Institute of Oceanology, Chinese Academy of Sciences
Article Information
- YE Lingtong(叶灵通), LU Mingmiao(卢明淼), QUAN Keyan(权可艳), LI Wenxiang(李文祥), ZOU Hong(邹红), WU Shangong(吴山功), WANG Jiangyong(王江勇), WANG Guitang(王桂堂)
- Intestinal disease of scattered mirror carp Cyprinus carpio caused by Thelohanellus kitauei and notes on the morphology and phylogeny of the myxosporean from Sichuan Province, southwest China
- Chinese Journal of Oceanology and Limnology, 35(3): 587-596
- http://dx.doi.org/10.1007/s00343-017-5312-5
Article History
- Received Dec. 9, 2015
- accepted in principle Mar. 19, 2016
2 Key Laboratory of Aquatic Biodiversity and Conservation, and the Institute of Hydrobiology, Chinese Academy of Sciences, Wuhan 430072, China;
3 Sichuan Fisheries School, Chengdu 611730, China
The taxon Myxosporea (Myxozoa) contains widely parasitic species with high species diversity and wide host range, mainly infecting a variety of freshwater and marine fishes (Kent et al., 2001). In most cases, myxosporean species can co-exist with or mildly harm their fish hosts (Lom, 1987; Chen and Ma, 1998; Longshaw et al., 2005). However, when large amounts of myxosporean parasites infect fish hosts, the infestation will impair the immune system of the hosts, inevitably leading to severe diseases and even causing mass mortality. In China, the rapid development of intensive culture modes have led to an increased prevalence of myxosporidiosis in the main fish culture districts. As a result, disease outbreaks have become more frequent, often with disastrous results. (Xie et al., 2000; Wang et al., 2001, 2003; Zhang et al., 2010; Liu et al., 2011, 2012; Xi et al., 2011). For instance, Thelohanellus wuhanensis, the agent of skin disease in juvenile Carassius gibelio, can easily infect the host skin, reach high prevalence, severely retard the growth and development of the host, and even cause severe mortality of juvenile C. gibelio (Xiao and Chen, 1993; Wang et al., 2001). Pharynx disease of C. gibelio recently became one of the most harmful myxosporidioses that often occurred at fish farms in the middle and lower reaches of Changjiang (Yangtze) River, especially in Jiangsu Province, at the eastern part of China (Xi et al., 2011; Liu et al., 2012). The agent of this disease was originally recognized as Myxobolus ampullicapsulatus (Xi et al., 2011) and was newly identified as Myxobolus honghuensis (Zhao et al., 2013). This "throat cancer" caused the mass mortality of pond-cultured C. gibelio at numerous fish farms in Jiangsu Province (Xi et al., 2011) and posed a grave threat to the aquaculture of C. gibelio all over the country. Myxosporidioses can cause serious pathological effects on host fish. Therefore, it is important to accurately report morphology, pathology and molecular characteristics of the disease causing agent in order to better understand the organism and develop effective control measures to manage or prevent future disease outbreaks.
Between the summer and autumn of 2011, we were informed that mass mortality of pond-reared scattered mirror carp, C. carpio, occurred for a short time at fish farms in southwest China. After we arrived, observation of diseased fish revealed large amounts of myxosporean cysts that accumulated in the guts of host fish. Preliminary microscopic examination indicated the infectious agent was Thelohanellus kitauei (Egusa and Nakajima, 1981). In this current study, morphological and molecular analysis were supplemented with histological evaluation of infected tissues to better understand the route of infection and the pathological effects of T. kitauei on the fish host.
2 MATERIAL AND METHOD 2.1 SamplingTen fish were collected from a pond in Hongxing Town, Mingshan County, Ya'an City, Sichuan Province in southwest China. The 20 hectare pond was stocked with 40 000 adult scattered mirror carp. Mortality had reached approximately 66% with the onset of disease with no signs of abating. Upon necropsy, 6 of 10 C. carpio were found to have myriad large protuberances along the intestinal tracts. These protuberances were composed of numerous small myxosporean cysts, likely the proximate cause of mortality.
2.2 Morphological and histological observationInfected intestinal tracts were isolated from the internal organs of diseased fish through careful dissections. Some of the infected tissues were fixed in 10% neutral formalin for species identification, some in Davidson's fluid for histological examination, and some in 2.5% glutaraldehyde solution for scanning electron microscopy (SEM) observations. The number and size of myxosporean cysts in the intestinal tracts were measured and recorded. Myxospores from infected tissues were photographed under light microscopy, with measurements of key morphological features recorded according to guidelines established by Lom and Arthur (1989). For histological examination, infected tissues were embedded in paraffin, cut into 5 μm sections, and stained with hematoxylin and eosin. For SEM, infected tissues were cut into small pieces, and washed thrice with 0.65% physiological saline. The pieces of infected tissues were then post-fixed in 1% OsO4 at 4℃ for 1 h, followed by serial dehydration in acetone, critical point drying, even coating with metallic gold, and examination under a Quanta 200 (FEI, Holand) SEM.
2.3 Molecular analysesCysts were isolated from infected intestinal tracts of C. carpio and preserved in 95% ethanol for molecular analyses. Individual cysts were cut into several fragments and placed in 1.5-mL Eppendorf tubes. After ethanol evaporated, cyst fragments were ground in the tubes with glass pestles. Total DNA was extracted using Tissue/Cell Genomic DNA Extraction Kit (Bioteke, China) according to the manufacturer's protocol. The 18S SSU rDNA gene was amplified with the primer pairs ERIB1 and ERIB10 (Barta et al., 1997). Polymerase chain reaction (PCR) was performed in 50 μL volumes consisting of 25 μL of 2×PCR Mixture (Bioteke, China), 19 μL of distilled water, 20 pmol of each primer, and approximately 2 μL of isolated DNA. The DNA was denatured at 95℃ for 5 min, followed by 35 cycles at 94℃ for 50 s, 56℃ for 50 s, and 72℃ for 90 s, with a final extension at 72℃ for 10 min. The PCR products were then purified using a PCR Purification Kit (Bioteke, China) according to the manufacturer's protocol. The purified products were cloned into PMD18-T vector (TaKaRa, Japan), and the inserts were sequenced on an ABI 3730XL automatic DNA sequencer (Applied Biosystems Inc., USA) using Bigdye Terminator V3.1 cycle sequencing kit (Applied Biosystems Inc., USA) according to the manufacturer's protocol.
Basic Local Alignment Search Tool (BLASTn) search was performed to compare newly obtained sequences with highly similar sequences in the NCBI database. A total of 47 taxa of sequences related with Thelohanellus kitauei were selected and aligned using Clustal X 1.83 (Thompson et al., 1997) with default parameters. The resulting alignments were manually edited using the program BioEdit (Hall, 1999). After alignment, transitions and transversions were plotted against sequence divergence in DAMBE 4.0.59 (Xia and Xie, 2001) to evaluate the possibility of sequence saturation. The best-fit nucleotide evolutionary model was selected using the Akaike Information Criterion, as implemented in Modeltest 3.6 (Posada and Crandall, 1998). Phylogenetic analyses were performed using Bayesian inference (BI) criteria as implemented in MrBayes v. 3.0 (Ronquist and Huelsenbeck, 2003). To construct a BI tree, the bestfit nucleotide evolutionary model, GTR+I+G model of evolution, was applied, and four independent Markov chains were simultaneously run for 4 000 000 generations, with every 100 trees being sampled for a total of 40 000 trees. As part of a burn-in procedure, the first 10% of trees were discarded, and the remaining samples were used to generate a 50% majority rule consensus tree.
3 RESULT 3.1 Host symptomsA large number of diseased scattered mirror C. carpio were gathered around the periphery of the fish pond. These fish could not maintain balance in the water and were usually unresponsive to even strong external disturbance. Most of the diseased fish swam sluggishly at the surface of the water, showed symptoms of lethargy, anorexia, and dysfunction. The surfaces of diseased fish were coarse, and the abdomens were slightly distended (Fig. 1a). After dissections, abdominal ascites was found among the internal organs, and the intestinal wall was very thin and fragile due to the presence of numerous myxosporean cysts (Figs. 1b, c). No food was present in the intestinal tracts.
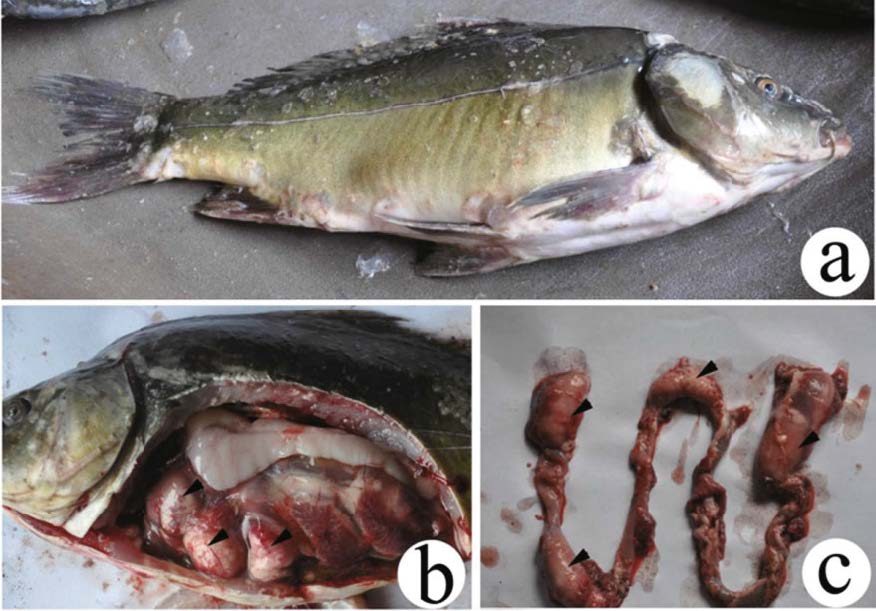
|
| Figure 1 Gross presentation of Thelohanellus kitauei cysts (arrowhead) in the intestine of the scattered mirror carp, Cyprinus carpio a. fish infected by T. kitauei; b. abdominal anatomy of the infected fish; c. infected intestine. |
The intestinal submucosa of C. carpio was first invaded by young plasmodia (Figs. 2a, b), thus leading to necrosis of submucosa cells, which were replaced by T. kitauei spores (Fig. 2c, d). As the cysts in the intestinal submucosa became increasingly developed, the size of the cysts also became increasingly larger. These cysts squeezed the intestinal mucosa, causing deformation, and finally invading the entire tissue layer (Fig. 2a). Upon destruction of the intestinal mucosa, the cysts of T. kitauei entered the intestinal tract and rapidly filled the entire tract. No cysts or spores were observed in the muscle layer of the intestine; nevertheless the fibrae circulares were found to be loose or necrotic, as caused by the infestation of the cysts or spores in other layers of the intestine (Fig. 2b).
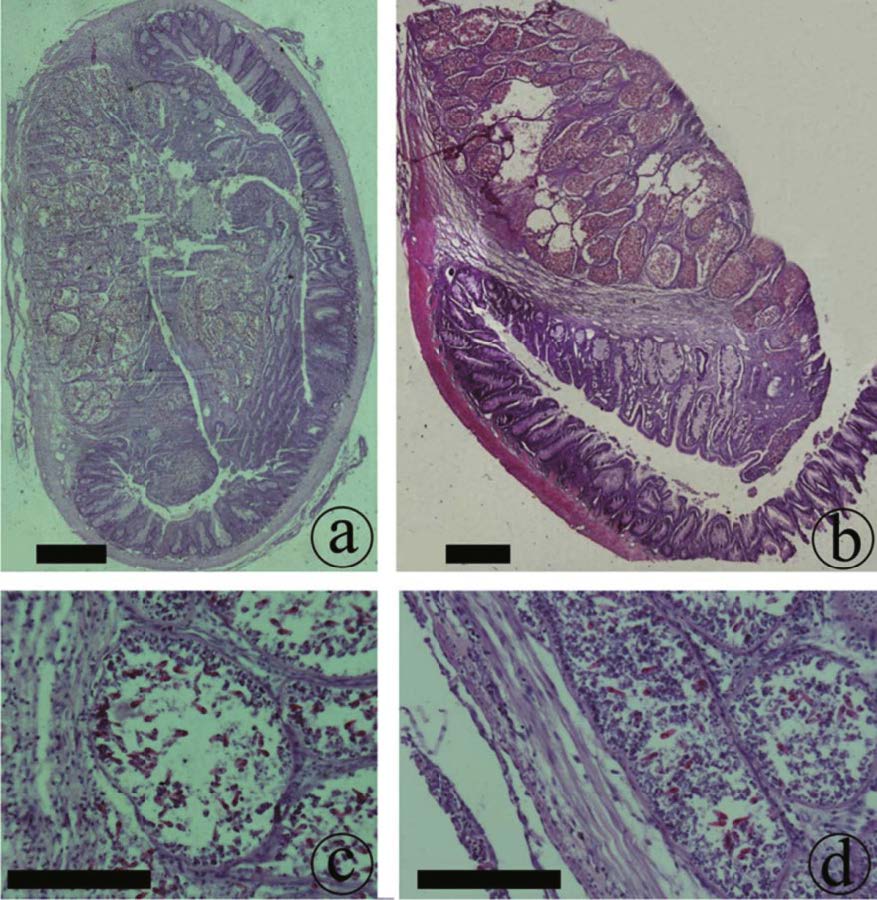
|
| Figure 2 Histological sections of Thelohanellus kitauei-infected intestines of the scattered mirror carp, Cyprinus carpio a. the intestinal submucosa invaded by young plasmodia; b. clusters of fibrae circulares loose and necrotic after invasion of spores; c. cells in intestinal mucosa replaced by T. kitauei cysts after invasion of spores; d. cells in intestinal submucosa replaced by T. kitauei cysts after invasion of spores. Scale bar=0.2 mm. |
The whitish cysts were oval or elongated, ranging from 2 cm to 3.6 cm in diameter. The contents of the cysts were dry and rough (Fig. 3b, c). Fresh spores were pyriform in frontal view, tapered at the anterior ends, and blunt at the posterior ends. The spore sheath surrounded the entire body of the spore, was thin and transparent in appearance, and had no substances within (Fig. 3a, b). The total length of the spores was 38.41 μm±2.45 μm (n=25, mean±SD), the total width was 13.30 μm±0.87 μm, and the total thickness was 13.38 μm±0.84 μm. The spore body length was 25.98 μm±0.95 μm, width was 8.72 μm±0.51 μm, and thickness was 7.86 μm±0.26 μm. The single polar capsule was similar in shape to the mature spore. (Fig. 3b). Two polar capsule nuclei were located in the frontal end (Fig. 3c), with eight to 10 turns of filament coils wound inside the polar capsule. The length of the polar capsule was 14.73 μm±0.92 μm, and the width was 6.82 μm±0.45 μm. Sporoplasm was single, with one round iodinophilous vacuole (Fig. 3b) in the middle part, one pyriform macronucleus in the upper part, and two globular micronuclei near the iodinophilous vacuole (Fig. 3d).
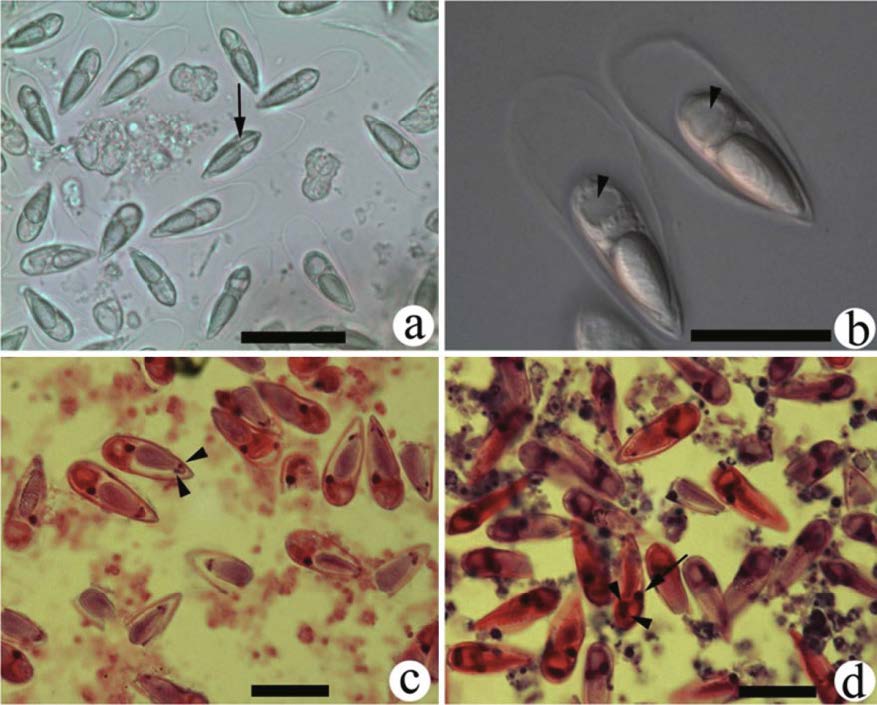
|
| Figure 3 Spores of Thelohanellus kitauei from the intestine of the scattered mirror carp, Cyprinus carpio a. fresh spores without pressing, photographed using inverted microscopy; the sutural line located between two valves (arrow); b. fresh spores observed under oil immersion, one round iodinophilous vacuole located in the middle of the sporoplasm (arrowhead); c. spores in histological sections stained with hematoxylin and eosin, two polar capsule nuclei located at the apex of polar capsule (arrowhead); d. spores observed under oil immersion, histological section, hematoxylin and eosin staining, one macronucleus (arrow) and two nuclei (arrowhead) located in the sporoplasm. Scale bar=20 μm. |
The plasmodium mass was located inside the intestinal mucosa (Fig. 4a, b), and the spores were scattered inside the intestinal mucosa after rupture of T. kitauei cysts (Fig. 4c). The spores were long elliptic or pyriform in overall appearance, and their surfaces were smooth (Fig. 4e), the spore sheath was not observed. The sutural ridges were wide and straight (Fig. 4e). The pyriform polar capsule was present, with eight to 10 turns of funiculose filaments wound inside (Fig. 4d, f). The sporoplasm was heart-shaped and was located beneath the polar capsule (Fig. 4f).
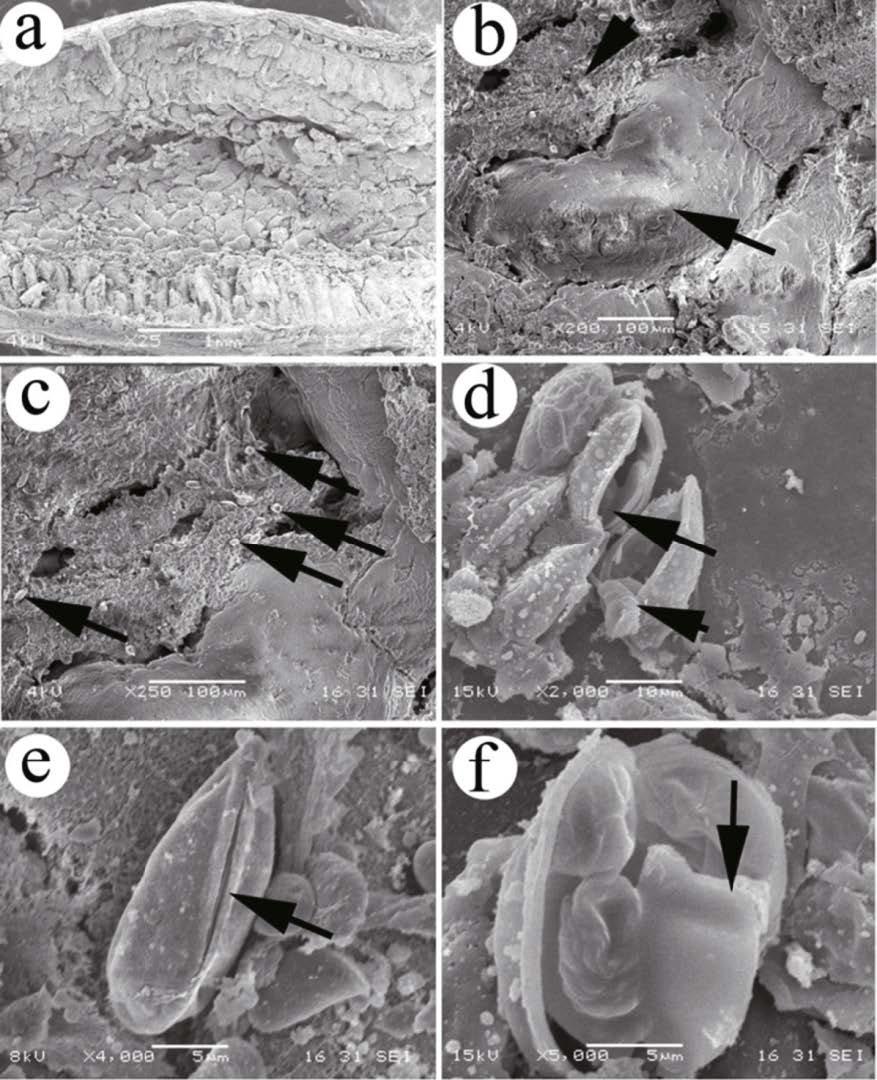
|
| Figure 4 Scanning electron micrographs of Thelohanellus kitauei from the intestine of the scattered mirror carp, Cyprinus carpio a. spores scattered inside intestinal mucosa after rupture of the cysts; b. plasmodium mass (arrow) located inside intestinal mucosa (arrowhead); c. spores (arrow) scattered between intestinal mucosa; d. ruptured spore and polar capsule (arrow), funiculose filaments (arrowhead) located in the polar capsule; e. wide and straight sutural ridge (arrow) located between two valves; f. heart-shaped sporoplasm (arrow) located inside the spore. |
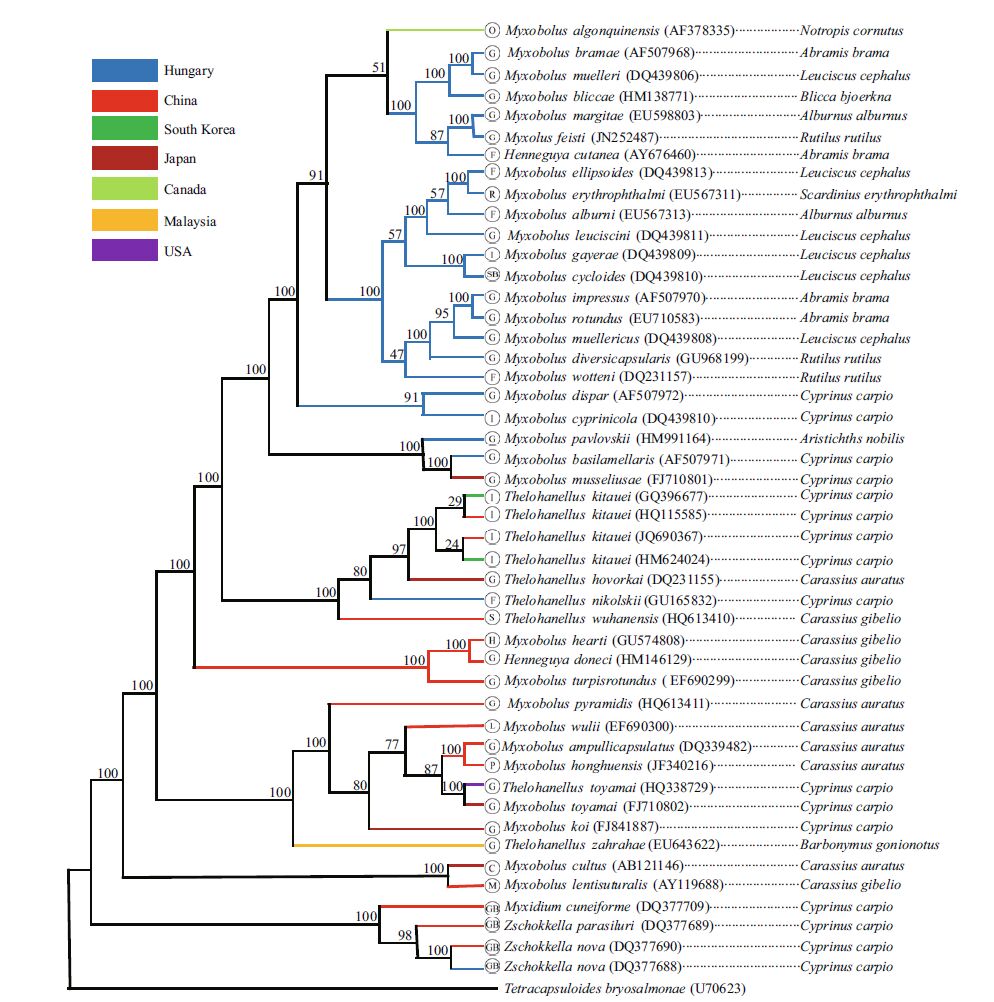
The length of the complete SSU rDNA sequence of T. kitauei (accession number JQ690367) was 2 051 bp with a GC content of 48.1%. The comparison of obtained sequence with those available in GenBank showed slight differences were present within T. kitauei species. The case isolate shared 100% similarity with the strains collected from South Korea (accession number GQ396677) and from Wuhan City in China (accession number HQ115585), and had 99.9% similarity (2046/2049) with another strain collected from South Korea (accession number HM624024). The species T. kitauei was most closely related (95.5%) to T. wuhanensis (accession number JQ690370) in the same genus, one of the most common myxozoan species in China, infecting the skins of juvenile gibel carp (Carassius gibelio). The SSU-based phylogenetic analyses showed that all T. kitauei strains grouped together within the wellsupported Thelohanellus clade, which included T. kitauei, T. hovokai, T. nikolskii, and T. wuhanensis (Fig. 5). After consideration of the parasite host, geographical position, and infection site, no obvious clustering was found to be related to these factors, and only a few species grouped together according to their geographical position, such as those of the Hungarian species (Fig. 5).
|
| Figure 5 Fifty percent major rule consensus tree obtained from Bayesian analysis based on SSU rRNA gene sequences of Myxozoa The GTR+I+G substitution model was used. Numbers beside the nodes represent Bayesian posterior probabilities (the posterior probabilities translated into percentages). Branch color indicates the countries of Myxozoa collection. Abbreviations in the cycle indicate the infected tissues of Myxozoa. G: gill; I: intestine: SB: swim bladder; F: fin; R: renal; S: skin; H: heart; L: liver; P: pharynx; C: cartilage; M: muscle; GB: gall bladder. |
A total of 108 nominal species in genus Thelohanellus Kudo, 1 933 had been reported all over the world; nevertheless only a small number of them are pathogenic to their hosts (Zhang et al., 2013). Thelohannelus kitauei is one of the more severely pathogenic myxosporean species, typically found infecting the intestinal tracts of C. carpio in Asia. The first report of T. kitauei was from Japan, where it attributed to significant losses in pond-reared C. carpio. (Kitaue, 1980; Egusa and Nakajima 1981). Subsequently, similar mortality events were reported in the fish farms of China and South Korea (Rhee et al., 1990; Zhou et al., 1998).
Some myxosporean species have a broad host range, but most exhibit strict host specific tropism and only infect closely related fish hosts (Molnár, 1994; Masoumian et al., 1996; Ye et al., 2014). In the present study, T. kitauei was found to infect the scattered mirror carp. However, T. kitauei has also been reported from Jian and Israeli carp in addition to the common carp (Egusa and Nakajima, 1981; Rhee et al., 1990; Zhou et al., 1998; Liu et al., 2011; Zhai et al., 2016). This is not surprising as scattered mirror carp, Jian carp and Israeli carp are all variants of the common carp derived from intervarietal hybridization (Yoon, 2001; Lou, 2007). They are all conspecific. The marine fish Agrammus agrammus was, strangely, also reported as one of fish hosts of T. kitauei (Zhao and Song, 1999). This so-called T. kitauei was coelozoic parasite infecting the urinary bladder of A. agrammus. However, all T. kitauei strains belonged to freshwater myxosporean species and histozoic parasites infecting the intestines of C. carpio (Egusa and Nakajima, 1981; Liu et al., 2011). Such great differences between the hosts and parasitic tissues seemed unlikely for the same myxosporean species. The coelozoic parasites reported by Zhao and Song (1999) may be another marine clade that shares high morphological similarity with T. kitauei. However, in the absence of confirmatory molecular data, this assertion is mostly inductive speculative.
Tissue-and site-preference of myxosporean species had been noted in various organs of fish, such as gills, hearts, muscles, etc. (Molnár, 1994, 2002; Molnár et al., 2006; Ye et al., 2014). The same preference also appeared in myxosporean species infecting the intestines of fish hosts. Thelohannelus kitauei in this study only formed the cysts in the submucosa and mucosa layer of the intestines, and no other host tissues were infected. Enteromyxum leei and E. scophthalmi have also been reported to exclusively infect the mucosa layer of the intestines (Diamant et al., 1994; Palenzuela et al., 2002; Redondo et al., 2002; Yanagida et al., 2004; Fleurance et al., 2008). In addition, the spores of Kudoa ciliatae, K.intestinalis, Myxoboluslubati, M.intestinalis, M.nodulointestinalis, Henneguya ghaffari, and Sphaerospora dicentrarchi have been observed in the muscle layer of the host intestines (Sitja-Bobadilla and Alvarez-Pellitero, 1992; Maeno et al., 1993; Masoumian et al., 1996; Hallett et al., 1997; Ali, 1999; Ali et al., 2002, 2007). Such distinct site preference of the parasites in the intestines may cause different degrees of destruction in the fish hosts. Muscle-infected parasites may be constricted in the muscle tissues as a reaction of the host, and therefore, these parasites often do not cause severe lesions to their hosts (Masoumian et al., 1996; Ali et al., 2007). Upon invasion of the mucosa layer of the intestines, the parasites may disrupt epithelium structure of the host intestines, thus making the host fish suffer from poor nutrient absorption, followed by emaciation of the hosts, or even mass mortality of the hosts (Diamant et al., 1994; Palenzuela et al., 2002; Fleurance et al., 2008). The case isolate demonstrated similar pathology, invading the mucosa layer and the enteric cavity. Infection by T. kitauei resulted in necrosis of the epithilal cells of the intestine, resulting in dysfunction of the intestinal tissues, malnutrition and eventually death.
The highly specialized structures and ambiguities of defining characters of myxosporean species, as well as the lack of universal criteria for taxonomic structure (for example, intraspecific vs. interspecific variation range of species size) suggests morphological classifications alone are insufficient to differentiate between similar species (Kent et al., 2001; Hogge et al., 2004; Lom and Dyková 2006; Ye et al., 2012). The same taxonomic problems were also present in the discrimination of species similar to T. kitauei (Zhao and Song, 1999; Xie et al., 2000; Liu et al., 2011). With respect to morphology, T. xinyangensis Xie et al., 2000 from the intestine of C. carpio was most similar to T. kitauei (Chen and Ma, 1998; Xie et al., 2000). Liu et al. (2011) rediscribed T. xinyangensis from the same infected tissues and hosts, and they found that SSU rRNA gene sequences shared 99.8%-100% identity between T. xinyangensis and T. kitauei from the intestine of C. carpio in the ponds of South Korea, with minor morphometric differences detected. Therefore, they concluded that T. xinyangensis was synonymous of T. kitauei. In the present study, the same minor differences in the spore dimensions were also present when comparing the present T. kitauei with those from Japan, China, and South Korea (Rhee et al., 1990; Zhou et al., 1998; Xie et al., 2000; Liu et al., 2011). However, only less than 0.1% variation was found within T. kitauei SSU rRNA gene sequences derived from different countries. Therefore, we agreed with the viewpoint that T. xinyangensis was synonymous with T. kitauei and that SSU rRNA gene was an effective molecular marker to support the validity of morphological taxonomy. The intraspecific variation in the spore dimensions of T. kitauei was probably caused by their different locations in the intestine. For example, due to higher pressure from intestinal tissues, the spores in the cysts infecting the submucosa and mucosa layers were smaller in sizes than those in the intestinal cavity. Different spore ages and fixative solutions used might have also resulted in the variation in T. kitauei.
Slight difference in SSU rDNA sequences of T. kitauei between China and South Korea samples implied that low genetic diversity exists across great distances. However, whether or not such geographical isolation caused intraspecific genetic difference needs the comparison of sequences through other molecular markers; for example, the ITS gene, a more variable region of intraspecies of Myxozoa (Whipps and Kent, 2006). We had obtained the ITS sequence of T. kitauei (accession number JQ690368); however the lack of ITS sequence of T. kitauei from South Korea or Japan made the intraspecies genetic comparison of T. kitauei impossible. Phylogenetic analyses of SSU rDNA sequences in the present study revealed close affinity between the genus Thelohanellus and Myxobolus (Fig. 5), as supported by numerous phylogenetic studies (Kent et al., 2001; Fiala, 2006; Fiala and Bartošová, 2010). In fact, the number of polar capsules (PCs) was the only morphological characteristic discriminating between the two genera. Myxobolus spp. possessed two PCs, but Thelohanellus spp. had only one. Lom and Dyková (2006) noted that the species of Thelohanellus spp. were morphologically similar to those of Myxobolus spp., with one PC extremely stunted, and have suggested the closeness of these genera. Fiala and Bartošová (2010) proposed that the one-PC character of the myxosporean spore evolved from the two-PC spore character, and have implied the separation of the genus Thelohanellus from the genus Myxobolus. Interestingly, we observed that two PC nuclei were present at the anterior end of the PC (Fig. 3c) after hematoxylin and eosin staining of T. kitauei spores. This suggested the possession of two PCs within the Thelohanellus spp., and the sizes of the second PC may have been too small to be recognized as a PC, as mentioned by Shulman (1966) and Griffin and Goodwin (2011). Numerous phylogenetic analyses of the myxosporean species based on 18S rDNA sequences have revealed trends to cluster according to site of infection (Andree et al., 1999; Holzer et al., 2004; Fiala, 2006). However, the present study did not found any convincing branch among the histozoic parasites clustering according to the site of infection, with exception to the gall-bladder parasites (Fig. 5). It is speculated this is a result of the coelozoic parasites possessing a slower evolutionary rate due to lower selective pressure inside the host coelom, whereas the evolutionary rate of the histozoic parasites may be expedited by heavier selective pressure from cells of those tissues. Therefore, species diversity of the histozoic parasites may be higher and genetic relationships among the histozoic parasites may be more complicated than those of coelozoic parasites.
The case isolate described here was associated with a severe mortality event in scattered mirror carp. At present, the alternate oligochaete host for this parasite remains unknown. Future studies will focus on the discovery and identification of the actinosporean stage of T. kitauei and the alternate host. Rapid detection of this parasite in its early stages using molecular techniques is another field of study that must explored. Elucidation of the complete T. kitauei life cycle of T. kitauei and the development of early detection techniques is critical to establish measures to effectively control and prevent disease outbreaks associated with this devastating parasite.
5 ACKNOWLEDGMENTWe want to express our sincere thanks to the staff of Sichuan Fisheries School for their help in fish sample collection and Mrs. LI Ranran from NingXia University for her help with the histopathological work. We also thank three anonymous reviewers for critical comments and suggestions on the manuscript. Special thanks is extended to one of reviewers, who spends a lot of energy to polish our manuscript.
| Ali M A, Abdel-Baki A S, Sakran T, Entzeroth R, AbdelGhaffar F, 2007. Myxobolus lubati n. sp. (Myxosporea: Myxobolidae), a new parasite of haffara seabream Rhabdosargus haffara (Forsskal, 1775), Red Sea, Egypt: a light and transmission electron microscopy. Parasitology Research, 100(4): 819–827. Doi: 10.1007/s00436-006-0318-5 |
| Ali M A, Al-Rasheid K A, Sakran T, Abdel-Baki A A, AbdelGhaffar F A, 2002. Some species of the genus Myxobolus (Myxozoa: Myxosporea) infecting freshwater fish of the River Nile, Egypt, and the impact on their hosts. Parasitology Research, 88(1): 9–15. |
| Ali M A, 1999. Henneguya ghaffari sp. n. (Myxozoa: Myxosporea), infecting the Nile perch Lates niloticus (Teleostei: Centropomidae). Diseases of Aquatic Organisms, 38(3): 225–230. |
| Andree K B, El-Matbouli M, Hoffman R W, Hedrick R P, 1999. Comparison of 18S and ITS-1 rDNA sequences of selected geographic isolates of Myxobolus cerebralis. International Journal for Parasitology, 29(5): 771–775. Doi: 10.1016/S0020-7519(99)00035-1 |
| Barta J R, Martin D S, Liberator P A, Dashkevicz M, Anderson J W, Feighner S D, Elbrecht A, Perkins-Barrow A, Jenkins M C, Danforth H D, Ruff M D, Profous-Juchelka H, 1997. Phylogenetic relationships among eight Eimeria species infecting domestic fowl inferred using complete small subunit ribosomal DNA sequences. The Journal of Parasitology, 83(2): 262–271. Doi: 10.2307/3284453 |
| Chen Q L, Ma C L. 1998. Fauna Sinica: Myxozoa, Myxosporea. Science Press, Beijing. p.1-993. (in Chinese) |
| Diamant A, Lom J, Dyková I, 1994. Myxidium leei n. sp., a pathogenic myxosporean of cultured sea bream Sparus aurata. Diseases of Aquatic Organisms, 20(2): 137–141. |
| Egusa S, Nakajima K, 1981. A new myxozoa Thelohanellus kitauei, the cause of intestinal giant cystic disease of carp. Fish Pathology, 15(3-4): 213–218. Doi: 10.3147/jsfp.15.213 |
| Fiala I, Bartošová P, 2010. History of myxozoan character evolution on the basis of rDNA and EF-2 data. BMC Evolutionary Biology, 10(1): 228. Doi: 10.1186/1471-2148-10-228 |
| Fiala I, 2006. The phylogeny of Myxosporea (Myxozoa) based on small subunit ribosomal RNA gene analysis. International Journal for Parasitology, 36(14): 1 521–1 534. Doi: 10.1016/j.ijpara.2006.06.016 |
| Fleurance R, Sauvegrain C, Marques A, Le Breton A, Guereaud C, Cherel Y, Wyers M, 2008. Histopathological changes caused by Enteromyxum leei infection in farmed sea bream Sparus aurata. Diseases of Aquatic Organisms, 79(3): 219–228. |
| Griffin M J, Goodwin A E, 2011. Thelohanellus toyamai (Syn. Myxobolus toyamai) infecting the gills of koi Cyprinus carpio in the eastern United States. Journal of Parasitology, 97(3): 493–502. |
| Hall T A, 1999. BioEdit: a user-friendly biological sequence alignment editor and analysis program for Windows 95/98/NT. Nucleic Acids Symposium Series, 41: 95–98. |
| Hallett S L, O'Donoghue P J, Lester R J G, 1997. Infections by Kudoa ciliatae (Myxozoa: Myxosporea) in Indo-Pacific whiting Sillago spp. Diseases of Aquatic Organisms, 30(1): 11–16. |
| Hogge C, Campbell M, Johnson K, 2004. Discriminating between a neurotropic Myxobolus sp. and M. cerebralis, the causative agent of salmonid whirling disease. Journal of Aquatic Animal Health, 16(3): 137–144. |
| Holzer A S, Sommerville C, Wootten R, 2004. Molecular relationships and phylogeny in a community of myxosporeans and actinosporeans based on their 18S rDNA sequences. International Journal for Parasitology, 34(10): 1 099–1 111. Doi: 10.1016/j.ijpara.2004.06.002 |
| Kent M L, Andree K B, Bartholomew J L, El-Matbouli M, Desser S S, Devlin R H, Feist S W, Hedrick R P, Hoffmann R W, Khattra J, Hallett S L, Lester R J G, Longshaw M, Palenzeula O, Siddall M E, Xiao C X, 2001. Recent advances in our knowledge of the Myxozoa. The Journal of Eukaryotic Microbiology, 48(4): 395–413. Doi: 10.1111/jeu.2001.48.issue-4 |
| Kitaue K, 1980. Intestinal giant-cystic disease affecting the carp, caused by Thellohanellus sp. Fish Pathology, 14(3): 145–146. Doi: 10.3147/jsfp.14.145 |
| Liu Y, Whipps C M, Gu Z M, Zeng C, Huang M J, 2012. Myxobolus honghuensis n. sp. (Myxosporea: Bivalvulida) parasitizing the pharynx of allogynogenetic gibel carp Carassius auratus gibelio (Bloch) from Honghu Lake, China. Parasitology Research, 110(4): 1 331–1 336. Doi: 10.1007/s00436-011-2629-4 |
| Liu Y, Whipps C M, Liu W S, Zeng L B, Gu Z M, 2011. Supplemental diagnosis of a myxozoan parasite from common carp Cyprinus carpio: synonymy of Thelohanellus xinyangensis with Thelohanellus kitauei. Veterinary Parasitology, 178(3-4): 355–359. Doi: 10.1016/j.vetpar.2011.01.008 |
| Lom J, Arthur J R, 1989. A guideline for the preparation of species descriptions in Myxosporea. Journal of Fish Diseases, 12(2): 151–156. Doi: 10.1111/jfd.1989.12.issue-2 |
| Lom J, Dyková I, 2006. Myxozoan genera: definition and notes on taxonomy, life-cycle terminology and pathogenic species. Folia Parasitologica, 53(1): 1–36. Doi: 10.14411/fp.2006.001 |
| Lom J, 1987. Myxosporea: a new look at long-known parasites of fish. Parasitology Today, 3(11): 327–332. Doi: 10.1016/0169-4758(87)90115-3 |
| Longshaw M, Frear P A, Feist S W, 2005. Descriptions, development and pathogenicity of myxozoan (Myxozoa: Myxosporea) parasites of juvenile cyprinids (Pisces: Cyprinidae). Journal of Fish Diseases, 28(8): 489–508. Doi: 10.1111/jfd.2005.28.issue-8 |
| Lou Y D, 2007. Close hybridization of fish and its application in aquaculture. Journal of Fisheries of China, 31(4): 532–538. |
| Maeno Y, Nagasawa K, Sorimachi M, 1993. Kudoa intestinalis n. sp. (Myxosporea: Multivalvulida) from the intestinal musculature of the striped mullet, Mugil cephalus, from Japan. The Journal of Parasitology, 79(2): 190–192. Doi: 10.2307/3283506 |
| Masoumian M, Baska F, Molnár K, 1996. Myxobolus nodulointestinalis sp. n. (Myxosporea, Myxobolidae), a parasite of the intestine of Barbus sharpeyi. Diseases of Aquatic Organisms, 24(1): 35–39. |
| Molnár K, Marton S, Eszterbauer E, Székely C, 2006. Comparative morphological and molecular studies on Myxobolus spp. infecting chub from the River Danube, Hungary, and description of M. muellericus sp. n. Diseases of Aquatic Organisms, 73(1): 49–61. |
| Molnár K, 1994. Comments on the host, organ and tissue specificity of fish myxosporeans and on the types of their intrapiscine development. Parasitologia Hungarica, 27: 5–20. |
| Molnár K, 2002. Site preference of fish myxosporeans in the gill. Diseases of Aquatic Organisms, 48(3): 197–207. |
| Palenzuela O, Redondo M J, Alvarez-Pellitero P, 2002. Description of Enteromyxum scophthalmi gen. nov., sp. nov. (Myxozoa), an intestinal parasite of turbot (Scophthalmus maximus L.) using morphological and ribosomal RNA sequence data. Parasitology, 124(Pt 4): 369–379. |
| Posada D, Crandall K A, 1998. Modeltest: testing the model of DNA substitution. Bioinformatics, 14(9): 817–818. Doi: 10.1093/bioinformatics/14.9.817 |
| Redondo M J, Palenzuela O, Riaza A, Macías Á, ÁlvarezPellitero P, 2002. Experimental transmission of Enteromyxum scophthalmi (Myxozoa), an enteric parasite of turbot Scophthalmus maximus. Journal of Parasitology, 88(3): 482–488. Doi: 10.1645/0022-3395(2002)088[0482:ETOESM]2.0.CO;2 |
| Rhee J K, Kim J O, Park B K, 1990. Prophylactic and therapeutic studies on intestinal giant-cystic disease of the Israel carp caused by Thelohanellus kitauei. Ⅱ. . Effects of physical and chemical factors on T. Kitauei spores in vitro. The Korean Journal of Parasitology, 28(4): 241–252. |
| Ronquist F, Huelsenbeck J P, 2003. MrBayes 3: Bayesian phylogenetic inference under mixed models. Bioinformatics, 19(12): 1572–1574. Doi: 10.1093/bioinformatics/btg180 |
| Shulman S S. 1966. Myxosporidia of the Fauna of the USSR. Nauka, Moscow. p.1-504. (in Russian) |
| Sitja-Bobadilla A, Alvarez-Pellitero P, 1992. Light and electron microscopic description of Sphaerospora dicentrarchi n. sp. (Myxosporea: Sphaerosporidae) from wild and cultured sea bass, Dicentrarchus labrax L. Journal of Eukaryotic Microbiology, 39(2): 273–281. |
| Thompson J D, Gibson T J, Plewniak F, Jeanmougin F, Higgins D G, 1997. The Clustal_X windows interface: flexible strategies for multiple sequence alignment aided by quality analysis tools. Nucleic Acids Research, 25(24): 4876–4882. Doi: 10.1093/nar/25.24.4876 |
| Wang G T, Yao W J, Gong X N, Wang J G, Nie P, 2003. Seasonal fluctuation of Myxobolus gibelioi (myxosporea) plasmodia in the gills of the farmed allogynogenetic gibel carp in China. Chinese Journal of Oceanology and Limnology, 21(2): 149–153. Doi: 10.1007/BF02843145 |
| Wang G T, Yao W J, Wang J G, Lu Y S, 2001. Occurrence of thelohanellosis caused by Thelohanellus wuhanensis (Myxosporea) in juvenile allogynogenetic silver crucian carp, Carassius auratus gibelio (Bloch), with an observation on the efficacy of fumagillin as a therapeutant. Journal of Fish Diseases, 24(1): 57–60. Doi: 10.1046/j.1365-2761.2001.00269.x |
| Whipps C M, Kent M L, 2006. Phylogeography of the cosmopolitan marine parasite Kudoa thyrsites (Myxozoa: Myxosporea). The Journal of Eukaryotic Microbiology, 53(5): 364–373. Doi: 10.1111/jeu.2006.53.issue-5 |
| Xi B W, Xie J, Zhou Q L, Pan L K, Ge X P, 2011. Mass mortality of pond-reared Carassius gibelio caused by Myxobolus ampullicapsulatus in China. Diseases of Aquatic Organisms, 93(3): 257–260. Doi: 10.3354/dao02297 |
| Xia X, Xie Z, 2001. DAMBE: software package for data analysis in molecular biology and evolution. Journal of Heredity, 92(4): 371–373. Doi: 10.1093/jhered/92.4.371 |
| Xie X R, Gong X N, Xiao W H, Guo Q L, Li L C, Guo X S, 2000. Studies on Thelohanellionsis (Myxosporidiosis) parasitized freshwater fish in China. I. Thelohanelliosis (Myxosporidiosis) parasitized Carp in Xinyang, Henan Province. Journal of Xinyang Agricultural College, 10(2): 1–5. |
| Yanagida T, Nomura Y, Kimura T, Fukuda Y, Yokoyama H, Ogawa K, 2004. Molecular and morphological redescriptions of enteric myxozoans, Enteromyxum leei (formerly Myxidium sp. TP) and Enteromyxum fugu comb. n. (syn. Myxidium fugu) from cultured tiger puffer. Fish Pathology, 39(3): 137–144. Doi: 10.3147/jsfp.39.137 |
| Ye L T, Li W X, Wang W W, Wu S G, Wang G T, 2014. Updated morphology, histopathology and molecular phylogeny of Myxobolus hearti, cardiac myxosporea in gibel carp, Carassius gibelio (Bloch). Journal of Fish Diseases, 37(1): 11–20. Doi: 10.1111/jfd.2013.37.issue-1 |
| Ye L T, Li W X, Wu S G, Wang G T, 2012. Supplementary studies on Henneguya doneci Schulman, 1962 (Myxozoa: Myxosporea) infecting the gill filaments of Carassius auratus gibelio (Bloch) in China: histologic, ultrastructural, and molecular data. Parasitology Research, 110(4): 1509–1516. Doi: 10.1007/s00436-011-2655-2 |
| Yoon J M, 2001. Genetic similarity and difference between common carp and Israeli carp (Cyprinus carpio) based on random amplified polymorphic DNAs analyses. Korean Journal of Biological Sciences, 5(4): 333–339. Doi: 10.1080/12265071.2001.9647624 |
| Zhai Y H, Gu Z M, Guo Q X, Wu Z Z, Wang H M, Liu Y, 2016. New type of pathogenicity of Thelohanellus kitauei Egusa & Nakajima, 1981 infecting the skin of common carp Cyprinus carpio L. Parasitology International, 65(1): 78–82. Doi: 10.1016/j.parint.2015.10.010 |
| Zhang J Y, Gu Z M, Kalavati C, Eiras J C, Liu Y, Guo Q Y, Molnár K, 2013. Synopsis of the species of Thelohanellus Kudo, 1933 (Myxozoa: Myxosporea: Bivalvulida). Systematic Parasitology, 86(3): 235–256. Doi: 10.1007/s11230-013-9449-0 |
| Zhang J Y, Yokoyama H, Wang J G, Li A H, Gong X N, RyuHasegawa A, Iwashita M, Ogawa K, 2010. Utilization of tissue habitats by Myxobolus wulii Landsberg & Lom, 1991 in different carp hosts and disease resistance in allogynogenetic gibel carp: redescription of M. wulii from China and Japan. Journal of Fish Diseases, 33(1): 57–68. |
| Zhao Y J, Li N N, Tang F H, Dong J L, 2013. Remarks on the validity of Myxobolus ampullicapsulatus and Myxobolus honghuensis (Myxozoa: Myxosporea) based on SSU rDNA sequences. Parasitology Research, 112(11): 3817–3823. Doi: 10.1007/s00436-013-3569-y |
| Zhao Y J, Song W B, 1999. Redescription of Thelohanellus kitauei Egusa & Nakajima, 1981 (Mxospores: Thelohanellidae), parasitic in marine fishers from the Yellow Sea, China. Journal of the Yellow Sea, 5: 73–76. |
| Zhou L, Gong Q L, Yu K K, Meng Q X, 1998. A preliminary study on the disease of Myxozoa, Thelohanellus Kitauei of Jian Carp (Cyprinus Carpio Var. Jian). Journal of Ocean University of Qingdao, 28(1): 59–62. |
 2017, Vol. 35
2017, Vol. 35


