic subtropical South Atlantic. Palaeogeography, Palaeoclimatology, Palaeoecology, 235(4): 321–329. Doi: 10.1016/j.palaeo.2005.10.026 |
| Round F E, Crawford R M, Mann D G. 1990. The Diatoms:Biology and Morphology of the Genera. Cambridge University Press, Cambridge. p.1-760. |
| Sancetta C, 1982. Distribution of diatom species in surface sediments of the Bering and Okhotsk seas. Micropaleontology, 28(3): 221–257. Doi: 10.2307/1485181 |
| Schlitzer R, 2007. Assimilation of radiocarbon and chlorofluorocarbon data to constrain deep and bottom water transports in the world ocean. Journal of Physical Oceanography, 37(2): 259–276. Doi: 10.1175/JPO3011.1 |
| Scott R B, Kroenke L, Zakariadze G et al. 1981. Evolution of the south Philippine Sea:deep Sea drilling project Leg 59 results. In:Kroenke L, Scott R B, Balshaw K et al eds.Initial Reports of the Deep Sea Drilling Project, vol. 59.U.S. Govt. Printing Office, Washington. p.803-815. |
| Sdrolias M, Roest W R, Müller R D, 2004. An expression of Philippine Sea plate rotation:the Parece Vela and Shikoku Basins. Tectonophysics, 394(1-2): 69–86. Doi: 10.1016/j.tecto.2004.07.061 |
| Shibamoto Y, Harada K, 2010. Silicon flux and distribution of biogenic silica in deep-sea sediments in the western North Pacific Ocean. Deep Sea Research Part I:Oceanographic Research Papers, 57(2): 163–174. Doi: 10.1016/j.dsr.2009.10.009 |
| Smol J P, Stoermer E F. 2010. The Diatoms:Applications for the Environmental and Earth Sciences. 2nd edn. Cambridge University Press, Cambridge, United Kingdom. 469p. |
| Stabell B, 1986. Variations of diatom flux in the eastern equatorial Atlantic during the last 400, 000 years ("Meteor" cores 13519 and 13521). Marine Geology, 72(3-4): 305–323. Doi: 10.1016/0025-3227(86)90125-8 |
| Strutton P G, Palacz A P, Dugdale R C, Chai F, Marchi A, Parker A E, Hogue V, Wilkerson F P, 2011. The impact of equatorial Pacific tropical instability waves on hydrography and nutrients:2004-2005. Deep Sea Research Part Ⅱ:Topical Studies in Oceanography, 58(3-4): 284–295. Doi: 10.1016/j.dsr2.2010.08.015 |
| Sun D Z, Fasullo J, Zhang T, Roubicek A, 2003. On the radiative and dynamical feedbacks over the Equatorial Pacific Cold Tongue. Journal of Climate, 16(14): 2425–2432. Doi: 10.1175/2786.1 |
| Sun H J, Li T G, Sun R T, et al, 2011. Calcareous nannofossil bioevents and microtektite stratigraphy in the Western Philippine Sea during the Quaternary. Chinese Science Bulletin, 56(25): 2732–2738. Doi: 10.1007/s11434-011-4603-z |
| Tang Z, Li T G, Chang F M, Nan Q Y, Li Q, 2013. Paleoproductivity evolution in the West Philippine Sea during the last 700 ka. Chinese Journal of Oceanology and Limnology, 31(2): 435–444. Doi: 10.1007/s00343-013-2117-z |
| Taylor B, Goodliffe A M. 2004. The West Philippine basin and the initiation of subduction, revisited. Geophysical Research Letters, 31(12), http://dx.doi.org/10.1029/2004GL020136. |
| Ter Braak C J F, Prentice I C, 1988. A theory of gradient analysis. Advance in Ecological Research, 18: 271–317. Doi: 10.1016/S0065-2504(08)60183-X |
| Ter Braak C J F, Smilauer P. 2002. Canoco 4.5. Biometrics.Wageningen University and Research Centre, Wageningen, The Netherlands. p.1-500. |
| Turk D, McPhaden M J, Busalacchi A J, Lewis M R, 2001. Remotely sensed biological production in the equatorial pacific. Science, 293(5529): 471–474. Doi: 10.1126/science.1056449 |
| Villareal T A, Joseph L, Brzezinski M A, Shipe R F, Lipschultz F, Altabet M A, 1999. Biological and chemical characteristics of the giant diatom Ethmodiscus (Bacillariophyceae) in the central North Pacific gyre. Journal of Phycology, 35(5): 896–902. Doi: 10.1046/j.1529-8817.1999.3550896.x |
| Villareal T A, Mckay R M L, Al-Rshaidat M M D, Boyanapalli R, Sherrell R M, 2007. Compositional and fluorescence characteristics of the giant diatom Ethmodiscus along a 3000 km transect (28°N) in the central North Pacific gyre. Deep Sea Research Part I:Oceanographic Research Papers, 54(8): 1273–1288. Doi: 10.1016/j.dsr.2007.04.018 |
| Villareal T A, 1993. Abundance of the giant diatom Ethmodiscus in the southwest Atlantic Ocean and central Pacific gyre. Diatom Research, 8(1): 171–177. Doi: 10.1080/0269249X.1993.9705248 |
| Wan S M, Yu Z J, Clift P D, Sun H J, Li A C, Li T G, 2012. History of Asian eolian input to the West Philippine Sea over the last one million years. Palaeogeography, Palaeoclimatology, Palaeoecology, 326-328: 152–159. Doi: 10.1016/j.palaeo.2012.02.015 |
| Wang Q Y, Hu D X, 2006. Bifurcation of the North Equatorial current derived from altimetry in the Pacific ocean. Journal of Hydrodynamics, Ser B, 18(5): 620–626. Doi: 10.1016/S1001-6058(06)60144-3 |
| Wu R, Gao Y H, Fang Q, Chen C P, Lan B B, Sun L, Lan D Z, 2013. Diatom assemblages in surface sediments from the South China Sea as environmental indicators. Chinese Journal of Oceanology and Limnology, 31(1): 31–45. Doi: 10.1007/s00343-013-1181-8 |
| Xu Z K, Li T G, Wan S M, Nan Q Y, Li A C, Chang F M, Jiang F Q, Tang Z, 2012. Evolution of East Asian monsoon:clay mineral evidence in the western Philippine Sea over the past 700 kyr. Journal of Asian Earth Sciences, 60: 188–196. Doi: 10.1016/j.jseaes.2012.08.018 |
| Yamasaki M, Sasaki A, Oda M, Domitsu H, 2008. Western equatorial Pacific planktic foraminiferal fluxes and assemblages during a La Niña year (1999). Marine Micropaleontology, 66(3-4): 304–319. Doi: 10.1016/j.marmicro.2007.10.006 |
| Yan Q S, Shi X F, Wang K S, Ren X W, Jiang X L, 2007. Provinces and material provenance of light detritus in the surficial sediments from the Western Philippine Sea. Geological Review, 53(6): 765–773. |
| Zabel M, Dahmke A, Schulz H D, 1998. Regional distribution of diffusive phosphate and silicate fluxes through the sediment-water interface:the eastern South Atlantic. Deep Sea Research Part I:Oceanographic Research Papers, 45(2-3): 277–300. Doi: 10.1016/S0967-0637(97)00073-3 |
| Zhai B, Li T G, Chang F M, Cao Q Y, 2009. Vast laminated diatom mat deposits from the west low-latitude Pacific Ocean in the last glacial period. Chinese Science Bulletin, 54(23): 4529–4533. |
| Zhai B, Li T G, Xiong Z F, Li J, 2012. Diatom records inferred from the diatom mat deposits from low-latitude Western Pacific in the last glacial period. Journal of Tropical Oceanography, 31(4): 75–82. |
| Zhai B. 2009. The Temporal and Spatial Distribution and Diatom Taxa of Vast Laminated Diatom Mat Deposits from the West Low-Latitude Pacific Ocean. University of Chinese Academy of Sciences, Qingdao. 119p. (in Chinese with English abstract) |
 2017, Vol. 35
2017, Vol. 35