Institute of Oceanology, Chinese Academy of Sciences
Article Information
- ZHENG Bin(郑斌), XIANG Xingwei(相兴伟), ZHOU Yufang(周宇芳), YANG Huicheng(杨会成), LUO Hongyu(罗红宇), LIAO Miaofei(廖妙飞), WEN Zhengshun(闻正顺)
- Characterization and antioxidant activities of marine pepsin soluble collagen from the skin of yellow goosefish Lophius litulon
- Chinese Journal of Oceanology and Limnology, 35(3): 634-644
- http://dx.doi.org/10.1007/s00343-017-5377-1
Article History
- Received Dec. 29, 2015
- accepted in principle Mar. 31, 2016
2 Zhejiang Marine Development Research Institute, Zhoushan 316000, China
Reactive oxygen species (ROS), such as superoxide anion and hydroxyl radicals, hydrogen peroxide, singlet oxygen, and hypochlorous acid, are produced inside cells under physiological and pathological conditions in response to external stimuli and chemicals (Sampath Kumar et al., 2012). Free oxygen radicals have been proposed as important causative agents of aging and many health disorders, such as diabetes mellitus, cancer, and neurodegenerative and inflammatory diseases (Butterfield et al., 2002). Endogenous cellular defense systems are equipped with enzymes to decrease ROS impacts (Ratnam et al., 2006).
At present the synthetic antioxidants butylated hydroxytoluene and butylated hydroxyanisole (BHT and BHA, respectively) are extensively used as food preservatives and have been suspected to threaten human health by causing liver damage and carcinogenesis (Sampath Kumar et al., 2012). Thus, research directed toward the development of natural, safer, and more effective antioxidants has attracted considerable attention. Collagen has significant antioxidant activities, including the ability to inactivate ROS, scavenge free radicals, chelate prooxidative transition metals, reduce hydroperoxides, and enzymatically eliminate specific oxidants (Elias et al., 2008; Sampath Kumar et al., 2012). Collagen not only plays an important physiological role but it is also utilized as an ingredient in foods, cosmetics, pharmaceuticals, and experimental reagents (Yamada et al., 2014).
Yellow goosefish (Lophius litulon) has become an economically important marine species. Its meat is used for the production of fish balls or fillets, and the remaining mass is considered a by-product (Wei et al., 2016). Collagen in by-products, including scales, fins, skins, bones, heads, guts, and frames, generated during fish processing has been reported to account for approximately 30% of the total protein (Wei et al., 2016). The biochemical properties of collagens extracted mainly from red snapper (Jongjareonrak et al., 2005), deep-sea redfish (Wang et al., 2007), Lates calcarifer (Sankar et al., 2008), bigeye snapper (Benjakul et al., 2010), cobia (Zeng et al., 2012), horse mackerel (Sampath Kumar et al., 2012), tilapia (Zhang et al., 2012), croceine croaker (Wang et al., 2013), amur sturgeon (Wang et al., 2014), yellowfin tuna (Kaewdang et al., 2014), and seabass (Sinthusamran et al., 2014) have been studied. To date, there have been no reports on collagen from yellow goosefish. Thus, the aim of this study was to identify and characterize potential antioxidant collagen peptides from pepsin-digested yellow goosefish skin and evaluate the antioxidant properties of these peptides in vitro and in vivo.
2 MATERIAL AND METHOD 2.1 Materials and chemicalsFrozen yellow goosefish (10 specimens) with an average body weight of 1.5-2.0 kg were provided by Zhejiang Xingye Group Co. Ltd., Zhejiang Province, China. Skins were prepared for collagen extraction by thawing frozen samples with running tap water until the fish core temperature reached 10℃. After manually removing the skin, it was washed with cold, distilled water and stored at -80℃ for no longer than 3 months. Bovine hemoglobin, β-mercaptoethanol (β- ME), pepsin (EC 3.4.23.1; powdered; 750 U/mg dry matter), acetic acid, and calfskin collagen (CSC) were purchased from Sigma-Aldrich, Inc. (St. Louis, MO, USA). Sodium dodecyl sulfate (SDS), N, N, N′, N′- tetramethylethylenediamine, and Coomassie Blue R-250 were procured from Bio-Rad Laboratories, Inc. (Hercules, CA, USA). High molecular protein weight markers were obtained from Shanghai Institute of Biochemistry, Chinese Academy of Sciences (Shanghai, China).
2.2 AnimalsSpecific pathogen-free female Kunming mice (18- 20 g, aged ~8 weeks) were provided by the Center for Experimental Animals of Zhoushan City (Zhoushan, Zhejiang, China) and incubated for 6 days in an approved mouse facility at 25±1℃ and in a room with 60% relative humidity. Those with dysplasia were eliminated by observation during the 6-day acclimatization. In this study, all experimental procedures were performed according to standard guidelines for animal care and approved by the Animal Welfare Committee of the Center for Experimental Animals of Zhoushan.
2.3 Collagen extractionCollagen was extracted using a previously established method (Zeng et al., 2012), with slight modifications. The following steps were performed at 4℃ unless otherwise indicated. First, frozen skin was thawed with running water and washed with cold tap water to remove the residual meat by knife. Subsequently, treated skins were cut into small 2-cm× 2-cm pieces using scissors, soaked in 0.1 mol/L NaOH solution for 3 h, and then placed in a blender at 8 000 r/ min for 5 min; this treatment removed nearly all scales. Non-collagenous proteins and pigments were removed by soaking scaled skin samples in 0.1 mol/L NaOH with a sample/solution ratio of 1/20 (w/v) and continuous stirring for 48 h using a Magnetic Stirrer (IT-08/09, Shanghai Yiheng Technical Co. Ltd., Shanghai, China) at 500 r/min. The cleaning solution was changed every 12 h. Skin pieces were washed with distilled water until at neutral pH.
The skin pieces (100 g wet wt) were washed twice with dd-H2O for 30 min and then soaked in an 0.5 mol/L acetic acid solution at a solid/solvent ratio of 1/15 (w/v) and with 20 U/g of added pepsin. After filtration through two layers of cheesecloth, the mixture was centrifuged at 9 000×g and 4℃ for 30 min. The residual liquid was extracted again in same manner and the filtrates pooled.
Collagen was precipitated by adding NaCl to a final 2.6 mol/L in the presence of Tris (0.05 mol/L, pH 7.5). Following centrifugation at 15 000×g for 30 min (CR21G refrigerated centrifuge, Hitachi Koki Co. Ltd., Tokyo, Japan), the precipitate was collected and resuspended in a minimum volume of 0.5 mol/L acetic acid, successively dialyzed against 0.1 mol/L acetic acid (25 volumes) for 2 days and then against distilled water for 1 day, changing the solution every 6 h. The dialysate was freeze-dried using a Labconco Freeze Dryer FreeZone 6 Liter (Labconco Corp., Kansas City, MO, USA) and stored at 4℃ for further analysis.
2.4 Amino acid composition analysisThe resulting pepsin-soluble collagen (PSC) was hydrolyzed using 6 mol/L HCl at 110℃ for 24 h in a sealed glass tube. After vacuum evaporation, the residue was dissolved in 30 mL of citric acid buffer solution (0.1 mol/L). Then, 0.06 mL of the resulting solution was applied to an automated amino acid analyzer (HITACHI 835-50, Hitachi Co. Ltd., Tokyo, Japan).
2.5 SDS-PAGE analysisSDS-PAGE was done according to the method of LaemmLi (1970) , with slight modifications, using a 7.5% separating gel and 4% stacking gel. Collagen samples were suspended in 5% (w/v) SDS at 85℃ for 1 h prior to centrifugation at room temperature. A 20- μL volume of the suspension was mixed with loading buffer (60 mmol/L Tris-HCl, pH 8.0, 0.1% bromophenol blue, 2% SDS, and 25% glycerol) at a ratio of 4/1 (v/v) in the presence of β-ME and then electrophoresed in an AE-6200 electrophoresis instrument (ATTO Corp., Tokyo, Japan) for 4 h using a constant voltage of 100 V. The resolved bands were stained using Coomassie Brilliant Blue R-250 solution (0.1%, w/v) for 40 min in an aqueous acetic acid and methanol solution (10% and 45%, respectively). High molecular protein weight markers were used to gauge the relative PSC molecular weights and CSC was used as a standard.
2.6 UV and FTIR spectroscopic analysesPSC was scanned in a UV spectrophotometer (UV- 1800, Mapada Instruments Co. Ltd., Shanghai, China) at 5 nm/min from 200 to 400 nm. The sample was in 0.5 mol/L acetic acid at 1/1 000 (w/v).
FTIR spectroscopy of PSC was performed using a Nicolet 6700 recording spectrophotometer (Thermo Fisher Scientific Inc., Waltham, Massachusetts, USA) at 2/cm per point from 4 000 to 500/cm. Lyophilized PSC (1 mg) was mixed with dry KBr (200 mg) and pressed into a tablet for 1 min before scanning.
2.7 Viscosity DeterminationPSC viscosity was investigated at different temperatures using the method of Kittiphattanabawon et al. (2005) . PSC was dissolved in 0.5 mol/L acetic acid at 1 mg/mL. The solution was heated from 4 to 40℃ at 4℃/min, and then held for 30 min before the viscosity was determined at a given temperature. The relative viscosity was calculated by taking the viscosity at 4℃ as the reference.
2.8 SolubilityCollagen solubility was determined according to a previous report (Zhao et al., 2015), with a slight modification. PSC at 3 mg/mL was dissolved in 0.5 mol/L acetic acid, stirred well at 4℃ for 1 d, and then centrifuged at 5 000×g for 20 min. The supernatant was used for the solubility study.
The effects of pH on solubility were examined by adding 8 mL of PSC solution into each of 12 centrifuge tubes (50 mL) and the pH value adjusted to create a pH range of 1-12 using 6 mol/L NaOH or HCl. Then, the solution volume was adjusted to 10 mL using deionized water and the centrifuged at 15 000×g at 4℃ for 1 h. The supernatant protein content was determined using the method of Zeng et al. (2012) , using bovine serum albumin as a protein standard.
The effects of NaCl concentration on solubility were examined by dissolving 5 mL of PSC solution (1 mg/mL) into 5 mL of 0.5 mol/L acetic acid, and NaCl was added at varying concentrations, from 0% to 6% (w/v). These mixtures were stirred continuously at 4℃ for 30 min and then centrifuged at 15 000×g at 4℃ for 1 h. The supernatants' protein content was determined and the relative solubilities computed according to the previous description (Zeng et al., 2012).
2.9 Hydroxyl radical scavenging activity assayPSC's scavenging activity against hydroxyl radicals was evaluated using the Fenton reaction system (Wang et al., 2013), with slight modifications. Briefly, 1, 10-phenanthroline solution (1.0 mL, 1.865 mmol/L) and FeSO4·7H2O solution (1.0 mL, 1.865 mmol/L) together with PSC solution (2.0 mL) were added sequentially into a vacuum-sealed tube, followed by homogeneous mixing. Then, 1.0 mL of H2O2 was added at a 3/13 (v/v) ratio to initiate the reaction. After incubation at 37℃ for 1 h, the A536 of the reaction mixture was determined against a reagent blank. The reaction mixture without antioxidant was used as a negative control while the mixture lacking H2O2 represented a blank control. The hydroxyl radical scavenging activity was defined as:
Hydroxyl radical scavenging (%) =[ (A1-A2) / (A3- A2) ]×100%,
where A1 represents the sample's A536, and A2 and A3 represent that of the negative and blank controls, respectively.
2.10 Superoxide anion scavenging activity assayPSC's scavenging activity for superoxide anion was measured using the pyrogallol-uminol system (Wang et al., 2013). PSC (0.1 mL) in cold distilled water and a luminol solution (850 μL, 1 mmol/L) 0.1 mol/L Na2CO3 together with fresh pyrogallol solution (50 μL, 0.625 mmol/L) were mixed in a reaction vacuum tube. The luminol chemiluminescence in the system was subsequently determined using a WDD-2 chemiluminometer (Beijing Rayleigh Analytical Instrument Corp., Beijing, China) and phosphate buffered saline (50 mmol/L, pH 7.8) used as the blank. Scavenging activity was calculated according to:
Superoxide scavenging activity (%) =[ (A0-A1) / A0]×100%,
where A1 and A0 represent the chemiluminescence of PSC and the blank, respectively.
2.11 Antioxidant activity of PSC in vivo 2.11.1 Establishment of D-galactose-induced aging mice and experiments with PSCFemale mice were randomLy divided into 4 groups of 10 each, the model, control, and two experimental (i.e., low- and high-dose) groups. All groups except the control group were injected subcutaneously with dosage of 1 000 mg/ (kg∙d) of D-galactose. Simultaneously, mice from the experimental groups were provided with 2 mL of PSC. Mice in the PSC dose group were given 50 mg/ (kg∙d) while those in the high-dose group received 100 mg/ (kg∙d). Concurrently, the control and model group mice were injected with normal saline (10 mL/ (kg∙d) ). All animals were fed with standard water and diet during the 30-day experiment.
2.11.2 Sample collectionSubcutaneous tissues were collected from the dorsal skin the day after the last PSC injection. Samples were defatted via an isopropanol treatment, weighed, cut into pieces, and homogenized after addition of normal saline (1/9, w/v) at 4℃. After centrifugation at 3 000×g for 15 min, the supernatant was collected and stored at -20℃ until further analysis.
2.11.3 DeterminationThe activities of superoxide dismutase, glutathione peroxidase, catalase, and glutathione (SOD, GSH-Px, CAT, and GSH, respectively) and the content of malondialdehyde (MDA) in the dorsal skin tissue homogenate were examined using reagent kits (Nanjing Jiancheng Bioengineering Inst., Nanjing, China). Analyses were performed according to manufacturer's instructions.
2.12 Statistical analysisAll experiments were conducted in triplicate, except for the amino acid composition analysis. Data were reported as means±standard deviations and further subjected to one-way ANOVA analyses. Values of P<0.05 were considered to indicate significant differences. Statistical analyses were performed using SPSS 13.0 for Windows (SPSS Inc., Chicago, IL, USA).
3 RESULT AND DISCUSSION 3.1 Optimization of PSC extractionAfter 24-h incubation with 20 U pepsin/g tissue at 4℃ and pH 2, PSC isolated from skin was 9.33±0.54% pure. The PSC therefore had purity higher than those isolated from Spanish mackerel (Li et al., 2013), bigeye and brownstripe red snappers (Jongjareonrak et al., 2005). These varied collagen yields might have resulted from differences between the species, their biological conditions, tissue compositions and structures, and even preparation methods.
3.2 PSC CharacteristicsThe PSC obtained here was investigated in terms of amino acid composition, SDS-PAGE, UV and FTIR spectroscopies, viscosity, and solubility (Fig. 1a-f).
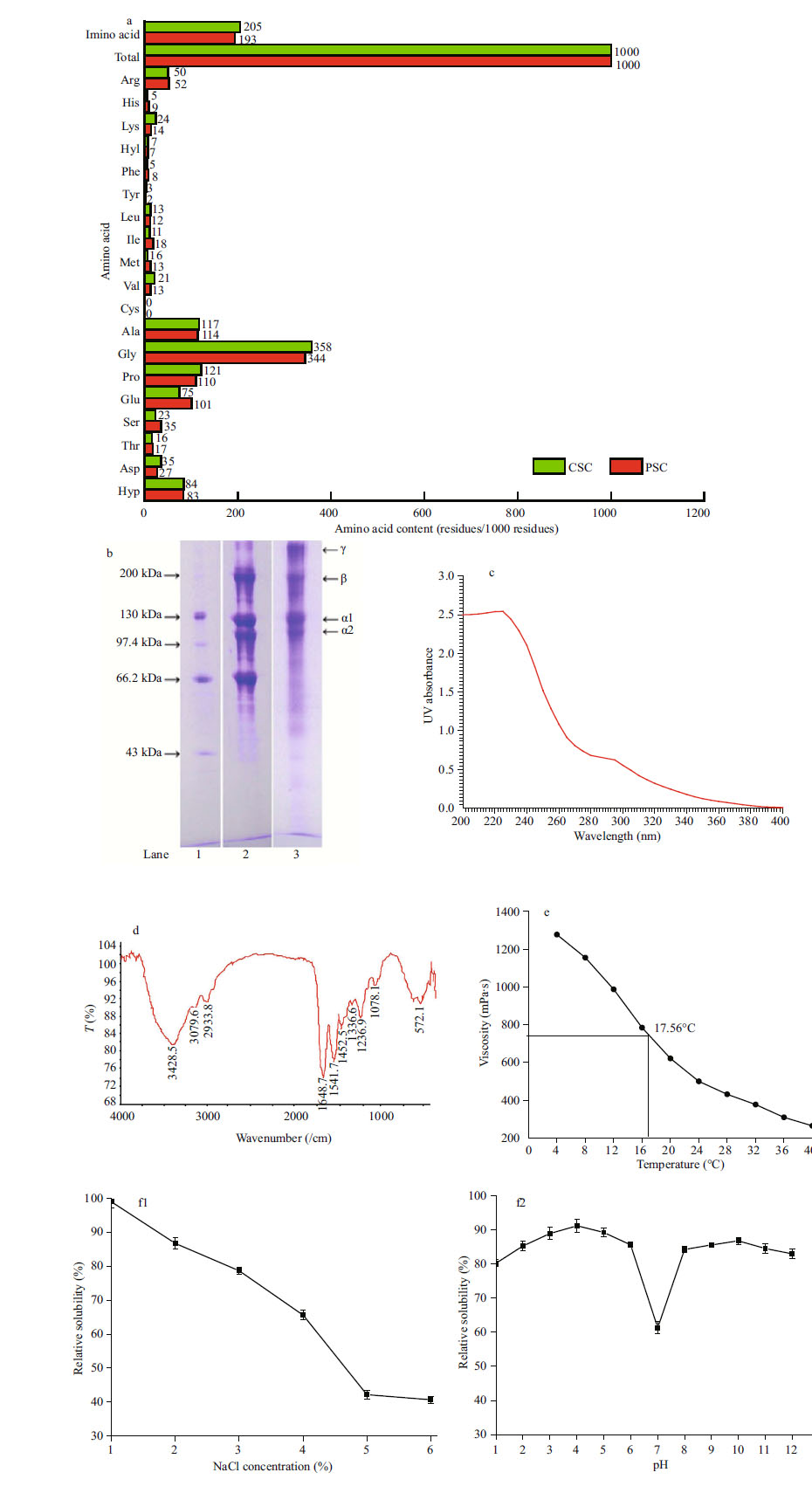
|
| Figure 1 Characteristics and profile of PSC from yellow goosefish Lophius litulon a. amino acid composition, expressed as specific residue/1 000 residues; b. SDS-PAGE: lane 1: protein marker; lane 2: PSC; lane 3: CSC; c. UV scanning spectroscopy; d. FTIR spectroscopy; e. thermal denaturation curve, as means±standard deviations; f. relative solubilities at different pH's and NaCl concentrations (f1 and f2, respectively), as mean±standard deviation. |
The PSC amino acid composition, expressed as residues per 1 000 total residues, showed a composition rich in Gly (344/1 000 residues) that was similar to other collagens, followed by Glu, Hyp, Ala, and Pro, and low proportions of Met, His, Hyp, Try, and Cys (Fig. 1a). The total content of imino acids (Pro and Hyp) was 193/1 000 residues, similar to those from the skin of grass carp (Zhang et al., 2007), croceine croaker (Wang et al., 2013), bigeye snapper (Benjakul et al., 2010), and seabass (Sinthusamran et al., 2014). Additionally, the total imino acids were lower compared with those from labeo rohita and catla (Pati et al., 2010). Imino acids contribute to collagen triple helix structural stability. Zones of collagen molecules rich in hydroxyproline and proline are involved in the formation of junctions stabilized by hydrogen bonding (Kaewdang et al., 2014). Observed differences in imino acid content might have been related to the species' living environments. Here, PSC's imino acid content was lower than those from CSC, thus yielding collagen with helices less stable than those in collagen from mammalian skin.
SDS-PAGE showed that PSC contained high proportions of low molecular weight chains that resulted from enzymatic conversion of large peptide chains to small chains (Fig. 1b). Moreover, α1 and α2 chains, β dimers, and γ trimers were delineated (Fig. 1b), showing a pattern that suggested that PSC possessed a molecular structure of (α1) 3 with a relative molecular mass of 130 kDa. There was an apparent low molecular weight band in PSC that is not found in CSC, which might have resulted from differences in the ease of enzymatic digestion among collagens from different sources and species. SDS-PAGE indicated that PSC isolated here was type I collagen, similar to collagen from Pagrus major and Oreochromis niloticus (Ikoma et al., 2003), croceine croaker (Wang et al., 2013), seabass (Sinthusamran et al., 2014), lizard fishes, horse mackerel, grey mullet, flying fish, and yellowback seabream (Minh Thuy Le et al., 2014) .
UV scanning spectroscopic results revealed that PSC had a maximum absorbance at 230 nm, which was similar to skin from arabesque greenling (233 nm; Nalinanon et al., 2010). Generally, the maximum absorption wavelength of protein in the near ultraviolet region is 280 nm. However, PSC showed is no obvious absorption at 280 nm, indicating that this collagen possessed few aromatic amino acids and was consistent with the present amino acid composition findings (Fig. 1a).
FTIR spectroscopy revealed amides A, B, Ⅰ, Ⅱ, and Ⅲ as the characteristic absorption bands, detected at 3 428.5, 3 079.6, 1 648.7, 1 541.7, and 1 236.9/cm, respectively (Fig. 1d), which were similar to those from other fish species (Benjakul et al., 2010; Kittiphattanabawon et al., 2010; Wang et al., 2013; Kaewdang et al., 2014). It is well known that amide A is associated with N-H stretching vibrations (Doyle et al., 1975). A slight shift to lower wavenumber was observed for PSC, compared to non-collagenous proteins, which indicated that it contained more N-H groups. The amide B band position from PSC was at 3 079.6/cm, which was related to the asymmetrical stretch of CH2.
The amide Ⅰ band was mainly associated with C=O stretching vibrations, with H-bond coupling with COO- or secondary protein structure. The amide Ⅰ band position was at 1 648.7/cm, which was consistent with characteristic frequencies ranging from 1 600 to 1 700/cm. Alternatively, the amide Ⅱ band was ~1 541.7/cm, which was associated with N-H deformation. In contrast, the amide Ⅲ band position was at 1 220-1 320/cm, which was not only related to N-H deformation but also due to C-N stretching vibrations. The absorption band between the amide Ⅲ and a 1 452/cm band implied the existence of helical structure. The FTIR spectrum was in accordance with the results from the present SDSPAGE analysis (Fig. 1b).
Examination of PSC relative viscosity at various temperatures showed that viscosity decreased rapidly at temperatures above 32℃ (Fig. 1e). However, as temperature continued to rise, the rate of viscosity decrease slowly declined. Hydrogen bonds between adjacent collagen polypeptide chains are disrupted by high temperature, causing transformation of intact trimers into individual chains or dimers, thus leading to collagen denaturation and declining collagen viscosity. The denaturation temperature (Td) refers to the temperature at the transition midpoint where the ratio of denatured to native state is 1/1, determined through viscosity measurements. Here, PSC's Td was judged to be 17.56℃, which was much lower than those of rohu and catla (Pati et al., 2010), tuna (Kaewdang et al., 2014), lizard fishes, horse mackerel, grey mullet, flying fish, and yellowback seabream (Minh Thuy Le et al., 2014). In addition, the present Td was notably lower than those of mammalian collagen (Td of ~41℃; Burjanadze, 1992). The variation in Td values might have resulted from differences between the species, living environments, body temperatures, and slight differences in measurement methods.
Examination of the effects of pH and NaCl concentration on PSC's relative solubility showed that its relative solubility in 0.5 mol/L acetic acid decreased as NaCl concentration increased to 5% (w/v; Fig.f1).This collagen preparation exhibited better solubility at <2% NaCl concentrations, which was similar to collagens from the skin of Spanish mackerel, brownstripe red snapper, and trout (Jongjareonrak et al., 2005; Li et al., 2013). As the ionic strength increased, hydrophobic interactions between protein chains and the competition with water for the salt was enhanced, thus inducing more protein precipitation (Minh Thuy Le et al., 2014).
Alternatively, better PSC relative solubility was observed at pH from 1 to 6, but it drastically declined at pH 6-7 (42.29±3.32%; Fig. 1f2), implying that PSC was more solubilized under acidic conditions. PSC's isoelectric point (pI) was ~7, within the range of isoelectric points of other collagens (pH 6-9). When the pH is lower or higher than the pI, the net residue charges of protein molecules are greater and the relative solubility enhanced by interchain repulsion forces between chains (Benjakul et al., 2010). The lowest solubility of PSC at neutral pH might have been because of this effect.
3.3 Radical scavenging activities in vitroPSC's abilities, as an antioxidant, to protect against oxidation at different concentrations were examined by measuring hydroxyl and superoxide radical scavenging activities. PSC exhibited the ability to scavenge hydroxyl radicals in a dose-dependent pattern at concentrations from 2 to 10 mg/mL (Fig. 2a), with an EC50 at 3.36 mg/mL. This scavenging activity by PSC was significantly higher than that of Vc (P<0.05), although lower than that of TPP and BHA (P<0.05). PSC also exhibited superoxide anion radical scavenging ability in a dose-dependent manner (Fig. 2b).
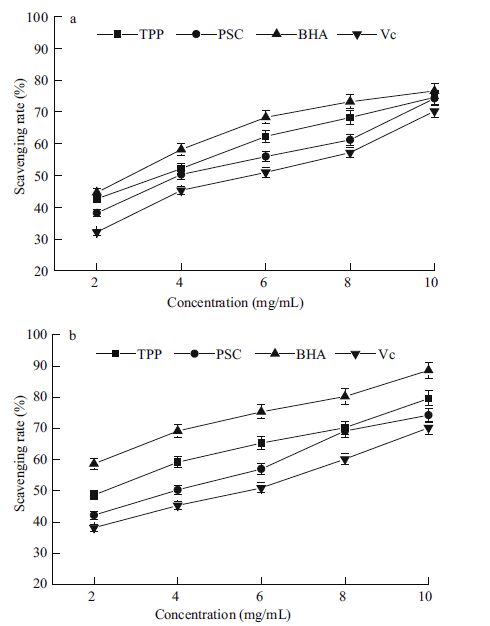
|
| Figure 2 Radical scavenging activity of PSC against hydroxyl radical (a) and super anion (b) All results as mean±standard deviation. |
The antioxidant abilities of collagen depend on specific amino acid residues and sequences. Active amino acid residues are transformed and exposed through enzyme hydrolysis, which contributes to reactions with oxidants (Kong and Xiong, 2006). High proportions of glycine, proline, and hydrophobic amino acids from collagen have been reported to be responsible for this antioxidant activity (Fu et al., 2010). PSC's amino acid composition possessed high proportions of these amino acid residues, which offered some clues that this collagen preparation could chemically inhibit hydroxyl radicals and superoxide anions.
The aging progress attributed to free radicals and ROS effects involves very complex biochemical mechanisms and the service of antioxidative compounds as anti-aging substances, thereby capturing these free radicals. As the present PSC from yellow goosefish possessed high antioxidant and free radical scavenging activities, this collagen showed great potential as a functional food supplement and in medicinal industries. The mechanism of PSC's impact on biochemical changes in skin-aged tissue need to be further investigated.
3.4 Biochemical activity in vivoOxidative stress caused by free radicals and ROS, chronically induced by D-galactose dosage, leads to the reduction of CAT, SOD, GPH-Px, and Hyp. MDA production is also enhanced, which ultimately leads to a variety of damages in skin cells (Inal et al., 2001). Different doses of D-galactose (50, 120, 200, 500, and 1 250 mg/kg) were used to establish a skin-aging model. Significant decreases in SOD, CAT, GSH-Px, and Hyp activities (16.7%, 27.3%, 25.1%, and 26.3%, respectively) were produced (Fig. 3a-e and Table 1), whereas MDA production was heightened (36.6%) in skin tissues after injection with 1 000 mg/ (kg∙d) of D-galactose.

|
| Figure 3 Biochemical activity of PSC in skin tissue homogenate of mouse back a-e. effects of PSC on SOD, GSH-Px, CAT, Hyp, and MDA, respectively. All results expressed as mean±standard deviation for 10 mice per group. Lowdosage group versus model group, a P<0.05; high dosage group versus model group, b P<0.01; and model group versus normal group, c P<0.05. |
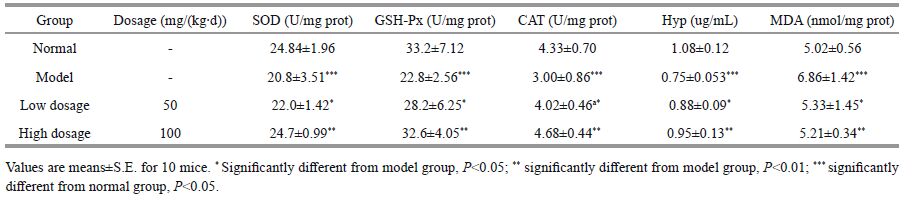 |
PSC's influence on SOD, GSH-Px, CAT, Hyp, and on MDA formation are shown in Fig. 3a-e and summarized in Table 1. The PSC-injected groups (both high and low dosage) were significantly different from the model group (P<0.05), indicating that PSC exerted effects on SOD, GSH-Px, CAT, and Hyp as well as MDA formation. In addition, the high dosage group presented more effects compared with the low dosage group in terms of SOD, GSH-Px, CAT, Hyp activities, and MDA formation (P<0.01). The increased activities of these enzymatic antioxidants were attributed to these biochemical reactions, as CAT acts as a constituent of peroxisomes and dismutates H2O2 into O2 and water to eliminate H2O2 in skin. SOD serves as the first gatekeeper in the antioxidant defense system by catalyzing the transition of superoxide anions into H2O2. Meanwhile, GSH-Px actively catalyzes GSH reactions that scavenge H2O2.
GSH is reported to be the principle nonprotein thiol serving as a free radical-scavenger or cofactor involved in antioxidant cellular defense (Moysan et al., 1993). In this study, D-galactose led to lower GSH concentrations due to leakage and oxidation of GSH, and GSH-Px activity was significantly increased, which suggested that this PSC played an effective role in both high and low dosage groups (P<0.05).
The degree of skin wrinkling is associated with the reduction in the Hyp content (Inal et al., 2001). Here, GSH-Px activity was affected by PSC (Fig. 3d and Table 1). The Hyp contents of both dosage groups were significantly different from the model group, which clearly indicated that PSC increased the Hyp content, with the greatest enhancement in the high dose group (P<0.01).
The antioxidative protective effects of collagen against the aged skin tissues have been suggested to be related to amino acid composition (Williams et al., 2002). Amino acids comprising Arg and Gln as well as Val, Ile, and Leu at specific ratios are useful for inhibiting skin-aging and accelerating wound-healing (Minh Thuy Le et al., 2014). The present PSC possessed protective abilities against skin aging, which might be attributed to its unique amino acid pattern.
MDA formation is the most widely used index of lipid peroxidation, with increased MDA indicating lipid peroxidative damage during skin aging (Inal et al., 2001). Here, the MDA concentration produced during both treatments were significantly different from the model group, which indicated that the extracted PSC inhibited MDA formation (P<0.05; Table 1 and Fig. 3e) and confirmed a previous report that collagen interacts with MDA to stop lipid peroxidation (Inal et al., 2001). The present results also clearly demonstrated that the high-dose group exhibited a greater reduction in MDA formation (P<0.01).
D-galactose induced skin modifications, including accelerated MDA formation and reduced SOD, GSHPx, Hyp, and CAT activities. This PSC inhibited wrinkle development and skin elasticity reduction caused by D-galactose; thus, it has a potential role in applications designed to slow skin aging.
4 CONCLUSIONExtraction of PSC from yellow goosefish (Lophius litulon) skin after pepsin digestion produced a high yield, with the optimal extraction conditions, including 20 U pepsin/g tissue, pH 2.0, 4℃, and 24-h incubation. The product was classified as type I collagen and shown to be rich in Gly, while Met, His, Hyp, Tyr, and Cys contents were low. The Td of PSC was 17.56℃, higher than those of cold-water fish species but lower than those of tropical fish.
The activities of PSC against hydroxyl radicals and superoxide anions responded in a dose-dependent manner. High proportions of Gly, Pro, and hydrophobic amino acids in PSC was associated with antioxidative activities. PSC's strong free radical scavenging activities suggested that this PSC possesses great potential for use as a functional food supplement and in medicinal industry applications.
PSC slowed the progress of wrinkle development and skin elasticity reduction at a dosage of 1 000 mg/ (kg∙d). The present evidence from this study indicated that PSC's effect against D-galactoseinduced skin damage was reasonably good. Further research studies are needed to investigate PSC's activity in human subjects.
| Benjakul S, Thiansilakul Y, Visessanguan W, Roytrakul S, Kishimura H, Prodpran T, Meesane J, 2010. Extraction and characterisation of pepsin-solubilised collagens from the skin of bigeye snapper (Priacanthus tayenus and Priacanthus macracanthus). J. Sci. Food Agric., 90(1): 132–138. Doi: 10.1002/jsfa.v90:1 |
| Burjanadze T V, 1992. Thermodynamic substantiation of water-bridged collagen structure. Biopolymers, 32(8): 941–949. Doi: 10.1002/(ISSN)1097-0282 |
| Butterfield D A, Castegna A, Pocernich C B, Drake J, Scapagnini G, Calabrese V, 2002. Nutritional approaches to combat oxidative stress in Alzheimer's disease. J. Nutr. Biochem., 13(8): 444–461. Doi: 10.1016/S0955-2863(02)00205-X |
| Doyle B B, Bendit E G, Blout E R, 1975. Infrared spectroscopy of collagen and collagen-like polypeptides. Biopolymers, 14(5): 937–957. Doi: 10.1002/(ISSN)1097-0282 |
| Elias R J, Kellerby S S, Decker E A, 2008. Antioxidant activity of proteins and peptides. Crit. Rev. Food Sci. Nutr., 48(5): 430–441. Doi: 10.1080/10408390701425615 |
| Fu L L, Chen H X, Dong P, Zhang X, Zhang M, 2010. Effects of ultrasonic treatment on the physicochemical properties and DPPH radical scavenging activity of polysaccharides from mushroom Inonotus obliquus. J. Food Sci., 75(4): C322–C327. Doi: 10.1111/(ISSN)1750-3841 |
| Ikoma T, Kobayashi H, Tanaka J, Walsh D, Mann S, 2003. Physical properties of type I collagen extracted from fish scales of Pagrus major and Oreochromis niloticas. Int. J. Biol. Macromol., 32(3-5): 199–204. Doi: 10.1016/S0141-8130(03)00054-0 |
| Inal M E, Kanbak G, Sunal E, 2001. Antioxidant enzyme activities and malondialdehyde levels related to aging. Clin. Chim. Acta, 305(1-2): 75–80. Doi: 10.1016/S0009-8981(00)00422-8 |
| Jongjareonrak A, Benjakul S, Visessanguan W, Nagai T, Tanaka M, 2005. Isolation and characterisation of acid and pepsin-solubilised collagens from the skin of brownstripe red snapper (Lutjanus vitta). Food Chem., 93(3): 475–484. Doi: 10.1016/j.foodchem.2004.10.026 |
| Kaewdang O, Benjakul S, Kaewmanee T, Kishimura H, 2014. Characteristics of collagens from the swim bladders of yellowfin tuna (Thunnus albacares). Food Chem., 155: 264–270. Doi: 10.1016/j.foodchem.2014.01.076 |
| Kittiphattanabawon P, Benjakul S, Visessanguan W, Nagai T, Tanaka M, 2005. Characterization of acid-soluble collagen from skin and bone of bigeye snapper (Priacanthus tayenus). Food. Chem., 89(3): 363–372. Doi: 10.1016/j.foodchem.2004.02.042 |
| Kittiphattanabawon P, Benjakul S, Visessanguan W, Kishimura H, Shahidi F, 2010. Isolation and characterisation of collagen from the skin of brownbanded bamboo shark (Chiloscyllium punctatum). Food Chem., 119(4): 1519–1526. Doi: 10.1016/j.foodchem.2009.09.037 |
| Kong B H, Xiong Y L, 2006. Antioxidant activity of zein hydrolysates in a liposome system and the possible mode of action. J. Agric. Food Chem., 54(16): 1059–1068. |
| LaemmLi U K, 1970. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature, 227(5259): 680–685. Doi: 10.1038/227680a0 |
| Li Z R, Wang B, Chi Q F, Zhang Q H, Gong Y D, Tang J J, Luo H Y, Ding G F, 2013. Isolation and characterization of acid soluble collagens and pepsin soluble collagens from the skin and bone of Spanish mackerel (Scomberomorous niphonius). Food Hydrocolloids, 31(1): 103–113. Doi: 10.1016/j.foodhyd.2012.10.001 |
| Minh Thuy Le T, Okazaki E, Osako K, 2014. Isolation and characterization of acid-soluble collagen from the scales of marine fishes from Japan and Vietnam. Food Chem., 149: 264–270. Doi: 10.1016/j.foodchem.2013.10.094 |
| Moysan A, Marquis I, Gaboriau F, Santus R, Dubertret L, Morlière P, 1993. Ultraviolet A-induced lipid peroxidation and antioxidant defense systems in cultured human skin fibroblasts. J. Invest. Dermatol., 100(5): 692–698. Doi: 10.1111/1523-1747.ep12472352 |
| Nalinanon S, Benjakul S, Kishimura H, 2010. Collagens from the skin of arabesque greenling (Pleurogrammus azonus) solubilized with the aid of acetic acid and pepsin from albacore tuna (Thunnus alalunga) stomach. J. Sci. Food Agric., 90(9): 1492–1500. Doi: 10.1002/jsfa.v90:9 |
| Pati F, Adhikari B, Dhara S, 2010. Isolation and characterization of fish scale collagen of higher thermal stability. Bioresour. Technol., 101(10): 1737–1742. |
| Ratnam D V, Ankola D D, Bhardwaj V, Sahana D K, Kumar M N V R, 2006. Role of antioxidants in prophylaxis and therapy: a pharmaceutical perspective. J. Control. Release, 113(3): 189–207. Doi: 10.1016/j.jconrel.2006.04.015 |
| Sampath Kumar N S, Nazeer R A, Jaiganesh R, 2012. Purification and identification of antioxidant peptides from the skin protein hydrolysate of two marine fishes, horse mackerel (Magalaspis cordyla) and croaker (Otolithes ruber). Amino Acids, 42(5): 1641–1649. Doi: 10.1007/s00726-011-0858-6 |
| Sankar S, Sekar S, Mohan R, Rani S, Sundaraseelan J, Sastry T P, 2008. Preparation and partial characterization of collagen sheet from fish (Lates calcarifer) scales. Int. J. Biol. Macromol., 42(1): 6–9. Doi: 10.1016/j.ijbiomac.2007.08.003 |
| Sinthusamran S, Benjakul S, Kishimura H, 2014. Characteristics and gel properties of gelatin from skin of seabass (Lates calcarifer) as influenced by extraction conditions. Food Chem., 152: 276–284. Doi: 10.1016/j.foodchem.2013.11.109 |
| Wang B, Wang Y M, Chi C F, Luo H Y, Deng S G, Ma J Y, 2013. Isolation and characterization of collagen and antioxidant collagen peptides from scales of croceine croaker (Pseudosciaena crocea). Mar. Drugs, 11(11): 1641–1661. |
| Wang L, An X, Xin Z, Zhao L, Hu Q, 2007. Isolation and characterization of collagen from the skin of deep-sea redfish (Sebastes mentella). J. Food Sci., 72(8): E450–E455. Doi: 10.1111/jfds.2007.72.issue-8 |
| Wang L, Liang Q F, Wang Z B, Xu J M, Liu Y, Ma H L, 2014. Preparation and characterisation of type I and V collagens from the skin of Amur sturgeon (Acipenser schrenckii). Food Chem., 148: 410–414. Doi: 10.1016/j.foodchem.2013.10.074 |
| Wei T, Wang R X, Shi G, Xu T J, 2016. Complete mitochondrial genome of Lophius litulon (Lophiiforms, Lophiidae) and comparison of light strand replication origin in Lophiidae. Mitochondr. DNA, 27(1): 286–288. Doi: 10.3109/19401736.2014.892080 |
| Williams J Z, Abumrad N, Barbul A, 2002. Effect of a specialized amino acid mixture on human collagen deposition. Ann. Surg., 236(3): 369–375. Doi: 10.1097/00000658-200209000-00013 |
| Yamada S, Yamamoto K, Ikeda T, Yanagiguchi K, Hayashi Y, 2014. Potency of fish collagen as a scaffold for regenerative medicine. Biomed. Res. Int., 2014: 302932. |
| Zeng S K, Yin J J, Yang S Q, Zhang C H, Yang P, Wu W L, 2012. Structure and characteristics of acid and pepsinsolubilized collagens from the skin of cobia (Rachycentron canadum). Food Chem., 135(3): 1975–1984. Doi: 10.1016/j.foodchem.2012.06.086 |
| Zhang Y F, Duan X, Zhuang Y L, 2012. Purification and characterization of novel antioxidant peptides from enzymatic hydrolysates of tilapia (Oreochromis niloticus) skin gelatin. Peptides, 38(1): 13–21. Doi: 10.1016/j.peptides.2012.08.014 |
| Zhang Y, Liu W T, Li G Y, Shi B, Miao Y Q, Wu X H, 2007. Isolation and partial characterization of pepsin-soluble collagen from the skin of grass carp (Ctenopharyngodon idella). Food Chem., 103(3): 906–912. Doi: 10.1016/j.foodchem.2006.09.053 |
| Zhao Y Q, Wang Y M, Wang B, Chi C F, Ding G F, 2015. Isolation and characterization of acid and pepsin soluble collagens from the head of bluefin leatherjacket (Navodon septentrionalis). Oceanologia et Limnologia Sinica, 46(3): 703–709. |
 2017, Vol. 35
2017, Vol. 35


