Institute of Oceanology, Chinese Academy of Sciences
Article Information
- ABDELOUAHAB Hinde, BERRAHO Amina, BAIBAI Tarik, AGOUZOUK Aziz, MAKAOUI Ahmed, ERRHIF Ahmed
- Autumn larval fish assemblages in the northwest African Atlantic coastal zone
- Chinese Journal of Oceanology and Limnology, 35(3): 515-527
- http://dx.doi.org/10.1007/s00343-017-5302-7
Article History
- Received Oct. 24, 2015
- accepted in principle Mar. 21, 2016
2 National Institute of Halieutic Research, Street Sidi Abderrahmane Club equestre Ould Jmel, Casablanca, 20100, Morocco
In the Morocco economic zone off the African coast, fisheries are exceptionally important, driven by upwelling through fertilization of the pelagic ecosystem. Moroccan catches are composed mainly of small pelagic fishes species, which are the most dominant and abundant resources in this area.
The Moroccan Atlantic coast, which extends between cape Spartel and cape Blanc, is characterized by the presence of the upwelling, which takes place throughout the year (Arístegui et al., 2009). The southern area, between Cap Blanc and Cap Boujdor (21°–26°30′N) (Fig. 1) is an area of a permanent upwelling (Makaoui et al., 2005; Benazzouz et al., 2014). In fact, in the north of Dakhla the upwelling activity is permanent but weaker in autumn; while in the region of Cap Blanc, in addition to the upwelling phenomenon, it is the seat of a thermal front influenced by the NACW (North Atlantic Central Water) and SACW (South Atlantic Central Water) (Makaoui et al., 2005). Moreover, the upwelling activity provides a considerable supply of nutrients which produces high primary and secondary production (phytoplankton and zooplankton) to the pelagic ecosystems (Somoue et al., 2003, 2005; Demarcq and Somoue, 2015) thus providing excellent conditions for the development of many larvae of pelagic and demersal fish species.
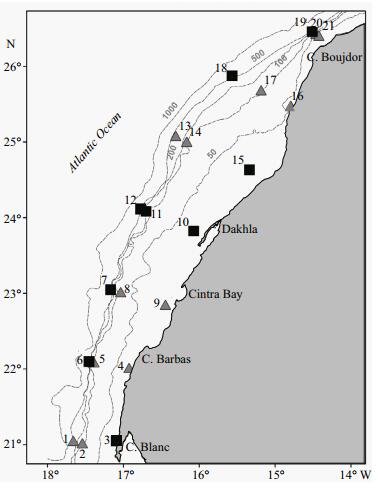
|
| Figure 1 Surveyed area curried out in November 2011 Grey triangles indicate oceanographic daylight hours sampling stations and black squares indicate night sampling stations. Dashed grey lines represent isobaths. |
An ichthyoplankton assemblage is by definition "a suite of species whose larvae are collected in the same area at the same time" (Miller, 2002). It is composed of multiple species associations that vary in time and depend on the spawning of adults (Smith et al., 1999). However, these associations may result from the shared or contrasting responses of different species to their pelagic environment (Somarakis et al., 2002) especially the local hydrodynamics (Smith et al, 1999; Sabatés et al., 2004; Rodríguez et al., 2006; Olivar et al., 2010; Moyano et al., 2014). Larval fish assemblages reflect the synchrony and overlapping of the spawning pattern of adults (Frank and Leggett, 1983) coupled with larval behavior (Gray and Miskiewicz, 2000). The maintenance of these assemblages depends on multiple factors promoting larval growth and survival especially in ecosystems characterized by a strong mesoscale activity (Moyano et al., 2014). Indeed, a combination of biological (prey availability e.g. zooplankton abundance and phytoplankton) and physical (such as temperature, water currents) factors define a larval habitat suitable for their survival (Somarakis et al., 2002; Carassou, 2008). Nevertheless, metamorphosis together with predation, that causes differential larval mortality between species within the assemblage, are the main factors leading to assemblage disruptions (Miller, 2002). Several studies have described the composition and spatio-temporal variation of larval fish assemblages, particularly in the Mediterranean Sea (Olivar et al., 2010; Somarakis et al., 2011a) and in the Canaries system (Moyano et al., 2009, 2014). On the Moroccan Atlantic coast, previous studies have focused on the horizontal eggs and larvae distribution of small pelagic fishes (Ettahiri, 1996; Berraho, 2007). In time, larval pelagic fish assemblages are strongly influenced by seasonality of the environmental parameters. Temperature, salinity and phosphate concentration are the parameters most involved in structuring these assemblages (Berraho et al., 2005; Berraho, 2007). However, qualitative changes in larval diet, even switching between trophic levels (phytoplankton and/or zooplankton) and this high flexibility of the adult diet may have a marked effect on ichthyoplankton associations (Berraho, 2007).
This paper presents the first results concerning the assemblage composition and vertical distribution of larval fish in the southern area of the Moroccan Atlantic coast in Autumn. Furthermore, we attempt to explore the influence of environmental variables on the larval fish associations in this period.
2 MATERIAL AND METHOD 2.1 Sample collection and analysisThe present study is based on the data of the multidisciplinary survey which was carried out on board the R/V Dr. Fridtjof Nansen, in the southern zone of the Moroccan Atlantic coast between cape Boujdor (26°N) and cape Blanc (21°N), in autumn (between 18 and 29 November) 2011 (Fig. 1). A total of 21 stations were sampled extending over seven transects. Ichthyoplankton and mesozooplankton samples were collected at five depths with a 'Multinet' (5 nets with 50 cm×50 cm surface mouth each and 180 μm mesh size) by oblique tows. At all stations, samples were collected from 200 m to the surface in five layers: (LⅤ) Surface–25 m, (LⅣ) 25–50 m; (LⅢ) 50–75 m; (LⅡ) 75–100 m and (LⅠ) 100–200 m, apart from at coastal stations where we took only one sample between the surface and 25 m as the total depth did not exceed 30 m. 12 stations were sampled in daytime hours and 9 stations at night. The water volume filtered was measured by 2 electronic flowmeters (Chelesa UV Aquatracka), one inside the opening of the underwater unit for the determination of the amount of water passing through the opened nets and one outside the opening for the determination of clogging effects. The ichthyoplankton samples were immediately preserved in 5% borax-buffered formalin. In the laboratory, all fish larvae were sorted from the ichthyoplankton samples, counted and identified to the lowest taxonomic level possible. The depth and water volume filtered were used to standardize larval catches to number/10 m2. The distribution maps of the larvae densities have been represented by quantiles. Mesozooplankton biomass was calculated as wet weight from 5% buffered formaldehyde preserved samples (Wiebe et al., 1975) as a proxy of zooplankton production (mg/m3).
Hydrographic sampling (temperature, salinity) and water samples for determination of the chlorophyll a concentration using a fluorometer, along the water column, was performed at each station using a multisonde CTD (Conductivity-Temperature-Density of water; SBE-911+) and carrousel with 24 5-L Niskin bottles.
2.2 Data analysisIn order to analyze the spatial and vertical patterns of environmental parameters, mean values for different parameters at each water column layer (temperature, salinity and chlorophyll a concentration) were calculated. The horizontal distribution of mean surface environmental variables was obtained with a standard optimal interpolation scheme, using inverse distance weighted (IDW) using ArcGis (Shepard, 1968).
In order to analyze the spatial structure of larval fish associations (LFAs) (at a family level), cluster analysis was used. Cluster analysis was performed on larval fish densities matrices and using the BrayCurtis distance measure (Bray and Curtis, 1957). An arbitrary cut-off level was chosen on dendrograms to produce ecologically interpretable clusters (Auth et al., 2007).
The relation between the environmental parameters: average temperature (Ta), average salinity (Sa), zooplankton biomass (Zb), depth (Dh), average concentration of chlorophyll a (Chl a) at each water column layer and LFA was assessed with Canonical Correspondence Analysis (CCA) using Xlstat v.10 (Ter Braak and Verdonschot, 1995). Canonical axes were tested for significance with a Monte Carlo permutation test (9 999 permutations).
In all analysis, only taxa found which had a relative abundance >0.5% were considered and larval abundance data were log10(x+1) transformed to down-weight the importance of highly abundant taxa. For the cluster analysis, Balistidae larvae were excluded because they were found at only one station.
One-way ANOVA was used to test the variability of hydrological and biological (zooplankton biomass and chlorophyll a) parameters in terms of spatial distribution between different depth layers, and to compare the difference between day/night larval abundance values.
3 RESULT 3.1 Hydrography (temperature and salinity)The horizontal distribution of sea surface temperature (SST) showed a differentiation into two coastal patterns of low temperature: the first one was observed between cape Boujdor and North of Dakhla, while the second one was located between cape Barbas and cape Blanc. Mean temperature in the 0–25 m layer in these two parts was 18℃ and 17℃ respectively (Fig. 2a) and varied considerably from the coast to offshore (respectively SD=±3.7 and 2.5℃).
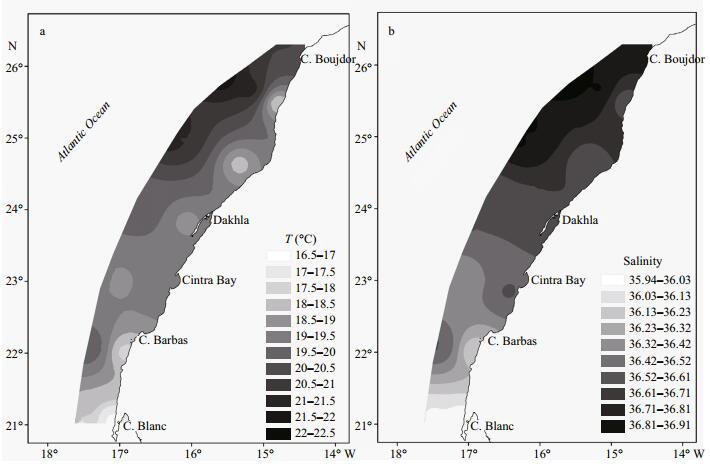
|
| Figure 2 Spatial distribution of average temperature (a) and average salinity (b) in surface layer (0-25 m) |
The mean salinity in the upper water column varied considerably between the north and the south part of the studied area and from the coast to offshore. Low salinities occurred in the southern part with a lowest value 35.94 observed at cape Blanc. In the central part, the salinities ranged between 36.42 and 36.61; while in northern part, even higher salinities were found (>36.61) with the highest value recorded offshore (36.91) (Fig. 2b).
Vertical temperature profiles (applied only for the offshore stations) showed considerable latitudinal variation. Indeed, a clear separation of the recorded temperature is observed between the south and the north part of the study area and between different depth layers (Fig. 3). In south part (st 1 and 6), the average temperature profile recorded ranged between 20.3 and 13.5℃, while in the north part (st 13, 18 and 19) average temperature was higher and varied between 22 and 15℃. In these two parts, the water column was thermally well stratified; the thermocline started around 20–50 m and continued until 90–100 m depth. Furthermore, in the central part (st 7 and 12), the thermocline was located in deeper layers (between 50–90 m to 150 m depth) with only small changes in average temperature, compared to the other parts. Similarly, vertical salinity profiles showed the same trends as temperature in the three parts of study area. At the shelf break stations, the vertical temperature and salinity distribution was more homogeneous due to their limited depth.
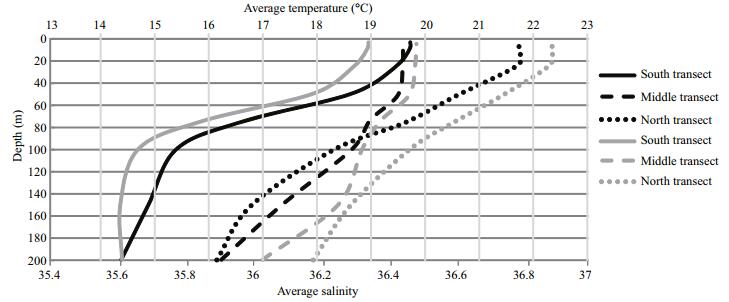
|
| Figure 3 Vertical average temperature and salinity profiles in offshore stations South transect (st 1 and 6), Middle transect (st 7 and 12) and North transect (st 13–18–19). Black line: temperature; grey line: salinity. |
The mean concentrations of chlorophyll a at the surface layer were low and did not exceed 0.8 μg/L, except in the north of Dakhla and at cape Blanc where a peak of chlorophyll concentrations reached 1.6 μg/L (Fig. 4a). Contrary to the observed hydrological parameters, the vertical distribution of chlorophyll a presented a small variation along the study area in the offshore stations (0.1–0.8 μg/L) and a high variability in the coastal stations (ranged between 0.3 and 1.76 μg/L).
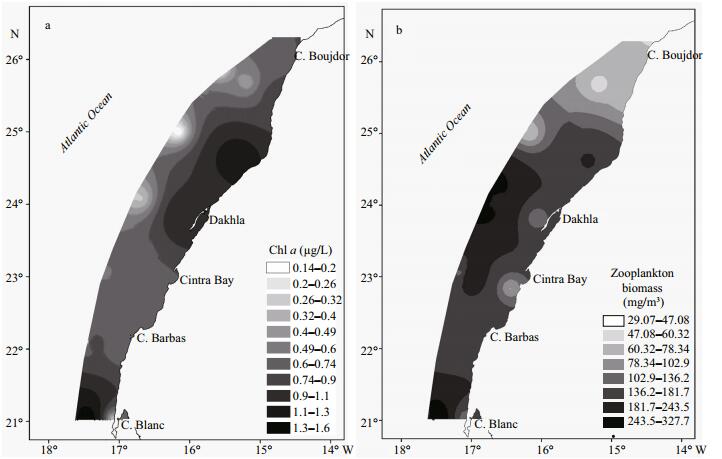
|
| Figure 4 Spatial distribution of average chlorophyll a concentration (a) and meso zooplankton biomass (b) in surface layer (0-25 m) |
Indeed, DCM (Deep Chlorophyll Maxima) level (recorded at all stations) occurred up to 10 m at coastal stations, except for stations 15 and 16 where it was detected at 26 m. At the offshore stations, however, the latitudinal DCM variation was higher and was recorded in the surface layer (10 m, except at station 2), between Cintra Bay and cape Blanc, and deeper (at 75 m) between Dakhla and cape Boujdor.
The spatial distribution of mesozooplankton biomass was heterogeneous and varied between 29.07 and 327.7 mg/m3. Higher mesozooplankton biomass was located particularly at some stations offshore off Dakhla and cape Blanc (Fig. 4b). The DZM (Deep Zooplankton Maxima) was recorded near the surface in the south, middle and north parts of the study area, where the maximum of mesozooplankton biomass was found. In addition, a relatively high mesozooplankton biomass was observed up to 80 m in both the south and north parts (Fig. 5).

|
| Figure 5 Vertical distribution of mesozoplankton biomass (mg/m3) in the South transect, Middle transect and North transect |
Significant variability in hydrological and biological parameters was recorded in term of spatial distribution and between different depth layers. Temperature and zooplankton biomass have showed an important variation between different depth layers (ANOVA, P < 0.001) while salinity and chlorophyll a recorded high significant spatial variability (among stations) (ANOVA, P < 0.001).
3.3 Taxonomic composition and abundance of fish larvaeA total of 1 680 fish larvae were identified from 21 families. Only 13 were identified to species level. The percentage of non-identified larval taxa was about 5% of the total larvae. Four families accounted for more than 80% of total abundance: Clupeids (the most abundant), followed by Myctophidae, Sparidae and Gadidae (Table 1). Seven families had an abundance between 1% and 3.5%, with an occurrence ranging between 4.5% and 16.4%; while for the other identified families, relative abundance did not exceed 1% (with total occurrence=23.1%).
|
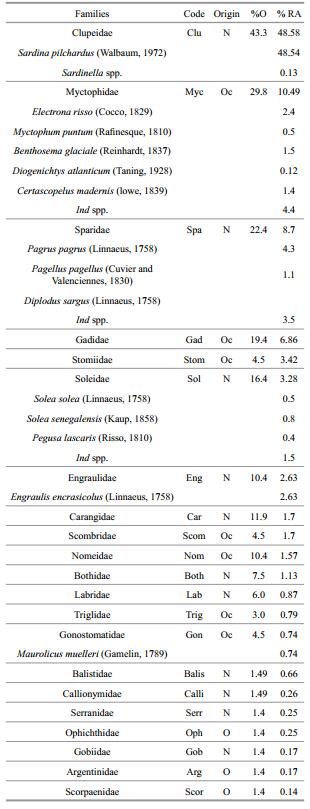
The horizontal distribution of fish larvae (at all depths) exhibited high densities in the north of Dakhla; where the highest value was recorded near cape Boujdor (1 369 larvae/10 m2) and moderate densities towards the south (Fig. 6a). In terms of specific spatial distribution, Sardina pilchardus larvae (99% of total Clupeids) was widely distributed through the study area and showed a major presence in the northern part; especially between Dakhla and cape Boujdor (Fig. 6b). Larvae of neritic families occurred particularly in the northern part, while larvae of oceanic families were mostly distributed at offshore stations (Fig. 6c, d).
|
| Figure 6 Spatial distribution of larvae fish abundance (N/10 m2): total fish larvae (a), S. pilchardus (b), neritic families larvae (c) and oceanic families larvae (d) |
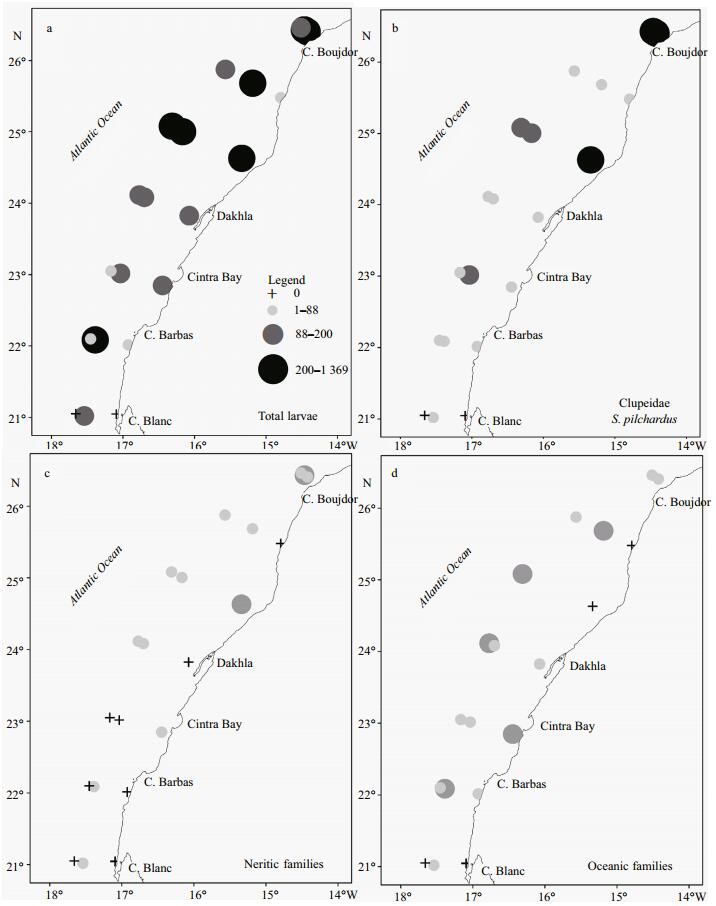
In the water column, the vertical distribution of fish larvae revealed that 57% were distributed in the surface layer (LⅤ) and almost 20% on the two underlying layers (LⅣ and LⅢ). The occurrence of larvae in the other depth layers (under 75 m) was low and the total density in both layers (LⅡ and LⅠ) did not exceed 200 larvae/10 m2 (Table 2).
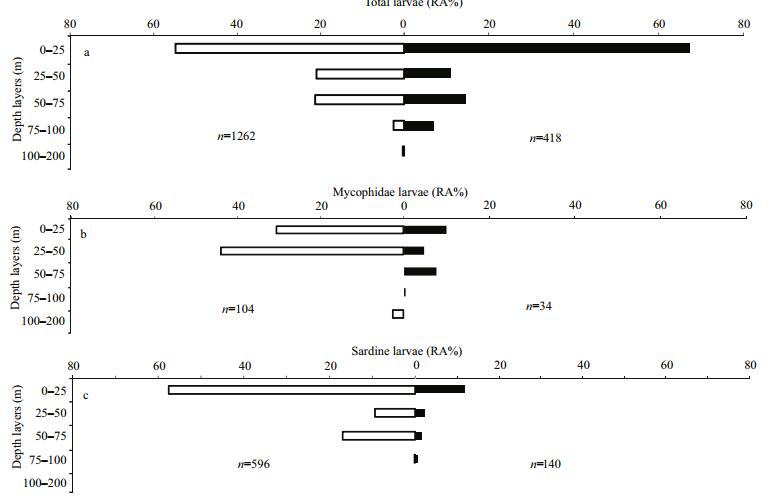
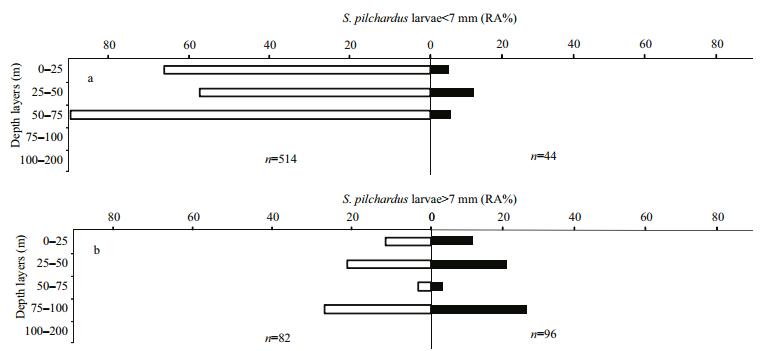
The abundance variability of total fish larvae sampled during daytime and at night showed almost the same preference for the surface layer (LⅤ), contrary to the low observed abundance in deeper layers that presented a small scale variation between day and night. Variation between day/night abundances values was not significant (one-way ANOVA test, P=0.18; α=0.05). Myctophidae larvae, the most abundant family among the oceanic group, presented a high abundance in the surface layers at stations sampled during both day and night (mainly at LⅣ and LⅤ respectively). Sardine larvae showed a maximum densities in the surface layer at both daylight-and night-sampled stations. Nevertheless, the relative abundance of sardine larvae was almost three times higher at stations during the day than at those sampled at night (Fig. 7a–c). Considering the threshold size of 7 mm as a limit between small and large sardine larvae, small S. pilchardus larvae were collected only between surface and 75 m, with the majority found in daylight hours. The vertical distribution of large larvae was extended to 100 m at both daytime-and night-sampled stations, and the greatest abundance was found between 75 and 100 m at night (Fig. 8a, b).
|
| Figure 7 Vertical distribution of the relative abundance of total fish larvae (a), Myctophidae larvae (b) and Sardine larvae (c) at daylight hours (white bars) and at night (black bars) |
|
| Figure 8 Vertical distribution of Sardina pilchardus larvae by size class in daytime (white bars) and at night (black bars) |
Analysis of larval richness (in terms of family), showed a variability in different depth layers. Indeed, in the three shallower layers (from 75 m to surface), almost the same number of fish larvae families were found (14 families in LⅤ and LⅣ, and 16 families in LⅢ) with nine common families in these three layers (Table 2). However, richness decreases considerably in the bottom layers where only Myctophidae larvae were identified over 100 m depth.

|
The relative abundance of the different fish larval taxa was variable from one to another layer, except for LⅠ which was characterized exclusively by Myctophidae larvae (Table 2). Six families appeared in the four layers (from surface to 100 m). Clupeidae was the most represented family in LⅤ (53%) and sees its abundance decrease in other depth layers. In another hand, Gadidae and Myctophidae larvae showed an increasing gradient toward deeper layers. Soliedae, Sparidae and Carangidae larvae were observed in the four layers but presented a variable abundance in each stratum. Various other families presenting the lowest abundance were found in variable position through the water column.
For the spatial structure of LFA, cluster analysis defined two main groups of families (Fig. 9). The first group (Ⅰ) represented by Clupeids, namely by S. pilchardus, Soleidae, Sparidae, Carangidae and Engraulidae belonging to neritic families and whose adults usually inhabit and reproduce on the shelf waters. Furthermore, in the same group we found also larvae of oceanic families: Myctophidae and Gadidae. The second group (Ⅱ) was represented essentially by oceanic species but included also larvae of Bothidae which is a neritic family.
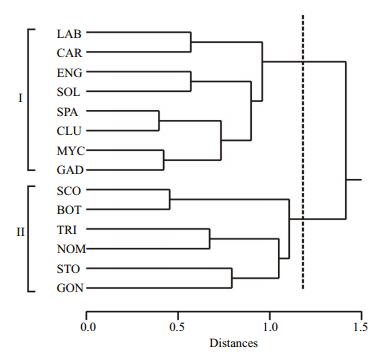
|
| Figure 9 Dendrogram representing two groups of fish larvae assemblages: Group Ⅰ and Group Ⅱ |
The Monte Carlo test showed a high significance of the two CCA biplot first axes (P < 0.05%). F1/F2 explained 58.6% of the variance of taxa-environment relationship. Temperature and salinity were the best correlated parameters with F2 (R=2.315 and-2.566 respectively) (Fig. 10). In general, the majority of taxa were grouped near the center of the CCA biplot with biological parameters (zooplankton biomass and chlorophyll a concentration) and depth. Neritic and oceanic families were present in the opposite sides of F2, excepted for Sparidae (a neritic family) which was localized on the same side as oceanic families. Only Soleidae (a neritic family) appeared at the left side of F1, with a contribution of 54%, while Stomiidae (an oceanic family) and Gadidae (a neritic family) were the two families that showed the highest correlation, with F2 (33% and 21% respectively). Myctophidae was the only family that presented the same high contribution with the two axes (20%).
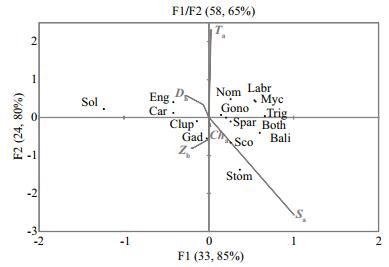
|
| Figure 10 Canonical correspondence analysis (CCA) results for the November survey Abbreviations explained in Table 1. |
The study was carried out in the southern area of the Moroccan Atlantic coast, namely between cape Blanc and cape Boujdor which is a subject of permanent upwelling (Makaoui et al., 2005; Benazzouz et al., 2014). The presence of upwelling provides this zone with high productivity (primary and secondary production) which is variable in time and space (Berraho, 2007). The hydrology of this zone, during the sampling period (November 2011), was marked by the presence of two fresh water masses. The first one was located in the south part, between cape Barbas and cape Blanc, and the second one was observed on the north part, between cape Boujdor and Dakhla. According to Makaoui et al. (2005), autumn is characterized by a very weak upwelling at cape Boujdor and the presence of thermal front drift located to the south of 25°N. Furthermore, in the south part, between cape Barbas and cape Blanc, the upwelling activity attains his maximum in autumn where an important quantity of phosphate was recorded. In addition, the influence of SACW (South Atlantic Central Water), characterized by an abundant nutrients and low salinity (less than 36) was marked in this area (Makaoui et al., 2005). In autumn 2011, temperature and salinity vertical profiles recorded in the studied area showed a wide variation between the north and the south parts. Higher values of temperature and salinity were observed at cape Blanc and cape Boujdor especially in the surface layers. However, the water column stratification explored between Dakhla and cape Boujdor, showed a presence of upwelled water offshore of Dakhla. Indeed, in this region, the presence of a coastal current of south-west direction, of which the velocity decreases from the north to the south, was the origin of a filament off Dakhla (Makaoui, 2008). The central part of the studied area, (Dakhla-Cintra Bay) presented intermediate hydrological characteristics compared to the other parts (north and south) and the water column stratification was adequate for phytoplankton and zooplankton development in the surface layers, justified by the DCM and DZM.
Diverse fish larvae taxa were identified in the southern part of the Moroccan Atlantic coast, in autumn 2011, concentrated particularly in the three uppermost layers (LⅤ, LⅣ and LⅢ). The specific composition of larvae presented a high abundance of Clupeidae, dominated mainly by sardine (Sardina pilchardus). The highest densities of this species were limited between cape Boujdor and Cintra Bay and a maximum was recorded in the northern part (1 257 larvae/10 m2) at station 21. Sardine is a pelagic neritic species whose main spawning period occurs in the cold season (autumn-winter) in the south part of the Moroccan Atlantic coast. In this area, the spawning location of sardine was identified between cape Boujdor and North of Dakhla (Berraho, 2007), while the larval development area may extend to the Cintra Bay (Ettahiri et al., 2012). In water column, a high larvae abundance of this species was observed in the surface layers during both day and night, and it decreases in the bottom layers. Considering the threshold size of 7 mm which corresponds to the dorsal fin apparition of the sardine larvae (Berraho et al., 2012) as a limit between small and large larvae, the majority of the sampled sardine larvae were small and were collected during daylight in the upper layers. In contrast, the highest abundance of large larvae occurred at night in greater depth (75–100 m). In the north-western part of the Mediterranean sea, sardine larvae mainly concentrate above 30m, during daylight and at night, and are found in deeper layers as they grow (Olivar et al., 2001; Sabatés, 2004). Although, on the West African Atlantic coast, neritic species show a vertical stratification during daylight, only sardine larvae show a significant diel vertical migration (Rodríguez et al., 2006).
The second most important family found in our samples was Myctophidae, whose larvae account for an elevated abundance and high diversity in the study area. In the Canary Islands, Clupeidae and Myctophidae larvae represent almost half of total larvae collected (21.9% and 20.5% respectively) (Moyano and Hernández-León, 2011). In the northern region of the NW Africa upwelling system, the Myctophidae family is characterized by a high diversity of species (Rodríguez et al., 1999). Our results revealed that Electrona risso (Cocco, 1829) was the most abundant taxa (23%) followed by Benthosema glaciale (Reinhardt, 1837) (15%), Myctophum punctatum (Rafinesque, 1810) (5%) and Diogenichtys atlanticus (Taning, 1928) (1%). The spawning period of E. risso occurs mainly in the summer and the autumn, while B. glaciale and M. punctatum spawn throughout the year in the Mediterranean Sea (Sabatés and Masó, 1990; Sabatés, 2004). The high abundance of Myctophidae larvae was recorded in the north part of studied area in the uppermost layers (LⅤ and LⅣ) during daytime, corresponding to the maximum observed mesozooplankton biomass. According to Sabatés (2004), larvae of meso and bathy-pelagic species have a deeper distribution in the water column and most of the larvae migrate from the bottom layers to spend the daytime in surface layers. A marked vertical distribution in the upper layers during the daytime coincides with the maximum concentrations of their potential food. Indeed, a correlation between Myctophidae larvae and high plankton production has been found in different ecosystems around the world (Doyle et al., 1993; Somarakis et al., 2011b). However, Olivar et al. (2010) note that in Catalan coast (NW Mediterranean Sea), the current mediated transport may regulate the vertical distribution of oceanic species more than food availability.
In addition to Clupeidae and Myctophidae, other identified families were found that contributed to LFA structure although they had low relative abundance. Spatially, LFA was well structured, illustrated by wide distribution of neritic families at both costal and offshore stations in the north part and by a distribution of oceanic families mostly at the offshore stations along the study area. Several factors have been identified as possible causes of changes in the composition and location of larval assemblage. In ecosystems characterized by a strong oceanographic mesoscale activity, horizontal distribution of planktonic community's structure, including fish eggs and larvae is dependent on the interactions between biological and physical aspects (Rodríguez et al., 2004). Consequently, LFAs were found to be spatially and temporally dynamic (Keane and Neira, 2008). Moreover, most LFA studies in different ecosystem have emphasized the influence of bathymetry and spawning habitat of adults on the spatial patterns (Somarakis et al., 2002; Sampey et al., 2004; Olivar et al., 2010). The southern Atlantic Moroccan coast is characterized by a large variation of bathymetry that could influence the established LFA, in addition to the spawning habitat specific to each taxon.
In spite of the capacity of fish larvae to choose their position in the water column for a better growth and survival condition (Lee et al., 2005), our results revealed that the majority of different fish larvae were distributed in the superficial layer. The day/night vertical distribution of the collected fish larvae, showed a preference for the upper layer, especially in the daylight hours compared to a dispersed distribution along the water column at night. These results confirm the importance of the light factor in larval vertical distribution which can be explained by the importance of the nutrition patterns of the majority of fish species (Gray and Kingsford, 2003; Morote et al., 2008). Referring to Ahlstrom (1959), fish larvae in stratified waters display a stratified vertical distribution where larvae of neritic species are distributed in the mixed layer and the upper layer of the thermocline, while oceanic larvae show a deeper distribution, usually below the thermocline. Given the fact that the hydrology of our study area is governed by the presence of upwelling, a thermal stratification was strong and a structured vertical larval distribution of different fish families throughout the water column was found. This water column stratification was observed between the surface and 80 m. Above this limit, both neritic and oceanic larvae families were found, whereas in the bottom layers, the proportion of neritic larvae was smaller in relation to oceanic larvae.
According to Smith and Suthers (1999), the thermocline position is important in acting as an upper or a lower barrier for the vertical distribution of the larvae of some species. In the Canaries-African Coastal Transition Zone, characterized by a strong mesoscale oceanographic activity, the vertical distribution of fish larvae was not conditioned by the seasonal thermocline (Rodríguez et al., 2006). Moyano et al. (2014) reported that the vertical distribution of neritic and oceanic larvae frequently showed a differentiated position in the water column and the effect of the upwelling front combined with the cyclonic-anticyclonic eddy dipole can present a successful retention mechanism for neritic larvae. In contrast, Gray (1996) has pointed that larval behavior and not the thermocline location, has a major influence on vertical distribution of fish larvae in waters characterized by high physical variability. The insignificant influence of the thermocline on the larval vertical distribution was also reported in the southeast of Australia (Gray and Kingsford, 2003).
Trophic variables may play a role in shaping LFAs. The availability of food (phytoplankton and zooplankton) in our study area is permanent due to the upwelling activity along the year. Therefore, the majority of fish larvae identified was found in the surface layer which coincided with the maximum concentrations of food (mesozooplankton as well as phytoplankton). Olivar et al. (2010) suggested that the trophic component influences associations of neritic larvae contrary to larvae of oceanic species which are more affected by the current fields (alongshelf and cross-shelf transport) in autumn. In the upwelling system, frontal area is likely acting as a concentration mechanism, providing a high food environment for both neritic and oceanic larvae (Moyano et al., 2014). Significant correlation between the spatial distribution of fish larvae and mesozooplankton suggests some kind of trophic relationship between them, while the prey condition would be the factor influencing the vertical distributions of fish larvae (Rodríguez et al., 2006). In the Mediterranean Sea, the vertical distribution of neritic species larvae, including sardine, is associated with high concentrations of mesozooplankton, especially of nauplii and copepodites, as potential food for these larvae (Sabatés, 2004; Olivar et al., 2010). According to CCA results, no clear relationship between LFA and trophic variables was distinguished but the temperature and salinity were most involved in structuring LFAs in relation to the hydrological features. Food availability, light factor, species specific ontogenetic development… etc are all factors which influence the vertical distribution of fish larvae, are still controversial given the wide variety and complexity of the ecosystems.
5 CONCLUSIONThe south part of the Moroccan Atlantic coast is a favorable environment for the spawning of multiples species in autumn, due to the particular hydrological features which provide a high productivity to this region. Indeed, a high diversity of larvae fish (in term of family) was reported during this period. LFA was generally structured into two larvae groups (neritic and oceanic families). No clear relationship between LFA and biological parameters (chlorophyll a and mesozooplankton) was found, contrary to hydrological parameters that was too contrasting in this region and seems to be the most involved factors in structuring this association. More investigations on circulation patterns, microzooplankton composition and diel vertical migration of larvae, may allow us to improve our understanding on ichthyoplankton assemblages and their dynamics in this region.
6 ACKNOWLEDGEMENTThis work was carried out in the context of the regional project CCLME "Canary Current Large Marine Ecosystem". The authors thank the project coordinators and all Moroccan-Norwegian scientific team that participated in the survey on aboard the R/V Dr Fridtjof Nansen.
| Ahlstrom E H, 1959. Vertical distribution of pelagic fish eggs and larvae off California and Baja California. U.S. Fish and Wildlife Service: Fishery Bulletin, 60(161): 107–146. |
| Arístegui J, Barton E D, Álvarez-Salgado X A, Santos A M P, Figueiras F G, Kifani S, Hernández-León S, Mason E, Machú E, Demarq H, 2009. Sub-regional ecosystem variability in the Canary current upwelling. Progress in Oceanography, 83(1-4): 33–48. Doi: 10.1016/j.pocean.2009.07.031 |
| Auth T D, Brodeur R D, Fisher K M, 2007. Diel variation in vertical distribution of an offshore ichthyoplankton community off the Oregon coast. Fishery Bulletin, 105(3): 313–326. |
| Benazzouz A, Mordane S, Orbi A, Chagdali M, Hilmi K, Atillah A, Pelegrí J L, Demarq H, 2014. An improved coastal upwelling index from sea surface temperature using satellite-based approach – The case of the Canary Current upwelling system. Continental Shelf Research, 81: 38–54. Doi: 10.1016/j.csr.2014.03.012 |
| Berraho A, Ettahiri O, Letourneur Y, Orbi A, Yahyaoui A, 2005. Importance des paramètres hydrologiques dans la distribution des œufs et larves des petits pélagiques du sud de l'Atlantique marocain. Cybium, 29(1): 21–31. |
| Berraho A, Ettahiri O, Brochier T, Benazzouz A, Larissi J, Makaoui A, Somoue L, Salah S, Hilmi K, Orbi A, 2012. Distribution des larves de sardine et d'anchois le long du filament du Cap Ghir (région nord-ouest Africaine). Journal des Sciences Halieutique et Aquatique, 6: 178–193. |
| Berraho A. 2007. Relations spatialisées entre milieu et ichtyoplancton des petits pélagiques de la côte Atlantique marocaine (zones centrale et sud). Ph. Doctor Thesis. Univ. Mohammed V Agdal, Rabat. 261p. |
| Bray R J, Curtis J T, 1957. An ordination of the upland forest communities of southern Wisconsin. Ecological Monographs Journal, 27(4): 325–349. Doi: 10.2307/1942268 |
| Carassou L. 2008. Les assemblages de larves de poissons dans le lagon de Nouvelle-Calédonie: structure spatiotemporelle et relations avec les facteurs abiotiques et biotiques de l'environnement. Ph. Doctor Thesis. Ecole pratique des hautes études. Centre IRD de Nouméa, Nouvelle-Calédonie. 292p. |
| Demarcq H, Somoue L. 2015. Phytoplankton and primary productivity off northwest Africa. In: Valdés L, DénizGonzález I eds. Oceanographic and Biological Features in the Canary Current Large Marine Ecosystem. IOCUNESCO, IOC Technical Series, Paris. p. 161-174. |
| Doyle M J, Morse W W, Kendall A W Jr, 1993. A comparison of larval fish assemblages in the temperate zone of the northeast Pacific and northwest Atlantic Oceans. Bulletin of Marine Science, 53(2): 588–644. |
| Ettahiri O, Berraho A, Houssa R, Ramzi A, Somoue L, Zizah S, Machu E, 2012. Characteristics of the spawning habitats of sardine, Sardina pilchardus, off the Moroccan Atlantic coast (21°N-26°N). FAO. Comptes rendues des pêches et de l'aquaculture, 18: 157–186. |
| Ettahiri O. 1996. Etude de la phase planctonique de la sardine, Sardina pilchardus (Walb. ), et de l'anchois, Engraulis encrasicolus (L. ) des côtes atlantiques marocaines. Ph. Doctor Thesis, Univ. Western Brittany, Brest. 252p. |
| Frank K T, Leggett W C, 1983. Multispecies larval fish associations: accident or adaptation?. Canadian Journal of Fisheries and Aquatic Sciences, 40(6): 754–762. Doi: 10.1139/f83-098 |
| Gray C A, Kingsford M J, 2003. Variability in thermocline depth and strength, and relationships with vertical distributions of fish larvae and mesozooplankton in dynamic coastal waters. Marine Ecology Progress Serial, 247: 211–224. Doi: 10.3354/meps247211 |
| Gray C A, Miskiewicz A G, 2000. Larval fish assemblages in south-east Australian coastal waters: seasonal and spatial structure. Estuarine, Coastal and Shelf Science, 50(4): 549–570. Doi: 10.1006/ecss.1999.0595 |
| Gray C A, 1996. Do thermoclines explain the vertical distributions of larval fishes in the dynamic coastal waters of south-eastern Australia?. Marine and Freshwater Research, 47(2): 183–190. Doi: 10.1071/MF9960183 |
| Keane J P, Neira F J, 2008. Larval fish assemblages along the south-eastern Australian shelf: linking mesoscale nondepth-discriminate structure and water masses. Fisheries Oceanography, 17(4): 263–280. Doi: 10.1111/fog.2008.17.issue-4 |
| Lee O, Nash R D M, Danilowicz B S, 2005. Small-scale spatiotemporal variability in ichthyoplankton and zooplankton distribution in relation to a tidal-mixing front in the Irish Sea. ICES Journal of Marine Science, 62(6): 1021–1036. |
| Makaoui A, Orbi A, Hilmi K, Zizah S, Larissi J, Talbi M, 2005. L'upwelling de la côte atlantique du Maroc entre 1994 et 1998. Comptes Rendus Geoscience, 337(16): 1518–1524. Doi: 10.1016/j.crte.2005.08.013 |
| Makaoui A. 2008. Etude de l'upwelling côtier de la côte atlantique marocaine et sa contribution à la sédimentologie du plateau continental. Ph. Doctor Thesis. Univ. Hassan II, Ben M'sik, Casablanca, Morocco. 163p. |
| Miller T J. 2002. Assemblages, communities and species interactions. In: Fuiman L A, Werner R G eds. Fishery science: the unique contributions of early life stages. Blackwell Science, Ltd. p. 183-205. |
| Morote E, Olivar M P, Pankhurst P M, Villate F, Uriarte I, 2008. Trophic ecology of bullet tuna Auxis rochei larvae and ontogeny of feeding-related organs. Marine Ecology Progress Series, 353: 243–254. Doi: 10.3354/meps07206 |
| Moyano M, Hernández-León S, 2011. Intra-and inter annual variability in the larval fish assemblage off Gran Canaria (Canary Islands) over 2005-2007. Marine Biology, 158(2): 257–273. Doi: 10.1007/s00227-010-1556-8 |
| Moyano M, Rodríguez J M, Benítez-Barrios V M, HernándezLeón S, 2014. Larval fish distribution and retention in the Canary Current system during the weak upwelling season. Fisheries Oceanography, 23(3): 191–209. Doi: 10.1111/fog.2014.23.issue-3 |
| Moyano M, Rodríguez J, Hernández-León S, 2009. Larval fish abundance and distribution during the late Winter bloom off gran Canaria Island, Canary Islands. Fisheries Oceanography, 18(1): 51–61. Doi: 10.1111/fog.2009.18.issue-1 |
| Olivar M P, Emelianov M, Villate F, Uriarte I, Maynou F, Álvarez I, Morote E, 2010. The role of oceanographic conditions and plankton availability in larval fish assemblages off the Catalan coast (NW Mediterranean). Fisheries Oceanography, 19(3): 209–229. Doi: 10.1111/(ISSN)1365-2419 |
| Olivar M P, Salat J, Palomera I, 2001. Comparative study of spatial distribution patterns of the early stages of anchovy and pilchard in the NW Mediterranean Sea. Marine Ecology Progress Series, 217: 111–120. Doi: 10.3354/meps217111 |
| Rodríguez J M, Barton E D, Hernández-León S, Arístegui J, 2004. The influence of mesoscale physical processes on the larval fish community in the Canaries CTZ, in summer. Progress in Oceanography, 62(2-4): 171–188. Doi: 10.1016/j.pocean.2004.07.006 |
| Rodríguez J M, Hernández-León S, Barton E D, 1999. Mesoscale distribution of fish larvae in relation to an upwelling filament off Northwest Africa. Deep-Sea Research Part I: Oceanographic Research Papers, 46(11): 1969–1984. Doi: 10.1016/S0967-0637(99)00036-9 |
| Rodríguez J M, Hernández-León S, Barton E D, 2006. Vertical distribution of fish larvae in the Canaries-African coastal transition zone in summer. Marine Biology, 149(4): 885–897. Doi: 10.1007/s00227-006-0270-z |
| Sabatés A, Masó M, 1990. Effect of a shelf-slope front on the spatial distribution of mesopelagic fish larvae in the Western Mediterranean. Deep-Sea Research Part A. Oceanographic Research Papers, 37(7): 1085–1098. Doi: 10.1016/0198-0149(90)90052-W |
| Sabatés A, Salat J, Masó M, 2004. Spatial heterogeneity of fish larvae across a meandering current in the northwestern Mediterranean. Deep-Sea Research Part I: Oceanographic Research Papers, 51(4): 545–557. Doi: 10.1016/j.dsr.2003.11.003 |
| Sabatés A, 2004. Diel vertical distribution of fish larvae during the winter-mixing period in the Northwestern Mediterranean. ICES Journal of Marine Science, 61(8): 1243–1252. Doi: 10.1016/j.icesjms.2004.07.022 |
| Sampey A, Meekan M G, Carleton J H, McKinnon A D, McCormick M I, 2004. Temporal patterns in distributions of tropical fish larvae on the north west Shelf of Australia. Marine and Freshwater Research, 55(5): 473–487. Doi: 10.1071/MF03160 |
| Shepard D. 1968. A two-dimensional interpolation function for irregularly-spaced data. In: Proceedings of the 23rd ACM National Conference. ACM, New York. p. 517-524. |
| Smith K A, Suthers I M, 1999. Displacement of diverse ichthyoplankton assemblages by a coastal upwelling event on the Sydney shelf. Marine Ecology Progress Series, 176: 49–62. Doi: 10.3354/meps176049 |
| Smith K S, Gibbs M T, Middleton J H, Suthers I M, 1999. Short term variability in larval fish assemblages of the Sydney shelf: tracers of hydrographic variability. Marine Ecology Progress Series, 178: 1–15. Doi: 10.3354/meps178001 |
| Somarakis S, Drakopoulos P, Filippou V, 2002. Distribution and abundance of larval fish in the Northern Aegean Seaeastern Mediterranean-in relation to early summer oceanographic conditions. Journal of Plankton Research, 24(4): 339–357. Doi: 10.1093/plankt/24.4.339 |
| Somarakis S, Isari S, Machias A, 2011a. Larval fish assemblages in coastal waters of central Greece: reflections of topographic and oceanographic heterogeneity. Scientia Marina, 75(3): 605–618. Doi: 10.3989/scimar.2011.75n3 |
| Somarakis S, Ramfos A, Palialexis A, Valavanis V D, 2011b. Contrasting multispecies patterns in larval fish production trace inter-annual variability in oceanographic conditions over the N. E. Aegean Sea continental shelf (Eastern Mediterranean). Hydrobiologia, 670: 275–287. |
| Somoue L, Elkhiati N, Ramdani M, Lam Haoi T, Ettahiri O, Berraho A, Do Chi, 2005. Abundance and structure of copepod communities along the Atlantic coast of southern Morocco. Acta Adriatica, 46(1): 63–76. |
| Somoue L, Elkhiati N, Vaquer A, Ramdani M, Ettahiri O, Makaoui A, Berraho A, 2003. Contribution à l'étude des Diatomées dans l'écosystème pélagique côtier au sud de l'Atlantique marocain (21°N-26°30′N). Journal de Recherche Océanographique, 28: 1–13. |
| Ter Braak C J F, Verdonschot P F M, 1995. Canonical correspondence analysis and related multivariate methods in aquatic ecology. Aquatic Sciences, 57(3): 255–289. Doi: 10.1007/BF00877430 |
| Wiebe P, Boyd S, Cox J L, 1975. Relationships between zooplankton displacement volume, wet weight, dry weight, and carbon. Fishery Bulletin, 73(4): 777–786. |
 2017, Vol. 35
2017, Vol. 35


