Institute of Oceanology, Chinese Academy of Sciences
Article Information
- LIAO Yibo(廖一波), SHOU Lu(寿鹿), TANG Yanbin(汤雁滨), ZENG Jiangning(曾江宁), GAO Aigen(高爱根), CHEN Quanzhen(陈全震), YAN Xiaojun(严小军)
- Macrobenthic assemblages of the Changjiang River estuary (Yangtze River, China) and adjacent continental shelf relative to mild summer hypoxia
- Chinese Journal of Oceanology and Limnology, 35(3): 481-488
- http://dx.doi.org/10.1007/s00343-017-5285-4
Article History
- Received Oct. 21, 2015
- accepted in principle Apr. 5, 2015
2 Key Laboratory of Applied Marine Biotechnology, Ministry of Education, Marine College of Ningbo University, Ningbo 315211, China
Dissolved oxygen (DO), which is essential for the metabolism of most marine organisms, has declined in more than 400 systems, leading to hypoxia and anoxia (Diaz and Rosenberg, 2008; Zhang et al., 2010). There are well-known hypoxic areas as the Gulf of Mexico (Rabalais et al., 2007), the Black Sea (Oguz et al., 2000), the Baltic Sea (Conley et al., 2009), and Chesapeake Bay (Kemp et al., 2005), as well as the Changjiang estuary in China (Li et al., 2002). Annual summertime hypoxia is the most common low DO event globally (Diaz and Rosenberg, 1995). The two principal factors that lead to the development of hypoxia, and that sometimes lead to anoxia, are water column stratification and decomposition of organic matter in the bottom water (Diaz, 2001; Li et al., 2002; Diaz and Rosenberg, 2008; Zhu et al., 2011). Hypoxia can have direct and indirect effects on the function and survival of organisms. Benthic organisms are particularly vulnerable to coastal hypoxia because they live farthest from the atmospheric oxygen supply and because coastal sediments tend to be depleted in oxygen relative to the overlying water column (Vaquer-Sunyer and Duarte, 2008).
Hypoxia is generally defined as DO concentration below 2.00 mg/L (Dauer et al., 1992; Rabalais et al., 2002). However, a number of behavioral and sublethal effects are found in benthic organisms at DO concentrations between 2.86 (2 mL/L) and 2.00 mg/L, such as reduced growth, physiological stresses, forced migration, and disruption of life cycles (Tyson and Pearson, 1991; Rabalais et al., 2002; Vaquer-Sunyer and Duarte, 2008; Sturdivant et al., 2014). Therefore, we define normoxia as DO concentrations >2.86 mg/L and additionally divide hypoxia into two categories of mild hypoxia (2.86–2.00 mg/L) and hypoxia (≤2.00 mg/L).
The Changjiang (Yangtze) River estuary is situated on the east coast of China and flows into the East China Sea (ECS). The Changjiang is the largest river in China and is ranked third in the world in terms of discharge, stretching for 6 200 km and draining an area of 1.8×106km2 (Rabouille et al., 2008). Annually, huge amounts of freshwater (~924×109 m3) and sediment (~486×106t) are conveyed into the northwest corner of the ECS (Rabouille et al., 2008). Like many other rivers in the world, the Changjiang suffers from eutrophication (Zhang et al., 1999), and dramatic oxygen depletion in the bottom waters off the estuary has occurred in recent decades (Li et al., 2002). Low DO concentration (2.57 mg/L) in the bottom waters off the Changjiang estuary was first reported in August 1959 (Gu, 1980; Li et al., 2002), and became more severe in recent decades, with the area of the hypoxic zone increasing from 1 900 km2 in 1959 to 15 400 km2 in 2006 (Zhu et al., 2011).
The extreme oxygen minimum zone (OMZ; 1.00 mg/L) had been observed in August 1999 off the Changjiang estuary (122°59′E, 30°51′N) (Li et al., 2002). However, the minimum oxygen concentration observed in this study was 2.02 mg/L (>2.00 mg/L), which we defined as mild hypoxia. In the present study, we analyzed environmental parameters and macrobenthos in the Changjiang estuary and the adjacent continental shelf. The objectives of our study were: (1) to identify the spatial patterns of the macrobenthic communities among the three zones: the estuarine zone (EZ), the mildly hypoxic zone (MHZ), and the normoxic zone (NZ) in the continental shelf; (2) to assess the relationship of macrobenthic communities with the physical and chemical parameters of DO concentration, temperature, salinity, and nutrients; and (3) to determine if mild hypoxia has significant impacts on marine benthic communities. This study improves our understanding of the levels of hypoxia that cause different impacts on marine benthic communities and provides information that will effectively allow the conservation coastal biodiversity as hypoxia continues to rise as a threat to coastal ecosystems.
2 MATERIAL AND METHOD 2.1 SamplingThirty-nine stations distributed along the estuarine gradient of the Changjiang estuary and adjacent continental shelf were sampled for macrobenthos and associated bottom-water characteristics in August 2006 (Fig. 1). These stations were then divided into three study zones according to the distribution and oxygen concentrations of each station: the estuarine zone (EZ; 11 stations), the mildly hypoxic zone in the continental shelf (MHZ; 16 stations), and the normoxic zone in the continental shelf (NZ; 12 stations).

|
| Figure 1 Location of the sampling stations in the Changjiang estuary and adjacent continental shelf Solid triangle: mildly hypoxic zone stations in continental shelf (MHZ); hollow triangle: normoxic zone stations in continental shelf (NZ); dot: estuarine zone stations (EZ). |
Three replicates were taken at each station using a van Veen grab sampler (0.1 m2) and grab contents were sieved using a 500-μm mesh and preserved with 5% buffered formalin. Macrofauna were sorted and identified to the lowest practical taxonomic level, counted, and weighed. Major taxa, including Polychaeta, Mollusca, Crustacea, Echinodermata, and a group of miscellaneous phyla, hereafter called "others" (e.g., Chordata, Coelentera, Nemertea, and Sipuncula) were found.
2.1.2 Environmental variablesFor each station, we also recorded or calculated the following abiotic environmental variables of the bottom water column: temperature, salinity, dissolved oxygen (DO), nitrate (NO3), nitrite (NO2), ammonium (NH4), and soluble reactive phosphate (PO4). Temperature and salinity were recorded using a conductivity/temperature/depth profiler (6 Hz XR-620 CTD, Richard Brancker Research Ltd., Canada). Bottom seawater (1 m above the seafloor) was collected in a 5-L Nisken bottle at each station. First, DO was extracted and measured using the Winkler method (Knap et al., 1996). The dissolved nutrients (NO3, NO2, NH4, and PO4) were then filtered through a 40-μm nylon pre-filter and then by pressure filtration onto 25 mm Whatman GF/F glass-fiber filters (precombusted for 4 h at 400℃). The filtrate was analyzed by segmented flow analysis (SFA) (Hansen and Koroleff, 1983).
2.2 Statistical analysesStatistical differences among zones were tested by a nonparametric ANOVA (Kruskal-Wallis test, KW) followed by stepwise step-down comparisons for physical and chemical measures (temperature, salinity, DO, NO3+NO2, NH4, and PO4) and macrobenthic measures (species richness (number of taxa, S), density, biomass, and Shannon diversity index (H′)).
The density (individuals/m2) of taxa was transformed with the fourth root, prior to performing cluster analysis and non-metric multidimensional scaling (nMDS) using the Bray-Curtis similarity index (Bray and Curtis, 1957). A one-way crossed analysis of similarity (ANOSIM) procedure using the sampling zone as a factor was used to determine differences in the macrobenthic communities. Data were fourth-root transformed to reduce the influence of numerically dominant taxa. The SIMPER ("similarity percentages"; Clarke, 1993) routine was used to identify the taxa that made the greatest contribution to differences among the macrobenthic communities.
Relationships of the macrobenthic communities with environmental variables in the Changjiang estuary and adjacent continental shelf were analyzed using canonical correlation analysis (CCA) (ter Braak and Smilauer, 1998). Data were log10(x+1) transformed before CCA analysis. Monte Carlo permutation tests were used to test the significance of the ordination axes.
All statistical analyses were performed by using STATISTICA 6 (StatSoft, Inc., Tulsa, Oklahoma, USA), PRIME R 6 (PRIMER-E Ltd., Plymouth, UK), and CANOCO 4.5 (Microcomputer Power, Ithaca, NY, USA) software.
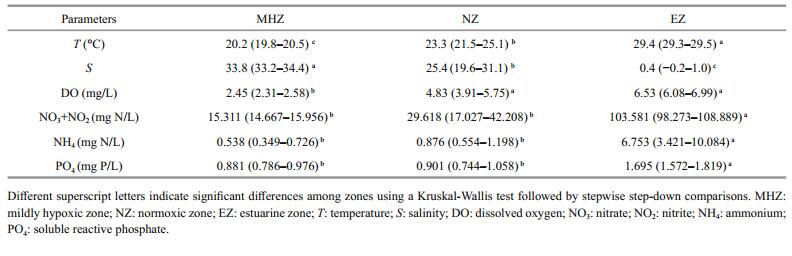
3 RESULT 3.1 Environmental parametersTable 1 shows physical and chemical variables of the bottom water column in the Changjiang estuary and adjacent continental shelf. The DO concentrations of the MHZ were significantly lower than the NZ and the EZ (Table 1; KW, P < 0.01). No hypoxic conditions (DO≤2.00 mg/L) were observed in this study, but mild hypoxia (2.86–2.00 mg/L) was found at 57% (16 of 28) of the sites in the continental shelf zone. The average temperature of the MHZ was 20.2℃, which was lower than that in the NZ and the EZ (KW, P < 0.01). Average salinity of the EZ was only 0.4 lower than the MHZ and the NZ (KW, P < 0.01). However, the concentrations of NO3+NO2, NH4, and PO4 of the EZ were significantly higher than the MHZ and the NZ. Results of stepwise comparisons showed that there was no significant difference in nutrients between the MHZ and the NZ (Table 1).
|
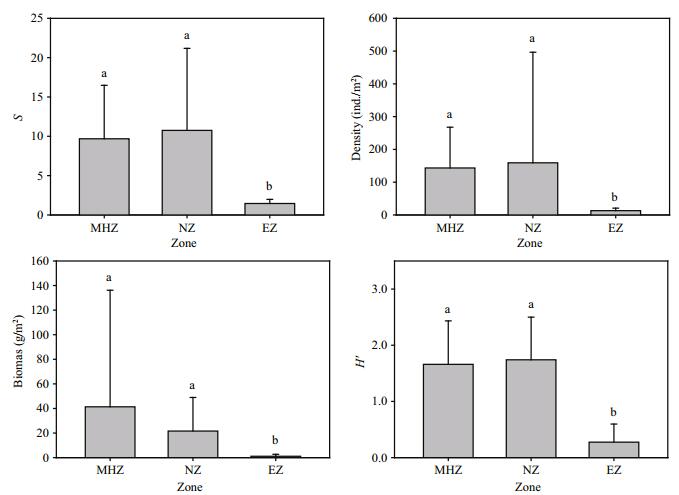
Figure 2 plots the spatial variations of S, density, biomass, and Shannon diversity index among the MHZ, NZ, and EZ. These biological parameters were significantly higher in the MHZ and NZ than in the EZ. However, there was no significant difference in the parameters between the MHZ and the NZ (KW, P>0.05, Fig. 2).
|
| Figure 2 Mean and standard deviation of species richness (S), density, biomass, and Shannon diversity index (H′) of the macrobenthic community in the three zones of the Changjiang estuary and adjacent continental shelf MHZ: mildly hypoxic zone; NZ: normoxic zone; EZ: estuarine zone. |
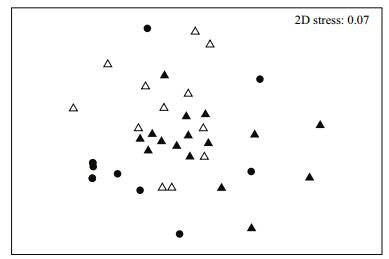
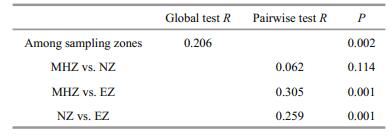
MDS analysis revealed that the samples in EZ were distinct from those in the MHZ and NZ, while samples from the MHZ and NZ were scattered in the MDS plot, with high similarity in macrobenthic community composition (Fig. 3). Results of one-way ANOSIM revealed significant differences in macrobenthic community composition among the three zones (Global R=0.206, P=0.002). The pairwise tests showed that the macrobenthic community composition of the EZ was significantly different from the MHZ (pairwise test R=0.305, P=0.001) and the NZ (pairwise test R=0.259, P=0.001). There was no significant difference in macrobenthic communities between the MHZ and the NZ (pairwise test R=0.062, P=0.114) (Table 2).
|
| Figure 3 Non-metric MDS plots of the benthic community structure at three zones in August 2006 at the Changjiang estuary and adjacent continental shelf Solid triangle: mildly hypoxic zone stations; hollow triangle: normoxic zone stations; dot: estuarine zone stations. |
|
The species making the greatest contribution to dissimilarity among the MHZ, NZ, and EZ were identified by means of a SIMPER analysis (Table 3). The community observed in the MHZ was markedly distinguished from the community in the NZ, mainly because of a lower abundance of Maldane sp. and higher abundances of Capitella capitata, Heterospio sinica, and Sternaspis scutata. The community observed in the MHZ was markedly distinguished from the EZ, mainly because of the higher abundance of Maldane sp. and Capitella capitata, and presence of Heterospio sinica, Sternaspis scutata, and Nucula sp. The community observed in the NZ was markedly distinguished from the community in the EZ, mainly due to the higher abundance of Maldane sp. and Capitella capitata, the presence of Sternaspis scutata, and the absence of Aglaophamus dibranchis. Taxa that included small, typically opportunistic polychaetes such as Maldane sp. and Capitella capitata contributed the most to the dissimilarity between the different zones (Table 3).
CCA analysis indicated that the environmental variables significantly explained the macrobenthic community in the first canonical axis (Monte Carlo test: F=1.940, P=0.036), but did not significantly explain all of the canonical axes (Monte Carlo test: F=1.096, P=0.226). All four canonical axes together explained 86.9% of the variability, with the first and second axes contributing 33.5% and 19.8%, respectively (Table 4). The first ordination axis reflected a gradient largely related to temperature, DO, and NO3+NO2 (in that order of importance) at the positive end of the axis and salinity at the negative end (Fig. 4). A clear discrimination between taxa characteristic of the typically estuarine zone (related to high nutrients, high temperature, high DO concentration, and low salinity [e.g., Aglaophamus dibranchis]) and taxa characteristic of the continental shelf zone (related to low nutrients, low temperature, low DO concentration, and high salinity [e.g., Maldane sp., Capitella capitata, Heterospio sinica, Sternaspis scutata, and Nucula sp.]) was observed in the ordination biplot (Fig. 4). With respect to DO, Ampharete acutifrons, Heterospio sinica, Laonice cirrata, and Lumbrineris shiinoi preferred lower DO concentrations. However, these species were arranged towards the center of the plot and comprised none of the species preferring extremely low DO concentrations (Fig. 4).

|
| Figure 4 Canonical correlation analysis (CCA) ordination diagrams for macrobenthos taxa abundance data The environmental variables are shown as arrows and taxa are shown as triangles. For results of the CCA analysis see Table 4. T: temperature; S: salinity; DO: dissolved oxygen; NO3: nitrate; NO2: nitrite; NH4: ammonium; PO4: soluble reactive phosphate. Only 14 species are displayed in this figure based on the SIMPE R analysis (Table 3). Maldane: Maldane sp.; Cap cap: Capitella capitata; Het sin: Heterospio sinica; Ste scu: Sternaspis scutata; Nucula: Nucula sp.; Hem lon: Hemigrapsus longitarsis; Lum shi: Lumbrineris shiinoi; Amp acu: Ampharete acutifrons; Ogy ori: Ogyrides orientalis; Lao cir: Laonice cirrata; Agl dib: Aglaophamus dibranchis; Nas siq: Nassarius siquijorensis; Barnea: Barnea sp.; Nas var: Nassarius variciferus. |

|
The mechanism of oxygen depletion off the Changjiang estuary has been receiving more and more attention from both scientists and managers (Li et al., 2002; Rabouille et al., 2008; Wei et al., 2015). The development of summer hypoxia in this location is related not only to stratification and input of suspended particulate matter, but also to the inflow of Taiwan Warm Current water and the bottom topography (Wang, 2009). Generally, severe hypoxia takes place in August, but after September much of the hypoxia has disappeared (Zhu et al., 2011). Interannual variation of the hypoxic area and extent off the Changjiang estuary has been unstable and irregular (Rabouille et al., 2008; Zhu et al., 2011). The most extreme DO concentration (1.00 mg/L) was observed there (122°59′E, 30°51′N) in August 1999 (Li et al., 2002). However, the minimum DO concentration (2.02 mg/L) of the bottom water observed in this study was higher than 2.00 mg/L (the value generally defined as the upper limit of hypoxia (Dauer et al., 1992; Rabalais et al., 2002). This provided us with the opportunity to study the effects of mild hypoxia on benthic organisms.
The abundance of macrobenthos and the DO in the hypoxic zone are closely related to the duration of hypoxia. Nilsson and Rosenberg (2000) found that, after 10 months of hypoxia in Gullmarsfjord (on the Swedish west coast), benthic community successional stages declined from equilibrium to virtually azoic conditions. Rosenberg et al. (2001) reported that, because of long-term hypoxia, there were very few benthic organisms in the sediment of Koljöfjord Gulf, Sweden except for two species of polychaetes (Pseudopolydora antennata and C. capitata). Dauer et al. (1992) found that hypoxia can alter macrobenthic community composition, and also causes lower species diversity, lower biomass, and a decrease in buried macrobenthos. The density and biomass of opportunistic species (e.g., euryhaline annelids) increased, while other later successional species (e.g., long-lived bivalves and maldanid polychaetes) decreased in dominance. The results presented here show that the macrobenthic communities at mildly hypoxic stations were not significantly different from those at normoxic stations in the continental shelf area. However, SIMPE R analysis showed that the macrobenthic communities at mildly hypoxic stations were characterized by a higher dominance of opportunistic species (e.g., C. capitata, H. sinica, and S. scutata). Nilsson and Rosenberg (2000) posited that the minimum DO content needed for estuarine macrobenthos to live was 0.98 mg/L. The DO concentration at mildly hypoxic stations in this study was in the range of 2.02–2.83 mg/L, a level which may not cause direct physical harm or lead to mortality of some of benthic organisms. However, different taxa probably reflect broad differences in adaptations to tolerate low oxygen conditions. Crustaceans, which showed the highest median lethal oxygen concentration (LC50) and the shortest median lethal time (LT50), were the most sensitive organisms to hypoxia (Vaquer-Sunyer and Duarte, 2008). Other benthic organisms (e.g., polychaetes, bivalves, and priapulids) can change their behavior or metabolism to avoid effects from hypoxia, such as leaving their burrows or tubes to move to the sediment surface or reducing their burial depth (Nilsson and Rosenberg, 1994).
There are many experimental studies on mortality, behavior, and metabolic rates of benthic organisms caused by reduced oxygen concentrations (Gray et al., 2002). However, effects of hypoxia in the field are complex. It is, therefore, important to understand how aquatic organisms are affected by fluctuating low oxygen concentrations in situ. Furthermore, differences in the cause of hypoxia can significantly affect macrobenthos abundance and community structure (Long and Seitz, 2009). The hypoxia off the Changjiang estuary is created by organic detritus decay (Wei et al., 2007). Maintenance of hypoxia results from a large density stratification caused by the significant salinity difference between the freshwater discharge plume and the salt water from the Taiwan Warm Current (Li et al., 2002; Wei et al., 2007). The stratification restricted the vertical supplementation of nutrients from surface to bottom waters. Results showed that, in addition to DO concentration, lower temperature and nutrient concentrations were also found in the bottom waters of hypoxic areas. We suggest that the occurrence of hypoxia, accompanied by other environmental factors, together contribute to benthic faunal changes. Results of CCA analysis showed that DO concentration, as well as temperature, salinity, and nutrient concentrations, were markedly correlated with the community composition of macrobenthos. Seitz et al. (2009) reported that density, biomass, and diversity of macrobenthic community were significantly and negatively correlated with water depth and positively correlated with the DO level. Kodama et al. (2012) demonstrated that the disturbance in the macrofauna correlated with organic enrichment in the sediment and bottom-water hypoxia. It is thus clear that the effects on the macrobenthic communities that are produced are not caused by a single factor, but are the interaction of a number of different factors in the mildly hypoxic area. It is not just reduced DO concentration that leads to the observed effects, but also the differences in environmental variables such as temperature, salinity, and nutrient concentrations, which are a result of stratification.
5 CONCLUSIONHypoxia has been a major feature of the Changjiang estuary and has had negative effects on its ecosystems. In recent decades, an increase in the area of the hypoxic zone has been a seasonally recurrent event off the Changjiang estuary. No hypoxic conditions (DO≤2.00 mg/L) were observed in August 2006, but mild hypoxia (2.86–2.00 mg/L) was found at 57% (16 of 28) of the sites in the continental shelf zone off the Changjiang estuary. There were no significant differences in macrobenthic parameters (species richness, density, biomass, and Shannon diversity index) and macrobenthic community composition between the mildly hypoxic zone (MHZ) and the normoxic zone (NZ). Taxa that included small, typically opportunistic polychaetes contributed the most to the dissimilarity among the different study zones. Reduced DO concentration is not the only contributor to the observed effects: differences in environmental variables such as temperature, salinity, and nutrient concentrations that result from stratification are also involved.
6 ACKNOWLEDGEMENTThe authors gratefully acknowledge CHEN Jianfang, LI Hongliang, and WANG Kui for their assistance in the laboratory and field, as well as the two anonymous reviewers for their valuable comments on the manuscript.
| Bray J R, Curtis J T, 1957. An ordination of the upland forest communities of southern Wisconsin. Ecological Monographs, 27(4): 325–349. Doi: 10.2307/1942268 |
| Clarke K R, 1993. Non-parametric multivariate analyses of changes in community structure. Australian Journal of Ecology, 18(1): 117–143. Doi: 10.1111/aec.1993.18.issue-1 |
| Conley D J, Björck S, Bonsdorff E, Carstensen J, Destouni G, Gustafsson B G, Hietanen S, Kortekaas M, Kuosa H, Meier H E M, Müller-Karulis B, Nordberg K, Norkko A, Nürnberg G, Pitkänen H, Rabalais N N, Rosenberg R, Savchuk O P, Slomp C P, Voss M, Wulff F, Zillén L, 2009. Hypoxia-related processes in the Baltic Sea. Environmental Science & Technology, 43(10): 3412–3420. |
| Dauer D M, Rodi A J, Ranasinghe J A, 1992. Effects of low dissolved oxygen events on the macrobenthos of the Lower Chesapeake Bay. Estuaries, 15(3): 384–391. Doi: 10.2307/1352785 |
| Diaz R J, Rosenberg R, 1995. Marine benthic hypoxia: a review of its ecological effects and the behavioural responses of benthic macrofauna. Oceanography and Marine Biology: An Annual Review, 33: 245–303. |
| Diaz R J, Rosenberg R, 2008. Spreading dead zones and consequences for marine ecosystems. Science, 321(5891): 926–929. Doi: 10.1126/science.1156401 |
| Diaz R J, 2001. Overview of hypoxia around the world. Journal of Environmental Quality, 30(2): 275–281. Doi: 10.2134/jeq2001.302275x |
| Gray J S, Wu R S, Or Y Y, 2002. Effects of hypoxia and organic enrichment on the coastal marine environment. Marine Ecology Progress Series, 238: 249–279. Doi: 10.3354/meps238249 |
| Gu H K, 1980. The maximum value of dissolved oxygen in its vertical distribution in Yellow Sea. Acta Oceanologica Sinica, 2(2): 70–79. |
| Hansen H P, Koroleff F. 1983. Determination of nutrients. In: Grasshoff K, Ehrhardt M, Kremling K eds. Methods of Seawater Analysis. 2nd edn. Verlag Chemie, Weinheim. |
| Kemp W M, Boynton W R, Adolf J E, Boesch D F, Boicourt W C, Brush G, Cornwell J C, Fisher T R, Glibert P M, Hagy J D, Harding L W, Houde E D, Kimmel D G, Miller W D, Newell R I E, Roman M R, Smith E W, Stevenson J C, 2005. Eutrophication of Chesapeake Bay: historical trends and ecological interactions. Marine Ecology Progress Series, 303: 1–29. Doi: 10.3354/meps303001 |
| Knap A, Michaels A, Close A, Ducklow H, Dickson A. 1996. Protocols for the Joint Global Ocean Flux Study (JGOFS) Core Measurements. JGOFS Report No. 19, Reprint of the IOC Manuals and Guides No. 29. UNESCO, Paris, France. |
| Kodama K, Lee J H, Oyama M, Shiraishi H, Horiguchi T, 2012. Disturbance of benthic macrofauna in relation to hypoxia and organic enrichment in a eutrophic coastal bay. Marine Environmental Research, 76: 80–89. Doi: 10.1016/j.marenvres.2011.08.007 |
| Li D J, Zhang J, Huang D J, Wu Y, Liang J, 2002. Oxygen depletion off the Changjiang (Yangtze River) estuary. Science in China Series D: Earth Sciences, 45(12): 1137–1146. Doi: 10.1360/02yd9110 |
| Long W C, Seitz R D, 2009. Hypoxia in Chesapeake Bay tributaries: worsening effects on macrobenthic community structure in the York River. Estuaries and Coasts, 32(2): 287–297. Doi: 10.1007/s12237-009-9132-5 |
| Nilsson H C, Rosenberg R, 1994. Hypoxic response of two marine benthic communities. Marine Ecology Progress Series, 115: 209–217. Doi: 10.3354/meps115209 |
| Nilsson H C, Rosenberg R, 2000. Succession in marine benthic habitats and fauna in response to oxygen deficiency: analysed by sediment profile-imaging and by grab samples. Marine Ecology Progress Series, 197: 139–149. Doi: 10.3354/meps197139 |
| Oguz T, Ducklow H W, Malanotte-Rizzoli P, 2000. Modeling distinct vertical biogeochemical structure of the Black Sea: dynamical coupling of oxic, suboxic, and anoxic layers. Global Biogeochemical Cycles, 14(4): 1331–1352. Doi: 10.1029/1999GB001253 |
| Rabalais N N, Turner R E, Sen Gupta B K, Boesch D F, Chapman P, Murrell M C, 2007. Hypoxia in the northern Gulf of Mexico: does the science support the Plan to Reduce, Mitigate, and Control Hypoxia?. Estuaries and Coasts, 30(5): 753–772. Doi: 10.1007/BF02841332 |
| Rabalais N N, Turner R E, Wiseman Jr W J, 2002. Gulf of Mexico hypoxia, A.K.A. "The dead zone". Annual Review of Ecology and Systematics, 33(1): 235–263. Doi: 10.1146/annurev.ecolsys.33.010802.150513 |
| Rabouille C, Conley D J, Dai M H, Cai W J, Chen C T A, Lansard B, Green R, Yin K, Harrison P J, Dagg M, McKee B, 2008. Comparison of hypoxia among four riverdominated ocean margins: the Changjiang (Yangtze), Mississippi, Pearl, and Rhône rivers. Continental Shelf Research, 28(12): 1527–1537. Doi: 10.1016/j.csr.2008.01.020 |
| Rosenberg R, Nilsson H C, Diaz R J, 2001. Response of benthic fauna and changing sediment redox profiles over a hypoxic gradient. Estuarine, Coastal and Shelf Science, 53(3): 343–350. Doi: 10.1006/ecss.2001.0810 |
| Seitz R D, Dauer D M, Llansó R J, Long W C, 2009. Broadscale effects of hypoxia on benthic community structure in Chesapeake Bay, USA. Journal of Experimental Marine Biology and Ecology, 381: S4–S12. Doi: 10.1016/j.jembe.2009.07.004 |
| Sturdivant S K, Díaz R J, Llansó R, Dauer D M, 2014. Relationship between hypoxia and macrobenthic production in Chesapeake Bay. Estuaries and Coasts, 37(5): 1219–1232. Doi: 10.1007/s12237-013-9763-4 |
| ter Braak C J F, Smilauer P. 1998. CANOCO Reference Manual and User's Guide to Canoco for Windows: Software for Canonical Community Ordination (Version 4). Microcomputer Power, Ithaca, NY, USA. |
| Tyson R V, Pearson T H, 1991. Modern and ancient continental shelf anoxia: an overview. Geological Society, London, Special Publications, 58(1): 1–24. Doi: 10.1144/GSL.SP.1991.058.01.01 |
| Vaquer-Sunyer R, Duarte C M, 2008. Thresholds of hypoxia for marine biodiversity. Proceedings of the National Academy of Sciences of the United States of America, 105(40): 15452–15457. Doi: 10.1073/pnas.0803833105 |
| Wang B D, 2009. Hydromorphological mechanisms leading to hypoxia off the Changjiang estuary. Marine Environmental Research, 67(1): 53–58. Doi: 10.1016/j.marenvres.2008.11.001 |
| Wei H, He Y C, Li Q J, Liu Z Y, Wang H T, 2007. Summer hypoxia adjacent to the Changjiang estuary. Journal of Marine Systems, 67(3-4): 292–303. Doi: 10.1016/j.jmarsys.2006.04.014 |
| Wei Q S, Wang B D, Chen J F, Xia C S, Qu D P, Xie L P, 2015. Recognition on the forming-vanishing process and underlying mechanisms of the hypoxia off the Yangtze River estuary. Science China Earth Sciences, 58(4): 628–648. Doi: 10.1007/s11430-014-5007-0 |
| Zhang J, Gilbert D, Gooday A J, Levin L, Naqvi S W A, Middelburg J J, Scranton M, Ekau W, Peña A, Dewitte B, Oguz T, Monteiro P M S, Urban E, Rabalais N N, Ittekkot V, Kemp W M, Ulloa O, Elmgren R, Escobar-Briones E, Van der Plas A K, 2010. Natural and humaninduced hypoxia and consequences for coastal areas: Synthesis and future development. Biogeosciences, 7(5): 1443–1467. Doi: 10.5194/bg-7-1443-2010 |
| Zhang J, Zhang Z F, Liu S M, Wu Y, Xiong H, Chen H T, 1999. Human impacts on the large world rivers: would the Changjiang (Yangtze River) be an illustration?. Global Biogeochemical Cycles, 13(4): 1099–1105. Doi: 10.1029/1999GB900044 |
| Zhu Z Y, Zhang J, Wu Y, Zhang Y Y, Lin J, Liu S M, 2011. Hypoxia off the Changjiang (Yangtze River) estuary: oxygen depletion and organic matter decomposition. Marine Chemistry, 125(1-4): 108–116. Doi: 10.1016/j.marchem.2011.03.005 |
 2017, Vol. 35
2017, Vol. 35



