Institute of Oceanology, Chinese Academy of Sciences
Article Information
- SUPRIYA Nagathinkal T, SUDHA Kappalli, KRISHNAKUMAR Velayudhannair, ANILKUMAR Gopinathan
- Molt and reproduction enhancement together with hemolymph ecdysteroid elevation under eyestalk ablation in the female fiddler crab, Uca triangularis (Brachyura: Decapoda)
- Chinese Journal of Oceanology and Limnology, 35(3): 645-657
- http://dx.doi.org/10.1007/s00343-017-5337-9
Article History
- Received Dec. 8, 2015
- accepted in principle Feb. 14, 2016
2 Department of Zoology, University of Madras, Guindy Campus, Chennai-600 025, India;
3 School of Biotechnology, Chemical and Biomedical Engineering, VIT University, Vellore 632014, India
In crustaceans, molt and reproduction are seasonal events, with physiological interaction and interdependence between these two highly energy demanding metabolic processes (Adiyodi and Adiyodi, 1970; Sudha and Anilkumar, 1996; Tsukimura, 2001; Kappalli et al., 2012; Shyamal et al., 2014). This interaction and interdependence are brought about by the co-ordinated function of stimulatory and inhibitory principles. The role of crustacean eyestalk hormones (MIH and GIH) for regulating molt and reproduction has been revealed by previous investigations (Okumura and Aida, 2001; Okumura, 2004; Tamone et al., 2005; Nazari et al., 2007; Sudha and Anilkumar, 2007; Zmora et al., 2009; Uawisetwathana et al., 2011). However, generally, the removal of inhibitory principles (GIH and MIH) through eyestalk extirpations enhances either molt or reproduction depending on the tendency prevailing at the time of eyestalk removal (Anilkumar and Adiyodi, 1980; Adiyodi, 1988; Laufer et al., 2002; Sudha and Anilkumar, 2007). Interestingly, there are some reports on differential response to eyestalk ablation even from individuals of the same species. For instance, in the snow crab, Chionoecetes opilio, de-eyestalking resulted in the commencement of premolt in immature males, while adult males showed no sign of molt initiation (Tamone et al., 2005). Eyestalk ablation was found to have significant effects on molting in the penaeid prawn, Metapenaeus dobsoni; the smaller prawns respond quicker than the bigger ones (Venkitraman et al., 2004). In Metopograpsus messor, a grapsid crab, eyestalk ablation resulted in simultaneous acceleration of growth and vitellogenesis irrespective of the season (Sudha and Anilkumar, 2007). Despite a number of reports available, endocrinologists are still unable to frame a common format for the role of eyestalk factors for the integration of growth and reproduction in decapod crustaceans. The situation is limiting our understanding of the exact interplay of hormones in accomplishing these major physiological functions, and this area clearly needs more research. As molt and reproduction, two interactive and interdependent events in crustaceans, are under the control of eyestalk hormones and are programmed seasonally (van Herp and Soyez, 1997; Sudha and Anilkumar, 1996, 2007; Kappalli et al., 2012), a year-round examination of the effect of eyestalk ablation on molt and reproduction in the candidate species, Uca triangularis, could improve our present understanding on the regulatory mechanisms of these two highly energy-demanding processes.
U. triangularis is a continuous breeder releasing 16-18 broods a year; the duration of each ovarian cycle is 13-14 days. The wild population of U. triangularis exhibits three discrete seasons for the programming of growth and reproduction (Kappalli et al., 2012). During the August-January season, almost all female members of the population engage in reproductive activities and premolt and vitellogenesis appear to be mutually exclusive events, akin to other brachyuran crabs reported so far. However, during February-May, the population follows a synergistic pattern for the programming of molt and reproduction inasmuch as the significant proportion of the premolt/postmolt females possess growing ovaries of different vitellogenic stages. During June-July, the whole population remains reproductively inactive and the ovaries appear as a translucent band with no sign of yolk deposition, but a significant proportion was active with respect to their molting processes (Kappalli et al., 2012).
Though the role of ecdysteroids in successful accomplishment of reproduction has been well documented in insects, a close phylogenic ally of crustaceans (Zhu et al., 2003; Žitňan et al., 2007; Dong et al., 2009; Swevers and Iatrou, 2009; Ables and Drummond-Barbosa, 2010; Mykles et al., 2010), the question of a possible role of ecdysteroids in crustacean reproduction has not been resolved (Wilder et al., 1990; Subramoniam, 2000, 2011a, b for reviews; Nagaraju, 2011), as they exhibit particularly high levels of diversity (even among the closely allied groups or sometimes even in the same species, but occupying different geographical areas) in seasonal programming of growth and reproduction. In palaemonid shrimps, for instance, ovarian growth occurs concomitantly with premolt activities (Phlippen et al., 2000; Tsukimura, 2001) implying that ecdysteroids could promote both molt and reproduction. In grapsid crabs, on the other hand, programming of molt and reproduction are temporally separated events inasmuch as females undergoing premolt activities do not possess growing ovaries (Sudha and Anilkumar, 1996, 2007), prompting us to suggest that ecdysteroids may have a restraining influence on ovarian growth. From our previous observations (Syama et al., 2010; Kappalli et al., 2012), it was revealed that during February-May season, there existed a concomitance between premolt growth and reproduction in U. triangularis (Kappalli et al., 2012). This situation appeared to be comparable with what was described in the palaemonid shrimp Macrobrachium rosenbergii (Young et al., 1993; Okumura, 2004; Sudha et al., 2010, 2011), but unlike other brachyuran crabs. Interestingly, we also found a positive correlation between hemolymph ecdysteroid titers and oocyte size during February-April, when several females (U. triangularis) were engaged in both molting and reproduction (Kappalli et al., 2012). Further, recent studies performed on the brachyuran crab Metopograpsus messor, have also demonstrated that, the hemolymph ecdysteroid level and the ovarian ecdysteroid receptor gene expression were higher during the breeding season than those examined in the non-breeding season in the same species (Shyamal et al., 2014, 2015). Nevertheless, our knowledge on the importance of co-ordination existing between ecdysteroids and eyestalk hormones for successful reproduction in brachyuran crabs is inadequate. At this juncture, it is expected that the present study on the assay of hemolymph ecdysteroid titer, together with the analysis of ovarian biochemistry under eyestalk ablation during the reproductive season of U. triangularis, would enable us to address the question of possible interaction between ecdysteroids and eyestalk hormones in accomplishing successful vitellogenesis, a matter that has been debated for the past several years (Subramoniam, 2000; Sudha and Anilkumar, 2007; Nagaraju, 2011; Subramoniam, 2011a, b for reviews; Kappalli et al., 2012).
2 MATERIAL AND METHOD 2.1 Collection and maintenance of crabsAdult females of U. triangularis, used for the present study, were collected from the intertidal zone of Muzhappilangad estuary (North Kerala, India) and the animals were brought to the laboratory and maintained in near natural conditions. During this period, crabs were fed ad libitum with clam meat and maintained in the laboratory for 2-3 days for acclimatization before the start of the experiment.
2.2 Stages of molt and vitellogenesisThe epipodite setogenic events of the 3rd maxilliped were used for the precise identification of the molt stages according to Sudha et al. (Sudha and Anilkumar, 2007; Kappalli et al., 2012). With utmost care, the epipodite was removed gently from the live animal and placed on a clean glass slide and examined through a microscope so as to observe clearly the epidermal retraction, development of new cuticle layer and the formation of new setae. The ovary was dissected out by cutting open the carapace dorsally and quickly rinsed in physiological saline and observed under the compound research microscope, Leica DM-1000. The measurement of oocyte diameter was done through micrometry and the ovarian index was determined by using the standard formula [ovarian index=(wet weight of the ovary/weight of the crab)×100]. Characterization and classification of ovarian stages in U. triangularis were performed based on the color of the ovary, oocyte diameter and ovarian index as the criteria (Kappalli et al., 2012). Accordingly, four stages were identified. Previtellogenic staged ovary (Pv Stage) contain proliferating oocytes with size less than 11 μm and during this stage, ovary appears white in color. The early vitellogenic staged ovary (Stage 1) appears yellow in color with oocyte size 12-90 μm. The ovary attains a brown hue at the mid stage (Stage 2) of its growth, the oocyte diameter ranging between 91 and 150 μm. As vitellogenesis progresses towards late stage (Stage 3), the ovary appears dark brown, the oocyte diameter being 151-200 μm.
2.3 Bilateral eyestalk extirpationThe intermolt crabs with average weight 1.5 g and possessing previtellogenic ovaries (oocyte diameter-6-10 μm) were used for the present study. Before eyestalk extirpation, the crabs were categorized into 6 groups (1-6), each group consisting of approximately 35 crabs. Groups 1, 3 and 5 were subjected to bilateral eyestalk ablation and treated as the experimentals for 0th day, 5th day and 10th day post-ablation respectively. Groups 2, 4 and 6 were maintained as respective controls (with intact eyestalks) for 0th day, 5th day and 10th day post-ablation respectively. Both eyestalks were removed from the crabs by excising them from the base using a fine pair of sterilized scissors. Both experimental and control crabs were maintained in cisterns laid with wet sand and were fed ad libitum on clam meat. Care was taken to remove the estuarine water and left over food twice a day. The duration of the experimental period was 10 days; the mortality rate was approximately 8%.
Crabs from Groups 1 (experimental) and 2 (control) were sacrificed soon after the eyestalk ablation (0th day) and their molt and ovarian stages were observed; the ovaries were processed for biochemical analysis. Crabs of the Groups 3 (experimentals) and 5 (controls) and 4 (experimentals) and 6 (controls) were sacrificed on the 5th and the 10th days post ablation respectively. The eyestalk ablation experiments were conducted in all three seasons, i.e., reproductive season (August-January), moltreproductive season (February-May) and nonreproductive season (June-July) separately (Supriya, 2011; Kappalli et al., 2012). (As per our preliminary observations, oocytes of the de-eyestalked crabs attained the size of fully matured normal oocytes (of the wild) (sometimes larger than that) within 10 days post-ablation maintenance of these experimental crabs for longer periods (up to 30 days) did not yield any significant results in terms of spawning or fecundity. Due to this reason, we limited the duration of the experiment to 10 days (post-ablation)).
2.4 Biochemical analysisThe ovary of both eyestalk-ablated and control crabs was subjected to quantitative analysis of biochemical reserves at 0, 5 and 10 days postoperation using standard procedures. Total protein in the tissue was precipitated by 10% cold TCA and subjected for centrifugation at 3 000 r/min for 15 min. The precipitate was dissolved in 0.1 N NaOH and used as the protein extract for colorimetric analysis with bovine serum albumin as the standard (after Lowry et al., 1951; Anilkumar and Adiyodi, 1980; Sudha and Anilkumar, 2007). In order to extract total lipid, the tissue was homogenized with chloroform:methanol (2:1) mixture to a final volume 20 times that of the tissue sample. The homogenate was centrifuged; the supernatant was washed briefly with 0.9% NaCl solution (by vortexing a few seconds), and centrifuged (2 000 r/min) again so as to separate the two phases. The lower chloroform phase (containing total lipid) was separated out and subjected to evaporation and gravimetric analysis (Folch et al., 1957). Carbohydrates of the tissue were separated into an ethanol-soluble oligosaccharide fraction and an ethanol-insoluble polysaccharide fraction (after Johnston and Davies, 1972). For this purpose, the tissue was homogenized in 95% ethanol and centrifuged at 3 000 r/min for 15 min. The supernatant was collected and subjected for evaporation at 60℃ and subsequently dissolved in an appropriate volume of distilled water for the estimation of oligosaccharide fraction. The left-over precipitate was also dissolved in an appropriate volume of distilled water and used as the sample for the estimation of the polysaccharide fraction. Both these fractions (polysaccharide and oligosaccharide) were estimated by the phenol-sulfuric acid method (Dubois et al., 1956). For the estimation of total free amino acid present in the tissue (ovary), the ethanolsoluble fraction was used. The fraction along with ninhydrin reagent prepared in citrate buffer was heated in a boiling water bath for 12 min to optimize color development which in turn was read colorimetrically at 540 nm, with glutamic acid as the standard (Lee and Takahashi, 1966).
2.5 Ecdysteroid titer assayThe hemolymph ecdysteroid titer of both experimental and control groups, was estimated by radioimmunoassay (RIA) as per the protocol adopted from Chang and O'Connor (1979), Sudha and Anilkumar (2007), Kappalli et al. (2012) and Shyamal et al. (2014). Fresh samples of hemolymph (10 μL) were collected in vials containing 100 μL borate buffer (pH 8.4) and the radioligand 3H-ecdysone (~12 000 dpm from PerkinElmer). A set of standards comprising the radioligand 3H-ecdysone, the borate buffer and the standard ecdysone (Sigma, USA) was also run along with the unknown samples for the purpose of plotting the standard graph. All the vials (the hemolymph samples and the standards) were vortexed to allow adequate mixing. To each vial, 100 μL antiserum (2-succinyl conjugate of ecdysone) was added. Prior to treatment with the antigen, the antiserum was subjected to titer assay to ensure optimal binding sensitivity; 1:500 was found to be the most appropriate dilution for the purpose. The mixture was then incubated for 12 h at 4℃, after which 200 μL saturated ammonium sulfate was added to each vial and incubated at 4℃ for 20 min to allow complete precipitation. The pellets resulting from the antigenantibody binding, were separated out by centrifugation at 5 000g for 20 min, and washed with 400 μL of 50% ammonium sulfate in borate buffer and centrifuged. The pellets were dissolved in 25 μL distilled water and mixed with 500 μL of scintillation cocktail (0.8% PPO and 0.02% POPOP in a tolune:triton [2:1] mixture) and read using an LKB Liquid Scintillation counter. A standard curve plotted with 25, 50, 125, 250, 500, 1 000 and 2 000, 4 000 pg ecdysone, on a semi-log paper, was used for accurate estimation of the ecdysteroid titer.
2.6 Statistical analysisFor assessing the statistical significance (if any) in the variations of oocyte size and biochemical resources of the ovary between experimental and control crabs and the fluctuation of ecdysteroid levels, the data were subjected to F-test (ANOVA) and Student's t-test, using InStat 3 (GraphPad Version 2.00, 1993) software. In all instances, null hypothesis was rejected at 95% confidence interval (P < 0.05).
3 RESULTIn the present study, bilateral eyestalk extirpation resulted in a spurty hyperphagia irrespective of the season noticeable from the first three hours of postoperation. The tendency for voracious feeding continued up to four days of post-operation; subsequently, the rate of feeding subsided to normal levels.
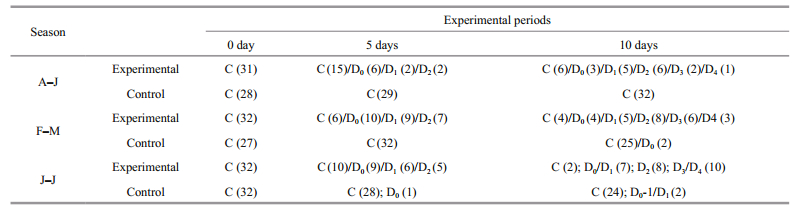
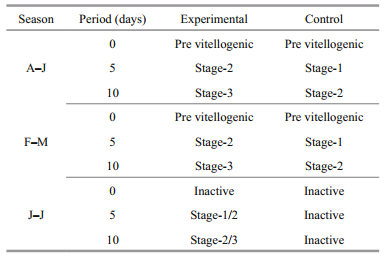
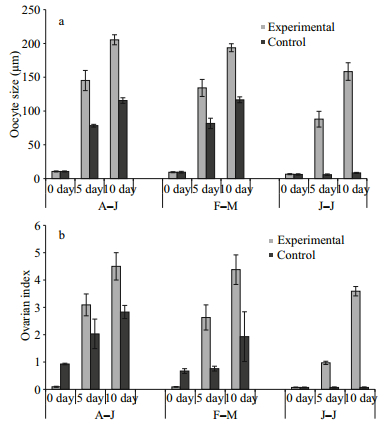
3.1 Effect of eyestalk ablation on molting and reproduction during different seasons 3.1.1 Reproductive season (August-January)During the reproductive season, more than 99% of the natural female population of U. triangularis was actively engaged in peak breeding activity and remained in intermolt stage. Bilateral eyestalk ablation during this season resulted in the simultaneous acceleration of both somatic and vitellogenic growth. At the beginning (0 day) of the eyestalk ablation experiment, all the crabs (Groups 1-2) were at intermolt stage with previtellogenic ovary and the oocyte size ranging from 7-11 μm. Within 5 days post-operation, out of 25 eyestalk ablated (experimental) crabs (Group 3) observed, 10 (40%) crabs entered premolt activities (D0-6/D1-2/D2-2) (Table 1). The remaining crabs (15; 60%), however, did not show any sign of premolt initiation and remained in intermolt stage. The control crabs on the other hand, were at intermolt stage, without showing any sign of premolt activities. Interestingly, all experimentals (both premolt and intermolt) also showed enhanced vitellogenic activities inasmuch as their ovaries attained mid-stage (Stage 2) with oocyte size (145.20±14.96) μm compared to that of control (Group 4) (Table 2; Fig. 1a)
|
|
|
| Figure 1 Oocyte size (a) and ovarian index (b) in U. triangularis under eyestalk ablation at 0, 5 and 10 days during different seasons A-J: August-January; F-M: February-May; J-J: June-July (mean±SE). Oocyte size: A-J: 0 day: C=E (NS); 5 day: C < E*; 10 day: C < E***; F-M: 0 day: C=E (NS); 5 day: C < E***; 10 day: C < E***; J-J: 0 day: C=E (NS); 5 day: C < E***; 10 days: C < E***; A-J and F-M: 0 day: E=E (NS); 5 day: E=E (NS); 10 day: E=E (NS); A-J and J-J: 0 day: E=E (NS); 5 day: E>E**; 10 day: E>E**; F-M and J-J: 0 day: E=E (NS); 5 day: E>E*; 10 day: E>E*. *P < 0.05; ** P < 0.01, *** P < 0.001. |
By 10 days post operation, out of 23 crabs (from Group 5) observed, 17 (74.29%) crabs were found to be at different stages of premolt activity; among which, 3 animals acquired the late stages (D3/D4) and the remaining 14 crabs were either at early (D0-1-8) or mid stage (D2-6) (Table 1). However, at this time, 6 crabs were found that had remained at intermolt stage. Interestingly, the ovary of all these experimental crabs attained late vitellogenesis stage with oocyte diameter (205.40±7.44) μm (Fig. 1a). Control crabs (Group 6), however, remained at intermolt (Table 1) and their ovaries were in ovitellogenesis mid stage (Stage 2) (Table 2; Fig. 1a).
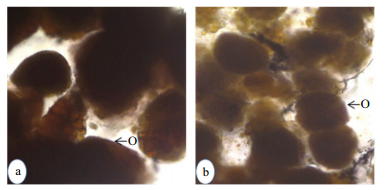
3.1.2 Molt-reproductive season (February-May)De-eyestalking when conducted in intermolt crabs in their previtellogenic stage during February-May, also resulted in precocious acceleration of both molting and reproduction. Within 5 days postoperation, more than 80% of experimentals (Group 3; 26 out of 32 crabs) reached the premolt stage (D0-10/D1-9/D2-7) (Table 1); while their ovaries were at Stage 2 ((134.19±12.59) μm) of vitellogenesis (Table 1; Fig. 1a). Controls (Group 4), on the other hand, remained at the intermolt stage and their oocyte diameter reached only up to (81.7±7.69) μm (Fig. 1a). Statistical evaluation showed that the oocyte size of the experimentals was considerably higher than that of the controls. Within 10 days post-operation, 26 (out of 30) (86.67%) eyestalk-ablated crabs (Group 5) showed the premolt activity ranging from D0- D4 (D0-4/D1-5/D2-8/D3-6/D4-3). On the other hand, excepting two crabs which showed very little sign of premolt initiation (D0), all the controls (Group 6) remained in the intermolt stage (Table 1). Apart from molting activity, within this period (10 days post ablation), all the experimental crabs (Group 5) also showed precocious acceleration of vitellogenesis, reaching the late stage (Stage 3), with oocyte diameter being (193.66±5.82) μm (Table 2; Figs. 1a, 2a, b). This increase in oocyte size is statistically significant (P < 0.01) when compared to that of the crabs (Group 3) at 5 days post operation. Significantly, the enhancement in oocyte size was reflected on the ovarian index as well (Fig. 1b). Even though, the oocyte size of the control (Group 6) females showed some increase during the experimental period, their size was significantly less (P < 0.001) than that of the experimental crabs (Fig. 1a).
|
| Figure 2a Ovary of U. triangularis showing growing oocytes after 10 days of postablation a. experimental; b. control. O: oocyte. |
|
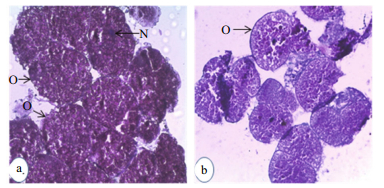
| Figure 2b Histological section through the ovary of U. triangularis after 10 days of postablation a: experimental; b. control. O: oocyte; N: nucleus. |
During this season, the natural female population of U. triangularis does not show any breeding activity and their inactive ovaries appear as a translucent band; however, approximately 24% of them are active in premolt-postmolt activity. Bilateral eyestalk ablation during this season resulted in the precocious acceleration of both molt and vitellogenesis. On the first day of the experiment, all crabs (both experimental and control) were at the intermolt stage and possessed inactive (oocyte size less than 10 μm) ovaries. By 5 days post-operation, 20 (out of 30) (66.67%) animals showed clear signs of premolt initiation (D0-9/D1-6/ D2-5) (Table 1). Apart from this, precocious acceleration of the oocyte size towards early/mid (Stage 1/2) vitellogenesis and a concomitant increase in the ovarian index could also be noticed in the experimental crabs (Group 3) (Fig. 1a, b). Within 10 days post extirpation, 92.59% (25 out of 27) of the experimental crabs (Group 5) entered premolt (D0-3/ D1-4/D2-8/D3-7/D4-3) among which 18 crabs were at mid and late stages of premolt (Table 1); while their ovaries were at different stages of vitellogenesis (Stage 2/Stage 3). However, except for few, all the control crabs remained in intermolt throughout the entire duration of the experiment, and their ovaries appeared with a translucent band showing the sign of inactivity (Tables 1 and 2).
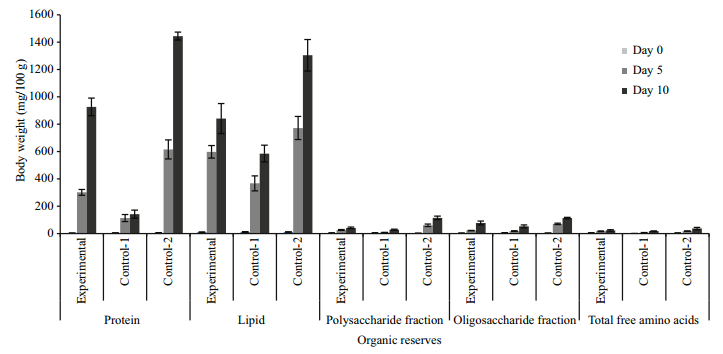
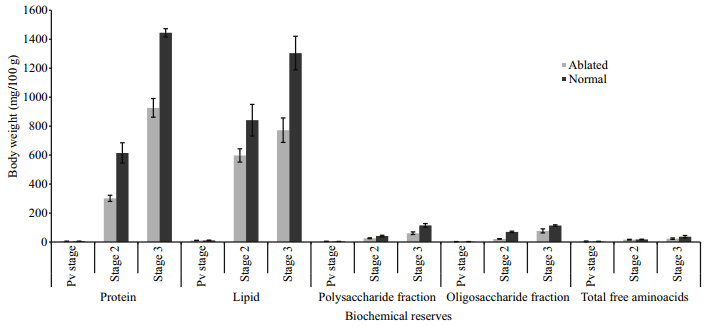
3.2 Ovarian biochemistry of eyestalk-ablated crabs during the reproductive season (August- January)Bilateral eyestalk ablation also resulted in significant increase in the biochemical reserves of the ovary throughout the experimental period. In experimental crabs, bilateral eyestalk ablation led to a statistically significant increase (P < 0.01) in total TCA-precipitable protein, chloroform-extractable lipids, the oligosaccharide fraction, the polysaccharide fraction and total free amino acid content of the ovaries compared to that of controls (Fig. 3). To evaluate whether the precociously incorporated yolk components after de-eyestalking is normal, we also compared our present data with those of individual females collected from the wild at the same stage of ovarian growth. The profiles of major biochemical compounds of the yolk were found to be impoverished in the ovaries of eyestalk ablated crabs compared to normal ovaries (Fig. 4).
|
| Figure 3 Profiles of organic reserves of U. triangularis ovary under eyestalk ablation at 0, 5 and 10 days in August-January season (mean±SE) (sample size 6) Protein: 0 day: C=E (NS), 5 day: C < E***, 10 day: C < E***; lipid: 0 day: C=E (NS), 5 day: C < E**, 10 day: C < E***; polysaccharide fraction: 0 day: C=E (NS), 5 day: C < E***, 10 day: C < E***; oligosaccharide fraction: 0 day: C=E (NS), 5 day C < E***, 10 day: C < E**; total free amino acid: 0 day: C=E (NS); 5 day: C < E***, 10 day: C < E** (*P < 0.05; **P < 0.01, ***P < 0.001). |
|
| Figure 4 Comparison of the ovarian biochemical reserves of the bilateral eyestalk ablated crabs with those of the individuals with respective ovarian stages collected from the wild at 0, 5 and 10 days in August-January season (sample size 6) (mean±SE) |
Hemolymph ecdysteroid assay shows that, in response to bilateral eyestalk ablation, the ecdysteroid titer of all females registered a substantial increase, coupled with precocious premolt and reproductive acceleration. At the commencement of the experiment (0 days) the profile of the ecdysteroid of the eyestalkablated crabs was (3.76±1.33) ng/mL, the value is comparable to that of controls with intact eyestalks (Fig. 5). By the fifth day postoperation, the level dramatically increased to (37.71±8.52) ng/mL coupled with premolt initiation and the acceleration of ovarian growth; the controls, however, remained at the intermolt stage and did not show any comparable change in their ecdysteroid levels (Tables 1, 2; Fig. 5). Interestingly, further significant increase of ecdysteroid titer could not be observed in eyestalkablated crabs by 10 days post operation and the level was found to be comparable with that of de-eystalked crabs at 5 days post operation (Fig. 4). However, compared to control crabs with intact eyestalks, the profile of ecdysteroid in eyestalk-ablated crabs was significantly higher (P < 0.000 1).

|
| Figure 5 Hemolymph ecdysteroid titer (ng/mL hemolymph); (mean±SE) in U. triangularis under bilateral eyestalk ablation during August-January season Serum Ecdysteroid titer: 0th day experimentals=controls (NS); 5th day controls < experimentals***, 10th day controls < experimentals***. Serum Ecdysteroid levels at 0th day experimentals < 5th day experimentals***; 5th day experimentals=10th day experimentals (NS). NS: not significant; *** highly significant (P < 0.001). |
Our present study clearly demonstrates that eyestalk ablation in the fiddler crab U. triangularis resulted in the simultaneous enhancement of molt and reproduction, thus severing the antagonistic relation between the molt and reproduction existing in the wild population during the reproductive season (August-January). This observation is akin to our previous results made in de-eyestalking experiments conducted with another brachyuran crab M. messor, wherein premolt and reproduction are mutually exclusive phenomena in the wild population (Sudha, 1992; Sudha and Anilkumar, 2007). We are thus encouraged to suggest the involvement of eyestalk inhibitory principles for maintaining the moltreproductive interaction in an appropriate way which in turn could be responsible for a balanced energy budget to accommodate successful breeding and somatic growth in the species. In a wild population of the freshwater shrimp, M. rosenbergii, molting and reproduction are synergistic events and a rise in their ecdysteroid level is reported to be concomitant with ovarian growth (Wilder and Aida, 1995). Even though de-eyestalking in this palaemonid species resulted in simultaneous enhancement of molt and reproduction, the normal relation between these two metabolic functions maintained significantly without much alteration, and implies there is no significant role of eyestalk hormones in integrating molt and reproduction (Okumura et al., 1992; Wilder and Aida, 1995; Okumura and Sakiyama, 2004). Previous investigations from our laboratory reveal that, contrary to that during the August-January season, in the wild population of U. triangularis, a significant proportion of the premolt/postmolt females possessed normal growing ovaries of different vitellogenic stages coupled with a high titer of ecdysteroids during the February-May season (Kappalli et al., 2012). Further, in the wild male population of M. messor, the relation between molting and reproduction does not appear to be completely antagonistic inasmuch as spurty spermatogonial proliferation under premolt and high ecdysteroid titer have been reported (Suganthi and Anilkumar, 1999).
Our comparative analysis of the effect of deeyestalking in U. triangularis conducted at various seasons of the year shows the existence of considerable difference in the intensity of the response of the somatic and ovarian tissues towards de-eyestalking. For instance, the degree of acceleration of vitellogenesis due to de-eyestalking was maximum during the reproductive season (August-January) and during molt--reproductive season (February-May). During these seasons, by 10 days post-ablation, a significant proportion of the eyestalk ablated crabs already had late-stage ovaries ready for spawning. The rate of ovarian growth (under de-eyestalking) was more or less comparable in both these seasons; during the June-July season, on the other hand, when the population in the wild would abstain from vitellogenesis, de-eyestalking precipitated only a slower enhancement of precocious ovarian growth. Further, the tendency for precocious premolt acceleration was observed to be at its maximum, if de-eyestalking was performed during June-July season. These results encourage us to suggest that the overriding physiological condition of the animal in question could be entrained with season-dependent (extrinsic) factors and this in turn could influence the degree of acceleration of ovarian growth in the absence of eyestalk inhibitory principles; apparently, the tissue's innate readiness for somatic growth in response to de-eyestalking seems to be minimal during the reproductive season. This observation seems to be comparable with the previous results obtained from the eyestalk ablation experiments on the estuarine crab Metopograpsus messor, conducted in our laboratory. The experiments revealed that the precocious precipitation of molting and/or vitellogenesis, as the case may be, was dependent not only on the presence or absence of the stimulatory/ inhibitory principles, but also on the respective tissue's responsiveness. To cite another example, removal of the eyestalk hormones (by de-eyestalking) in the freshwater crab Paratelphusa hydrodromous during the prebreeding and the breeding seasons resulted in distinct emphasis on precocious vitellogenesis, but not on molting. However, after spawning, the physiology of the population dramatically switched over to molting activity, resulting in precocious premolt acceleration due to de-eyestalking (Anilkumar and Adiyodi, 1980, 1985). Furthermore, in penaeid prawns, eyestalk ablation resulted in optimal enhancement of ovarian maturation, if the operation was conducted in warmer months (Muthu et al., 1984; Sherly and Kakati, 2003; Babu et al., 2013). These reports support our suggestion that the degree of acceleration of somatic and/or reproductive growth could be dependent on the respective tissue's readiness.
The present eyestalk extirpation experiments also reveal that the ovaries of eyestalkless U. triangularis have been responsive for yolk uptake throughout the year including during the reproductively inactive (June-July) season, evinced by the enhanced ovarian wet weight and oocyte size of the experimentals. There has also been a perceptible increase (P < 0.01) in the profiles of yolk materials such as total proteins, total lipids, oligosaccharide and polysaccharide fractions and total free amino acids due to eyestalk ablation. Nevertheless, significantly, the yolk profiles of the precociously grown ovaries of de-eyestalked crabs were found to be impoverished, when compared with those of the individuals from the wild at the same stages of ovarian growth.
Further, in spite of the oocytes attaining the 'full growth', [in 10 days post-ablation, the ovaries of the de-eyestalked crabs reached the final stage (Stage 3) (Table 2; Fig. 1a, b), comparable to the mature oocytes of the wild pending spawning], the experimentals did not oviposit. This implies either that the eyestalk hormones have no influence on spawning, or that spawning in U. triangularis is regulated other than by the eyestalk hormones. Similar situations of abstinence from spawning in de-eyestalked females have been reported also in the intertidal grapsid crabs Metopograpsus messor and Chasmagnathus granulata (Stella et al., 2000; Sudha and Anilkumar, 2007). The biochemical impoverishment of the yolk could be a factor responsible for the lack of spawning in the de-eyestalked females. There are further reports demonstrating changes in the biochemical constituents of eggs released under eyestalk ablation in penaeid shrimps (Palacios et al., 1999; Reddy et al., 2004; Marsden et al., 2008). Although the exact reason for the abnormal ovarian biochemistry of the experimentals is not clear, these results draw our attention to previous reports of deterioration in spawn quality and hatching success in eyestalk-ablated prawns (Emmerson, 1980; Primavera, 1985; Browdy, 1992; Zacharia and Kakati, 2003; Babu et al., 2013). It is tempting to suggest that this impoverished ovarian biochemistry could be a major contributory factor for the deficient spawn quality, leading to poor hatching success in these species.
In view of the observations of inadequate yolk deposition in the eyestalkless individuals, eyestalk hormones may be necessary for normal ovarian physiology. A 'priming' of the ovary under the (restraining) influence of the eyestalk hormones could be a requisite for successful vitellogenesis to culminate into spawning. Further investigations are, however, necessary to examine the role of eyestalk hormones in maintaining normal ovarian growth in crustaceans.
Bilateral eyestalk ablation resulted not only in precocious enhancement of premolting and vitellogenesis, but also in the elevation in ecdysteroid levels in all the experimental crabs (U. triangularis). The occurrence of precocious vitellogenesis under a high ecdysteroid titer (present study) attracts considerable interest, in view of the fact that reproduction and molting are suggested to be antagonistic among brachyuran crabs (Adiyodi and Adiyodi, 1970; Anilkumar and Adiyodi, 1985; Adiyodi, 1988). Inasmuch as ecdysteroids are known to be molt promoters, one may be inclined to believe that a high (premolt) titer of ecdysteroids could have a restraining influence on reproduction. However, the present observation of occurrence of precocious vitellogenesis under the premolt ecdysteroid titer, clearly demonstrates that ovarian growth in U. triangularis will not be restrained even under a high ecdysteroid titer. The gonadotropic role of ecdysteroids in eliciting the yolk-protein precursor (YPP) gene is well documented in insects (Zhu et al., 2003; Swevers and Iatrou, 2009), but poorly known in crustaceans. A recent study from our laboratory, however, has demonstrated that the ecdysteroid levels fluctuate significantly in concert with the secretory activity of the Y organ, with respect to seasons of molting and reproduction in M.messor. The study has demonstrated that hemolymph ecdysteroid levels (together with the Y organ secretory activity) were higher during the breeding season (August-December) than during the non-breeding season (June-July), implying a possible role of ecdysteroids in reproduction in this species (Shyamal et al., 2014). Further studies have identified the ovary of this brachyuran crab as a putative ecdysteroid target, judged from the ecdysteroid receptor gene (EcR) expression in the tissue. Interestingly, the ovaries of breeding crabs exhibited higher EcR steady state transcript levels than the ovaries of nonbreeding ones, suggesting a possible ecdysteroid role in reproduction (Durica et al., 2014; Shyamal et al., 2015). However, a stimulatory role of ecdysteroids in brachyuran vitellogenesis is yet to be demonstrated (Subramoniam, 2011a, b, in review; Shyamal et al., 2014, 2015). In this context, it should be recalled that, during the Feb.-May season, molting and reproduction in U. triangularis occur in a synergistic fashion inasmuch as a significant proportion of premoult-to-postmoult females possessed normally growing ovaries of different vitellogenic stages coupled with high ecdysteroid titer (Kappalli et al., 2012) comparable to the situation in the palaemonid shrimp M. rosenbergii (Young et al., 1993; Okumura, 2004; Sudha et al., 2010, 2011). The progress of yolk deposition from stages 1 to 3, coupled with an escalating ecdysteroid titer (from ~4 to ~34 ng/mL hemolymph; P < 0.05), is closely entrained with premolt growth (from D1 to D4) in the normal population of U. triangularis during the moltreproductive season (Kappalli et al., 2012). This observation also strengthens our present contention that a high level of ecdysteroids does not impede reproduction in U. triangularis. Indeed, the percentage of crabs carrying broods was almost the same (~70%- 80%) in both the Aug.-Jan. season (when ecdysteroids in the population are generally at low levels) and in mid-the Feb.-May season (when the molting frequency is at its maximum) (Kappalli et al., 2012). Further, it is tempting to ask whether having ecdysteroids at least at low levels (as seen in the Aug.-Jan. season), are a prerequisite for successful ovarian maturation in U. triangularis. Addressing this question would require hormone administration, gene expression and gene silencing studies.
5 CONCLUSIONThe present study suggests that induction of molt and/or vitellogenesis in U. triangularis depends not only on the presence or absence of eyestalk principles, but also on the respective tissue's responsiveness. Plausibly, the biochemical impoverishment of the yolk observed in the precociously grown ovaries, could be one of the factors responsible for the inability of spawning under de-eyestalking; these results also suggest that eyestalk hormones could be necessary for successful seasonal programming of growth and reproduction, as well as maintenance of normal ovarian physiology in U. triangularis. The study also clearly demonstrates that a high (premolt) ecdysteroid titer does not impede reproduction in U. triangularis. These observations further prompt us to ask if the ecdysteroids, at least in a low profile, could be a requisite for successful vitellogenesis. Future studies involving gene expression and silencing, could help us address this question effectively.
| Ables E T, Drummond-Barbosa D, 2010. The steroid hormone ecdysone functions with intrinsic chromatin remodeling factors to control female germline stem cells in Drosophila. Cell Stem Cell, 7(5): 581–592. Doi: 10.1016/j.stem.2010.10.001 |
| Adiyodi K G, Adiyodi R G, 1970. Endocrine control of reproduction in decapod Crustacea. Biol. Rev., 45(2): 121–164. Doi: 10.1111/brv.1970.45.issue-2 |
| Adiyodi R G. 1988. Reproduction and development. In: Burggren W W, McMahon B R eds. Biology of the Land Crabs. ambridge University Press, New York, USA, p. 139-185. |
| Anilkumar G, Adiyodi K G, 1980. Ovarian growth induced by eyestalk ablation during the prebreeding season, is not normal in the crab, Paratelphusa hydrodromous (Herbst). Int. J. Invert. Reprod., 2(2): 95–105. Doi: 10.1080/01651269.1980.10553345 |
| Anilkumar G, Adiyodi K G, 1985. The role of eyestalk hormones in vitellogenesis during the breeding season in the crab, Paratelphusa hydrodromous (Herbst). Biol. Bull., 169(3): 689–695. Doi: 10.2307/1541310 |
| Babu K N, Reddy D C, Kalarani V, 2013. Effect of eyestalk ablation on ovarian maturation in the tiger shrimp, Penaeus monodon (Fabricius) under different environmental conditions. Int. Res. J. Pharm. App. Sci., 3(4): 149–151. |
| Browdy C L. 1992. A review of the reproductive biology of Penaeus species: perspectives on controlled shrimp maturation systems for high quality nauplii production. In: Wyban J ed. Proceedings of the Special Session on Shrimp Farming. Florida, USA. p. 22-51. |
| Chang E S, O'Connor J D. 1979. Arthropod molting hormones. In: Jaffe B M, Behrman H R eds. Methods of Hormone Radioimmunoassay. Academic Press, Inc. , New York, USA. p. 797-814. |
| Dong S Z, Ye G Y, Guo J Y, Hu C, 2009. Roles of ecdysteroid and juvenile hormone in vitellogenesis in an endoparasitic wasp, Pteromalus puparum (Hymenoptera: Pteromalidae). Gen. Com. Endocrinol., 160(1): 102–108. Doi: 10.1016/j.ygcen.2008.11.007 |
| DuBois M, Gilles K A, Hamilton J K, Rebers P A, Smith F, 1956. Colorimetric method for determination of sugars and related substances. Anal. Chem., 28(3): 350–356. Doi: 10.1021/ac60111a017 |
| Durica D S, Das S, Najar F, Roe B, Phillips B, Kappalli S, Anilkumar G, 2014. Alternative splicing in the fiddler crab cognate ecdysteroid receptor: variation in receptor isoform expression and DNA binding properties in response to hormone. Gen. Comp. Endocrinol., 206: 80–95. Doi: 10.1016/j.ygcen.2014.05.034 |
| Emmerson W D, 1980. Induced maturation of prawn Penaeus indicus. Mar. Ecol. Prog. Ser., 2: 121–131. Doi: 10.3354/meps002121 |
| Folch J, Lees M, Sloane Stanley G H, 1957. A simple method for the isolation and purification of total lipides from animal tissues. J. Biol. Chem., 266: 497–509. |
| Johnston M A, Davies P S, 1972. Carbohydrates of the hepatopancreas and blood tissues of the Carcinus. Comp. Biochem. Physiol. Part B, 41(2): 433–443. Doi: 10.1016/0305-0491(72)90046-6 |
| Kappalli S, Supriya N T, Krishnakumar V, Gopinathan A, Chang E S, 2012. Hemolymph ecdysteroid titers in a brachyuran crab Uca triangularis that concomitantly undergoes molting and reproduction. Zool. Stud., 51(7): 966–976. |
| Laufer H, Ahl J, Rotllant G, Baclaski B, 2002. Evidence that ecdysteroids and methyl farnesoate control allometric growth and differentiation in a crustacean. Insect Biochem. Mol. Biol., 32(2): 205–210. Doi: 10.1016/S0965-1748(01)00104-7 |
| Lee Y P, Takahashi T, 1966. An improved colorimetric determination of Amino acids with the use of ninhydrin. Anal. Biochem., 14(1): 71–77. Doi: 10.1016/0003-2697(66)90057-1 |
| Lowry O H, Rosebrough N J, Farr A L, Randall R J, 1951. Protein measurement with the Folin phenol reagent. J. Biol. Chem., 193(1): 265–275. |
| Marsden G, Hewitt D, Boglio E, Mather P, Richardson N, 2008. Methyl farnesoate inhibition of late stage ovarian development and fecundity reduction in the black tiger prawn, Penaeus monodon. Aquaculture, 280(1-4): 242–246. Doi: 10.1016/j.aquaculture.2008.04.031 |
| Muthu M S, Laxminarayana A, Mohamed K H, 1984. pH as a factor influencing maturation of Penaeus indicus in captivity. Indian J. Fish., 31(2): 217–222. |
| Mykles D L, Adams M E, Gäde G, Lange A B, Marco H G, Orchard I, 2010. Neuropeptide action in insects and crustaceans. Physiol. Biochem. Zool., 83(5): 836–846. Doi: 10.1086/648470 |
| Nagaraju G P C, Rajitha B, Borst D W, 2011. Molecular cloning and sequence of retinoid X receptor in the green crab Carcinus maenas: a possible role in female reproduction. J. Endocrinol., 210(3): 379–390. Doi: 10.1530/JOE-11-0154 |
| Nagaraju G P C, 2011. Reproductive regulators in decapod crustaceans: an overview. J. Exp. Biol., 214(1): 3–16. Doi: 10.1242/jeb.047183 |
| Nazari E M, Bainy A C D, Ammar D, Müller Y M R, 2007. Ovarian staging in eyestalk ablated females of Farfantepenaeus paulensis: a histologic, morphometric and biochemical analysis. J. Crust. Biol., 27(2): 296–303. Doi: 10.1651/S-2702.1 |
| Okumura T, Aida K, 2001. Effects of bilateral eyestalk ablation on molting and ovarian development in the giant freshwater prawn, Macrobrachium rosenbergii. Fish. Sci., 67(6): 1. Doi: 10.1046/j.1444-2906.2001.00370.x |
| Okumura T, Hans C H, Suzuki Y, Haynu I, 1992. Changes in hemolymph vitellogenin and ecdysteroid levels during the reproductive and non-reproductive molt cycles in the freshwater prawn Macrobrachium nipponense. Zool. Sci., 9(1): 37–45. |
| Okumura T, Sakiyama K, 2004. Hemolymph levels of vertebratetype steroid hormones in female kuruma prawn Marsupenaeus japonicus (Crustacea: Decapoda: Penaeidae) during natural reproductive cycle and induced ovarian development by eyestalk ablation. Fish. Sci., 70(3): 372–380. Doi: 10.1111/fis.2004.70.issue-3 |
| Okumura T, 2004. Perspectives on hormonal manipulation of shrimp reproduction. Jpn. Agric. Res., 38(1): 49–54. Doi: 10.6090/jarq.38.49 |
| Palacios E, Carreno D, Rodríguez-Jaramillo M C, Racotta I S, 1999. Effect of eyestalk ablation on maturation, larval performance and biochemistry of white pacific shrimp, Penaeus vannamei broodstock. J. Appl. Aquacul., 9(3): 1–23. Doi: 10.1300/J028v09n03_01 |
| Phlippen M K, Webster S G, Chung J S, Dircksen H, 2000. Ecdysis of decapod crustaceans is associated with a dramatic release of crustacean cardioactive peptide into the haemolymph. J. Exp. Biol., 203(3): 521–536. |
| Primavera J H. 1985. A review of maturation and reproduction in closed thelycum Penaeids. In: Taki Y, Primavera J H, Llobera J A eds. Proceedings of the First International Conference on the culture of Penaeid Prawn/Shrimp sp. Aquaculture Department Southeast Asian Fisheries Development Center, Iloilo City, Philippines. p. 47-64. |
| Reddy P R, Nagaraju G P C, Reddy P S, 2004. Involvement of methyl farnesoate in the regulation of molting and reproduction in the freshwater crab Oziotelphusa senex senex. J. Crust. Biol., 24(3): 511–515. Doi: 10.1651/C-2478 |
| Shyamal S, Anilkumar G, Bhaskaran R, Doss G P, Durica D S, 2015. Significant fluctuations in ecdysteroid receptor gene (EcR) expression in relation to seasons of molt and reproduction in the grapsid crab, Metopograpsus messor (Brachyura: Decapoda). Gen. Comp. Endocrinol., 211: 39–51. Doi: 10.1016/j.ygcen.2014.11.006 |
| Shyamal S, Sudha K, Gayathri N, Anilkumar G, 2014. The Y-organ secretory activity fluctuates in relation to seasons of molt and reproduction in the brachyuran crab, Metopograpsus messor (Grapsidae): ultrastructural and immunohistochemical study. Gen. Comp. Endocrinol., 196: 81–90. Doi: 10.1016/j.ygcen.2013.11.016 |
| Stella V S, Greco L S L, Rodríguez E M, 2000. Effects of eyestalk ablation at different times of the year on molting and reproduction of the estuarine grapsid crab Chasmagnathus granulata (Decapoda, Brachyura). J. Crust. Biol., 20(2): 239–244. Doi: 10.1163/20021975-99990036 |
| Subramoniam T, 2000. Crustacean ecdysteriods in reproduction and embryogenesis. Comp. Biochem. Physiol. Part C, 125(2): 135–156. Doi: 10.1016/S0742-8413(99)00098-5 |
| Subramoniam T, 2011a. Mechanisms and control of vitellogenesis in crustaceans. Fish. Sci., 77(1): 1–21. Doi: 10.1007/s12562-010-0301-z |
| Subramoniam T, 2011b. In search of a gonadotropic hormone in Crustacea. Curr. Sci., 101(1): 18–19. |
| Sudha K, Anilkumar G, Brown J H. 2011. Ecdysteroid levels related to growth and reproduction in the freshwater prawn Macrobrachium rosenbergii. In: Proceedings AsiaPacific Aquaculture 2011. Kochi, India. |
| Sudha K, Anilkumar G, 1996. Seasonal growth and reproduction in a highly fecund brachyuran crab, Metopograpsus messor (Forskal) (Grapsidae). Hydrobiologia, 319(1): 15–21. Doi: 10.1007/BF00020967 |
| Sudha K, Anilkumar G, 2007. Elevated ecdysteroid titer and precocious molt and vitellogenesis induced by eyestalk ablation in the estuarine crab, Metopograpsus messor (Brachyura: Decapoda). J. Crust. Biol., 27(2): 304–308. Doi: 10.1651/S-2750.1 |
| Sudha K, Supriya N T, Krishnakumar V, Anilkumar G, Brown J H. 2010. Ecdysteroid levels in three decapod species in relation to growth and reproduction: comparative study. In: Proceedings 7th International Crustacean Congress (ICC7). Qingdao, China. |
| Sudha K, 1992. Studies on oogenesis and the role of storage tissues in Decapod crustaceans. Calicut University, Kerala, India. |
| Suganthi A S, Anilkumar G, 1999. Moult related fluctuation in ecdysteroid titre and spermatogenesis in the crab, Metopograpsus messor (Brachyura: Decapoda). Zool. Stud., 38(3): 314–321. |
| Supriya N T, 2011. Studies on hormonal regulation and storage physiology of growth and reproduction in decapod crustaceans. Kannur University, Kannur, India. |
| Swevers L, Iatrou K. 2009. Ecdysteroids and ecdysteroid signaling pathways during insect oogenesis. In: Smagghe G ed. Ecdysone: Structures and Functions. Springer, Netherlands. p. 127-164. |
| Syama V P, Supriya N T, Sudha K, Anilkumar G. 2010. Seasonal growth and reproduction in two brachyuran species inhabiting diverse ecosystems. In: Gupta V K, Verma A K, Singh J D eds. Perspectives in Animal Ecology and reproduction, Vol. 6. Daya publications, New Delhi. |
| Tamone S L, Adams M M, Dutton J M, 2005. Effect of eyestalkablation on circulating ecdysteroids in hemolymph of snow crabs, Chionoecetes opilio: physiological evidence for a terminal molt. Integr. Comp. Biol., 45(1): 166–171. Doi: 10.1093/icb/45.1.166 |
| Tsukimura B, 2001. Crustacean vitellogenesis: it's role inoocyte development. Am. Zool., 41(3): 465–476. Doi: 10.1093/icb/41.3.465 |
| Uawisetwathana U, Leelatanawit R, Klanchui A, Prommoon J, Klinbunga S, 2011. Insights into Eyestalk Ablation Mechanism to Induce Ovarian Maturation in the Black Tiger Shrimp. PLoS One, 6(9). Doi: 10.1371/journal.pone.0024427 |
| van Herp F, Soyez D. 1997. Arthropoda-Crustacea. In: Adiyodi K G, Adiyodi R G eds. Progress in Reproductive Endocrinology, Vol. Ⅷ. Reproductive Biology of Invertebrates. Wiley and Sons, New York, USA. |
| Venkitraman P R, Jayalakshmy K V, Balasubramanian T, Nair M, Nair K K, 2004. Effects of eyestalk ablation on moulting and growth of penaeid prawn Metapenaeus dobsoni (de man). Indian J. Exp. Biol., 42(4): 403–412. |
| Wilder M N, Aida K, 1995. Crustacean ecdysteroids and juvenoids: chemistry and physiological roles in two species of prawn, Macrobrachium rosenbergii and Penaeus japonicus. Israeli J. Aquacul. Bamidgeh, 47(3-4): 129–136. |
| Wilder M N, Okumura T, Aida K, Hanyu I, 1990. Ecdysteroid fluctuations during embryogenesis in the giant freshwater prawn, Macrobrachium rosenbergii. Gen. Comp. Endocrinol., 80(1): 93–100. Doi: 10.1016/0016-6480(90)90152-C |
| Young N J, Webster S G, Rees H H, 1993. Ovarian and hemolymph ecdysteroid titers during vitellogenesis in Macrobrachium rosenbergii. Gen. Comp. Endocrinol., 90(2): 183–191. Doi: 10.1006/gcen.1993.1073 |
| Zacharia S, Kakati V S, 2003. Effect of eyestalk ablation on ovarian maturation in the banana prawn, Fenneropenaeus merguiensis de Man under different environmental conditions. J. Mar. Biol. Ass. India, 45(1): 111–114. |
| Zhu L S, Miura K, Chen L, Raikhel A S, 2003. Cyclicity of mosquito vitellogenic ecdysteroid-mediated signaling is modulated by alternative dimerization of the RXR homologue Ultraspiracle. Proc. Natl. Acad. Sci. U. S. A., 100(2): 544–549. Doi: 10.1073/pnas.0235695100 |
| Žitňan D, Kim Y J, ŽitňanováI, Roller L, Adams M E, 2007. Complex steroid-peptide-receptor cascade controls insect ecdysis. Gen. Comp. Endocrinol., 153(1-3): 88–96. Doi: 10.1016/j.ygcen.2007.04.002 |
| Zmora N, Trant J, Zohar Y, Chung J S, 2009. Molt-inhibiting hormone stimulates vitellogenesis at advanced ovarian developmental stages in the female blue crab, Callinectes sapidus 1: an ovarian stage dependent involvement. Saline Syst., 5(1): 7. Doi: 10.1186/1746-1448-5-7 |
 2017, Vol. 35
2017, Vol. 35


