Institute of Oceanology, Chinese Academy of Sciences
Article Information
- XU Rui(徐蕊), LI Xiaoming(李晓明), XU Gangming(徐刚明), WANG Bingui(王斌贵)
- Optimizing production of asperolide A, a potential anti-tumor tetranorditerpenoid originally produced by the algal-derived endophytic fungus Aspergillus wentii EN-48
- Chinese Journal of Oceanology and Limnology, 35(3): 658-663
- http://dx.doi.org/10.1007/s00343-017-6028-2
Article History
- Received Feb. 1, 2016
- accepted in principle Mar. 31, 2016
2 University of Chinese Academy of Sciences, Beijing 100049, China
Norditerpenoids are a class of widely distributed natural products with diverse chemical structures and important biological properties (Sun et al., 2012; Jeker et al., 2013). We recently isolated and described asperolide A (Fig. 1), a tetranorlabdane diterpenoid with cytotoxic activities, from the marine algaderived endophytic fungus Aspergillus wentii EN-48 (Sun et al., 2012). We very recently proved that asperolide A inhibited the growth of NCI-H460 lung cancer cells by G2/M arrest through the activation of the Ras/Raf/MEK/ERK signaling pathway, and in vivo studies showed a marked inhibition of tumor growth with lower toxicity than conventional treatment (Lv et al., 2013). These findings support the therapeutic use of asperolide A as a treatment of nonsmall-cell lung cancer, the leading cause of cancerrelated deaths worldwide (Ferlay et al., 2015; Gomes et al., 2015).

|
| Figure 1 Chemical structure, extracted UV spectrum and HPLC analysis of purified asperolide A |
The first total synthesis of asperolide C, a simpler congener of asperolide A, has been recently reported (Jeker et al., 2013). However, the supply of asperolide A relies on isolation from natural sources and production of asperolide A in A. wentii EN-48 is very low (only ~0.3 mg/L), obstructing preclinical and mode of action studies. A. wentii is a common terrestrial-sourced species used for industrial enzyme and toxin production (Brian et al., 1965; Bräse et al., 2009) and is rarely found in the marine environment (Sun et al., 2012). Asperolide A is only produced by static cultures in very low yield, which indicates that the pathway for the synthesis of this compound is silent under normal fermentation conditions. Production of this silent natural product might be activated by environmental factors, which is very interesting and justifies further investigation (Miao et al., 2014).
This paper describes the optimization of the fermentation conditions of A. wentii EN-48 for the production of asperolide A. We study different environmental factors such as various culture media, initial pH, salinity, culture time, temperature, and light conditions to simulate the original habitat of A. wentii in order to improve the production of asperolide A. The individual and interactive effects of three factors (initial pH, salinity, and culture time) are further optimized by response surface methodology (RSM) to consider the feasibility of these conditions for large scale fermentation. We verify the results of our model by scale-up fermentation of A. wentii for production of asperolide A. Considering that A. wentii EN-48 is a marine alga-derived endophytic fungus, we investigate the effects of plant hormones on the production of asperolide A (Prusty et al., 2004; Pu et al., 2013).
2 MATERIAL AND METHOD 2.1 Fungal strain and chemicalsThe endophytic fungus A. wentii EN-48 was isolated from a marine brown alga (Sun et al., 2012). Modified potato dextrose broth (PDB) medium was used for liquid static fermentation of A. wentii EN-48 as described previously (Sun et al., 2012). All chemicals used were obtained from commercial sources.
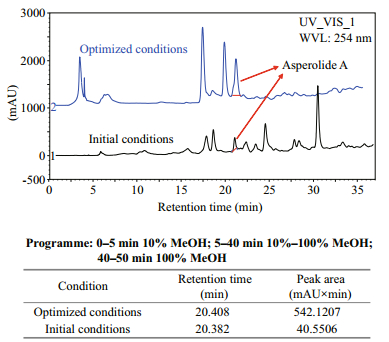
2.2 Fermentation, extraction, and high performance liquid chromatography (HPLC) analysisThe general procedure for fermentation and extraction of metabolites was a protocol we have previously reported (Xu et al., 2015). The mycelia and culture broth were separated by filtration after fermentation. The mycelia were extracted with acetone aided by an ultrasonic cell crusher, then extracted with ethyl acetate and the supernatant was extracted with ethyl acetate. The organic phases of the extracts were combined and concentrated under reduced pressure. The metabolites in the resulting residue were analyzed by HPLC (Dionex, C18 column) with detection at 254 nm using gradient elution of water-methanol (0-5 min 10% MeOH, 5-40 min 10%-100% MeOH, 40-50 min 100% MeOH) at a flow rate of 1.0 mL/min (Fig. 1). A standard calibration curve was generated from purified asperolide A dissolved in methanol as standard solution and used for quantification.
2.3 Culture conditions for asperolide A productionThe effects of different environmental factors were studied to determine the optimal culture conditions for the cultivation of A. wentii to maximize the production of asperolide A (Pu et al., 2013). Singlefactor optimization experiments were performed using various salinity (70.0, 35.0, 17.5, 8.75, and 0), initial pH (10, 8, 6, 4, and 2), temperature (30, 28, 25, 23, and 20℃), and culture time (40, 30, 25, 20, and 15 d). All experiments were carried out in triplicate, and the average values±standard deviations (SD) are presented in the experimental results.
2.4 Optimization of the asperolide A production by response surface methodologyA Box-Behnken design (BBD) and response surface methodology (RSM) were employed to identify experiments to further optimize our fermentation (Zhu et al., 2014). We identified three factors (initial pH, salinity, and culture time) for optimization for the asperolide A production from the results of single-factor experiments and considering feasible conditions for scale-up fermentation in our lab (Table 1). The experiments were designed with three factors and three levels. The complete design was carried out randomly and consisted of 15 combinations including three central points. The value of each dependent response was the average of three independent experiments. The data obtained from the RSM were analyzed by multiple regression and fitted to a quadratic polynomial model as below:
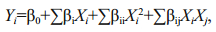 (1)
(1)where Yi is the predicted response; Xi and Xj are levels of the independent variables; β0 is the offset term; and βi, βii and βij are the linear, quadratic and interactive coefficients, respectively. Quadratic polynomial regression fitting was carried out using the statistical software Design Expert (version 8.0.6, Stat-Ease Inc., Minneapolis, MN, USA). The response surface analysis of asperolide A production and the verification tests were performed according to the predicted optimized conditions. Analysis of variance (ANOVA) was used to process the data from the model. All the experiments were performed three times, and the mean values±SD were used for statistical analysis.
The effects of different plant hormones on the induction of asperolide A production, including gibberellic acid (GA), indole-3-acetic acid (IAA), methyl jasmonate (MeJA), and salicylic acid (SA), were studied using normal culture conditions (Prusty et al., 2004; Pu et al., 2013). Plant hormones were exogenously added to the medium with final concentrations of 10 and 100 nmol/L and 10 and 100 μmol/L. The extraction and metabolite analyses were performed as described previously (Sun et al., 2012).
3 RESULT AND DISCUSSION 3.1 Effects of environmental factors on the production of asperolide ADifferent environmental factors were tested to determine the optimal culture conditions to improve the production of asperolide A by simulation of A. wentii's original habitat (Pu et al., 2013). We have previously established that the fungus A. wentii EN-48 grows and produces spores well in modified PDB medium to give the largest biomass and total extracts (Sun et al., 2012) and we chose the modified PDB medium for further investigations on this basis. Static fermentations were undertaken in modified PDB medium to identify the effects of various salinity, initial pH, temperature, and culture time to enhance production of asperolide A.
A. wentii is a common terrestrial species and is rarely found in the marine environment (Brian et al., 1965; Bräse et al., 2009). Marine-derived fungi are more tolerant to salinity than terrestrial species (Wang et al., 2011) and the salinity of the culture medium could significantly influence the mycelial growth and metabolite production from a marine-derived microorganism. It is reasonable that a suitable salinity level is required for the marine-derived fungus to produce the target metabolite in higher yield. The effects of medium salinity on the production of asperolide A in A. wentii EN-48 were investigated. Moderate salinity was favorable for formation of asperolide A, and maximum production was obtained at a salinity of 17.5 (Fig. 2a).
|
| Figure 2 Effects of various environmental factors on the asperolide A production a. salinity; b. initial pH; c. temperature; d. culture time. Values are means±SD from three replicates. |
A. wentii EN-48 was also inoculated using modified PDB medium with the initial pH values of 10, 8, 6, 4, and 2, respectively. The optimal pH value for the production of asperolide A was about pH 6 (Fig. 2b). Although the initial pH of the medium was 2-10, the final pH value of all the fermentations was around 3-4 after cultivation. We speculate that during the course of fermentation this fungus might produce some metabolites that make the media slightly acidic. Changes to the pH during fermentation might also have some influence on the accumulation of asperolide A (Andersen et al., 2009).
The temperature is closely related to the growth and metabolism of fungi. The production of asperolide A was increased with decreased culture temperature (Fig. 2c). Maximal asperolide A production occurred at 20-23℃. However, 24-26℃ is the most appropriate temperature for the growth of A. wentii strains (Brian et al., 1965), suggesting temperature lower than that required for optimal growth is favorable to active the biosynthetic pathway of asperolide A.
The fungus was fermented in modified PDB medium and harvested at 40, 30, 25, 20, and 15 d to establish the optimal culture time. Asperolide A was produced at a high level between 25-30 d (Fig. 2d) and a cultivation time of 30 d was used for further experiments. Asperolide A is likely synthesized during the later stage of fermentation after active growth has ceased, unlike many other fungal metabolites (Pettit, 2011).
3.2 Optimized production of asperolide A by RSMThe effects of initial pH value, salinity, and culture time on the production of asperolide A were further optimized by employing RSM based on the results obtained from the single-factor experiments (Pu et al., 2013; Zhu et al., 2014). The regression equation describing the relationship between the production of asperolide A and the test variables in coded units is:
 (2)
(2)where Y is the predicted response of asperolide A production; and X1, X2, and X3 are the coded values of three factors in the BBD.
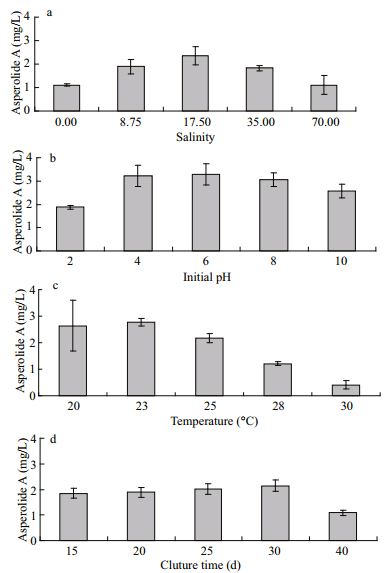
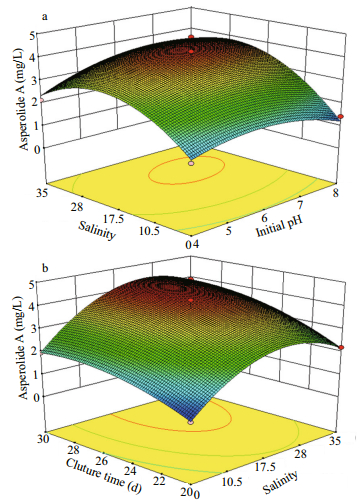
The model F-value (32.55) and P-value (0.000 2) indicate that the model terms are significant (Table 2). The lack of fit F-value (1.10) implies that this model could predict variation accurately. A good regression coefficient (R2) was calculated (0.977 5), indicating that the three fermentation factors had a quadratic relation to asperolide A production and an adequate precision of 15.444 was obtained for the data. The predicted R2 is consistent with the value of the adjusted R2, suggesting that the experimental production of asperolide A are in good agreement with the predicted values. The results of the regression parameter estimate revealed that the linear terms of salinity (X2) and culture time (X3), as well as the interaction terms of pH (X1) and salinity (X2) had significant positive effects, while the square terms of pH (X1), salinity (X2) and culture time (X3) had significant negative effects on asperolide A production (P < 0.05). The relationship between factors and asperolide A production is indicated by the 3D surface in Fig. 3.
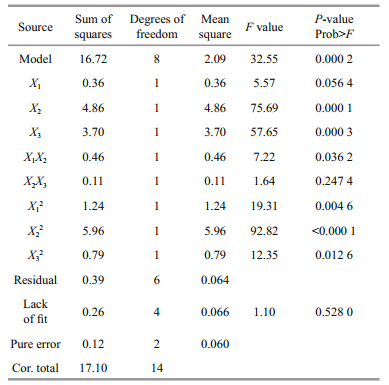
|
|
| Figure 3 Response surface plot for maximal production of asperolide A a. initial pH and salinity; b. salinity and culture time. |
The optimum fermentation conditions from the solved quadratic equation were an initial pH of 6.6, salinity 24.5, and culture time of 29 d. The theoretical highest production of asperolide A during static culture of A. wentii EN-48 was calculated at about 4.43 mg/L. The average actual production of asperolide A was 4.48 mg/L in the verification tests of repeated experiments employing the optimized culture conditions, which was 13.9-fold higher than that obtained under initial culture condition (Fig. 4). The optimum conditions were used in scale-up fermentation for asperolide A production in three batches. The fermentation was carried out at room temperature with natural light conditions, with each batch in 500 flasks. The results suggested that the RSM optimization for the asperolide A production was effective and feasible. The purified asperolide A from the scale-up fermentation was used for further drug evaluation and anti-tumor mechanism studies.
|
| Figure 4 HPLC profiles and peak areas of asperolide A under optimized culture conditions compared with initial conditions |
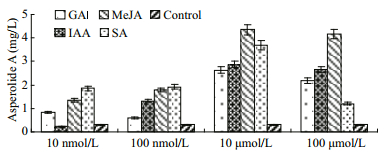
The effects of plant hormones, including GA, IAA, MeJA, and SA, on the asperolide A production during fermentation were determined. Final concentrations (10 nmol/L, 100 nmol/L, 10 μmol/L, and 100 μmol/L) of each plant hormone were added into the cultures of A. wentii EN-48. Exogenous addition of MeJA at 10 μmol/L increased asperolide A production to 4.85 mg/L under normal culture conditions (Fig. 5). Different concentrations of plant hormones might have different effects on the asperolide A production: at lower concentrations they might act as an induction signal, whereas at higher concentrations they might cause stress response. Plant hormones might affect the fungal growth and development, and also affect complex regulation of secondary metabolism (Akiyama et al., 2005). The rational design for addition time and concentration of plant hormones for the induction of secondary metabolites still requires further studies.
|
| Figure 5 Effects of plant hormones on asperolide A production |
Static culture conditions were optimized for the production of asperolide A from marine alga-derived fungus A. wentii EN-48 by employing RSM. The optimal fermentation conditions were: initial pH 6.6, salinity 24.5, culture time 29 d, in modified PDB medium, at room temperature (23±1℃) and with natural light. These conditions gave a 13.9-fold yield enhancement of asperolide A compared with the initial conditions and the improvement was further verified by scale-up fermentation. Exogenous addition of plant hormones (especially 10 μmol/L of MeJA) also stimulated asperolide A production. The results from this study might provide useful for optimizing the culture conditions of other fungal strains to enhance the production of related metabolites. Further attempts such as a genomic approach aimed at enhancing production yields should be carried out.
| Akiyama K, Matsuzaki K I, Hayashi H, 2005. Plant sesquiterpenes induce hyphal branching in arbuscular mycorrhizal fungi. Nature, 435(7043): 824–827. Doi: 10.1038/nature03608 |
| Andersen M R, Lehmann L, Nielsen J, 2009. Systemic analysis of the response of Aspergillus niger to ambient pH. Genome Biol., 10(5): R47. Doi: 10.1186/gb-2009-10-5-r47 |
| Bräse S, Encinas A, Keck J, Nising C F, 2009. Chemistry and biology of mycotoxins and related fungal metabolites. Chem. Rev., 109(9): 3903–3990. Doi: 10.1021/cr050001f |
| Brian P W, Elson G W, Hemming H G, Radley M, 1965. An inhibitor of plant growth-produced by Aspergillus wentii Wehmer. Nature, 207(5000): 998–999. Doi: 10.1038/207998a0 |
| Ferlay J, Soerjomataram I, Dikshit R, Eser S, Mathers C, Rebelo M, Parkin D M, Forman D, Bray F, 2015. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int. J. Cancer, 136(5): E359–E386. Doi: 10.1002/ijc.29210 |
| Gomes N G M, Lefrance F, Kijjoa A, Kiss R, 2015. Can some marine-derived fungal metabolites become actual anticancer agents?. Mar. Drugs, 13(6): 3950–3991. |
| Jeker O F, Kravina A G, Carreira E M, 2013. Total synthesis of (+)-asperolide C by iridium-catalyzed enantioselective polyene cyclization. Angew. Chem. Int. Ed., 52(46): 12166–12169. Doi: 10.1002/anie.201307187 |
| Lv C T, Sun W X, Sun H X, Wei S J, Chen R H, Wang B G, Huang C G, 2013. Asperolide A, a marine-derived tetranorditerpenoid, induces G2/M arrest in human NCI-H460 lung carcinoma cells, is mediated by p53-p21 stabilization and modulated by Ras/Raf/MEK/ERK signaling pathway. Mar. Drugs, 11(2): 316–331. Doi: 10.3390/md11020316 |
| Miao F P, Liang X R, Liu X H, Ji N Y, 2014. Aspewentins A-C, norditerpenes from a cryptic pathway in an algicolous strain of Aspergillus wentii. J. Nat. Prod., 77(2): 429–432. Doi: 10.1021/np401047w |
| Pettit R K, 2011. Small-molecule elicitation of microbial secondary metabolites. Microbiol. Biotechnol., 4(4): 471–478. Doi: 10.1111/j.1751-7915.2010.00196.x |
| Prusty R, Grisafi P, Fink G R, 2004. The plant hormone indoleacetic acid induces invasive growth in Saccharomyces cerevisiae. Proc. Natl. Acad. Sci. U. S. A, 101(12): 4153–4157. Doi: 10.1073/pnas.0400659101 |
| Pu X, Qu X X, Chen F, Bao J K, Zhang G L, Luo Y G, 2013. Camptothecin-producing endophytic fungus Trichoderma atroviride LY357: isolation, identification, and fermentation conditions optimization for camptothecin production. Appl. Microbiol. Biotechnol., 97(21): 9365–9375. Doi: 10.1007/s00253-013-5163-8 |
| Sun H F, Li X M, Meng L, Cui C M, Gao S S, Li C S, Huang C G, Wang B G, 2012. Asperolides A-C, tetranorlabdane diterpenoids from the marine alga-derived endophytic fungus Aspergillus wentii EN-48. J. Nat. Prod., 75(2): 148–152. Doi: 10.1021/np2006742 |
| Wang Y, Zheng J K, Liu P P, Wang W, Zhu W M, 2011. Three new compounds from Aspergillus terreus PT06-2 grown in a high salt medium. Mar. Drugs, 9(8): 1368–1378. |
| Xu R, Xu G M, Li X M, Li C S, Wang B G, 2015. Characterization of a newly isolated marine fungus Aspergillus dimorphicus for optimized production of the anti-tumor agent wentilactones. Mar. Drugs, 13(11): 7040–7054. |
| Zhu Y X, Yao L Y, Jiao R H, Lu Y H, Tan R X, 2014. Enhanced production of Fumigaclavine C in liquid culture of Aspergillus fumigatus under a two-stage process. Bioresour. Technol., 152: 162–168. Doi: 10.1016/j.biortech.2013.10.089 |
 2017, Vol. 35
2017, Vol. 35